The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites
Abstract
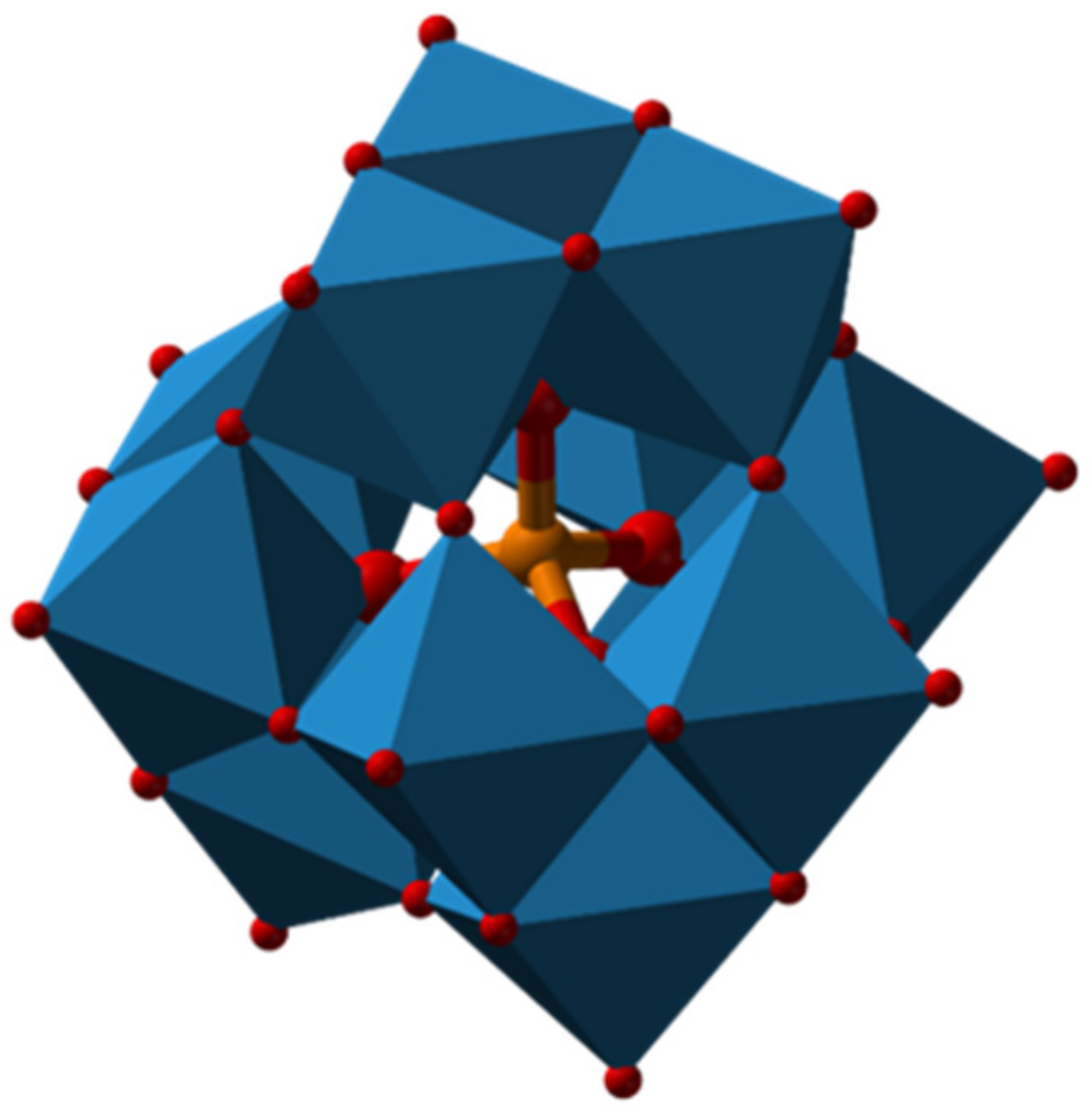
:1. Introduction
2. Experiments
2.1. Materials and AMP–PAN Composite Preparation
2.2. Irradiation
2.3. Characterisation
3. Results and Discussion
3.1. Chemical Changes Induced in AMP upon Irradiation
3.2. Effect or Irradiation on the Structure of the AMP–PAN Composite
3.3. Effect of Irradiation on the Thermal Stability of AMP and AMP–PAN
3.4. Implications of Radiolysis Mechanisms
3.5. Considerations for Use of AMP–PAN in Spent Fuel Recycling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A






References
- Carlton, J.S.; Smart, R.; Jenkins, V. The nuclear propulsion of merchant ships: Aspects of engineering, science and technology. J. Mar. Eng. Technol. 2011, 10, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Tavoni, M.; van der Zwaan, B. Nuclear Versus Coal plus CCS: A Comparison of Two Competitive Base-Load Climate Control Options. Environ. Model. Assess. 2011, 16, 431–440. [Google Scholar] [CrossRef]
- National Research Council. Nuclear Wastes: Technologies for Separations and Transmutation; National Academy Press: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Aoyama, M. Evidence of stratospheric fallout of caesium isotopes from the Chernobyl accident. Geophys. Res. Let. 1988, 15, 327–330. [Google Scholar] [CrossRef]
- Livens, F.R.; Loveland, P.J. The influence of soil properties on the environmental mobility of caesium in Cumbria. Soil Use Manag. 1988, 4, 69–75. [Google Scholar] [CrossRef]
- Maguire, S.; Pulford, I.D.; Cook, G.T.; Mackenzie, A.B. Caesium sorption—Desorption in clay—Humic acid systems. J. Soil Sci. 1992, 43, 689–696. [Google Scholar] [CrossRef]
- Desmet, G.M.; van Loon, L.R.; Howard, B.J. Chemical speciation and bioavailability of elements in the environment and their relevance to radioecology. Sci. Tot. Environ. 1991, 100, 105–124. [Google Scholar] [CrossRef]
- Mahendra, C.; Satya Sai, P.M.; Babu, C.A.; Revathy, K.; Rajan, K.K. Analysis and modeling of fixed bed sorption of cesium by AMP-PAN. J. Environ. Chem. Eng. 2015, 3, 1546–1554. [Google Scholar] [CrossRef]
- Dyer, A.; Pillinger, M.; Amin, S. Ion exchange of caesium and strontium on a titanosilicate analogue of the mineral pharmacosiderite. J. Mater. Chem. 1999, 9, 2481–2487. [Google Scholar] [CrossRef]
- Vlasselaer, S.; D’Olieslager, W.; D’Hont, M. Caesium ion exchange equilibrium on potassium-zinc-hexacyanoferrate(II), K2Zn3[Fe(CN)6]2. Selectivity for alkali ions. J. Radioanal. Chem. 1977, 35, 211–222. [Google Scholar] [CrossRef]
- Amphlet, C.B.; McDonald, L.A. Equilibrium studies on natural ion-exchange minerals—I. Caesium and strontium. J. Inorg. Nucl. Chem. 1956, 2, 403–414. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U.; Allulli, S.; Massucci, M.A. Crystalline insoluble acid salts of tetravalent metals—XX Forward and reverse Cs+/H+ and Rb+/H+ ion exchange on crystalline zirconium phosphate. J. Inorg. Nucl. Chem. 1975, 37, 1779–1786. [Google Scholar] [CrossRef]
- Valsalaer, T.P.; Roy, S.C.; Shah, J.G.; Gabriel, J.; Raj, K.; Venugopal, V. Removal of radioactive caesium from low level radioactive waste (LLW) streams using cobalt ferrocyanide impregnated organic anion exchanger. J. Haz. Mater. 2009, 166, 1148–1153. [Google Scholar] [CrossRef]
- Saberi, R.; Nilchi, A.; Garmarodi, S.R.; Zarghami, R. Adsorption characteristic of 137Cs from aqueous solution using PAN-based sodium titanosilicate composite. J. Radioanal. Nucl. Chem. 2010, 284, 461–469. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Garmarodi, S.R.; Bagheri, A. Evaluation of PAN-based manganese dioxide composite for the sorptive removal of cesium-137 from aqueous solutions. Appl. Radiat. Isotop. 2012, 70, 369–374. [Google Scholar] [CrossRef]
- Smit, J.V.R.; Jacobs, J.J.; Robb, W. Cation exchange properties of the ammonium heteropolyacid salts. J. Inorg. Nucl. Chem. 1959, 12, 95–103. [Google Scholar] [CrossRef]
- Smit, J.V.R.; Jacobs, J.J.; Robb, W. Cation exchange on ammonium molybdophosphate—I: The alkali metals. J. Inorg. Nucl. Chem. 1959, 12, 104–112. [Google Scholar] [CrossRef]
- Sebesta, F.; Stefula, V. Composite ion exchanger with ammonium molybdophosphate and its properties. J. Radioanal. Nucl. Chem. 1990, 140. [Google Scholar] [CrossRef]
- Tranter, T.J.; Herbst, R.S.; Todd, T.A.; Olson, A.L.; Eldredge, H.B. Evaluation of ammonium molybdophosphate-polyacrylonitrile (AMP-PAN) as a cesium selective sorbent for the removal of 137 Cs from acidic nuclear waste solutions. Adv. Environ. Res. 2002, 6, 107–121. [Google Scholar] [CrossRef]
- Todd, T.A.; Mann, N.R.; Tranter, T.J.; Sebesta, F.; John, J.; Motl, A. Cesium sorption from concentrated acidic tank wastes using ammonium molybdophosphate-polyacrylonitrile composite sorbents. J. Radioanal. Nucl. Chem. 2002, 254, 47–52. [Google Scholar] [CrossRef]
- Eccles, H.; Emmott, J.D.; Bond, G. Advanced Reprocessing–The Potential for Continuous Chromatographic Separations. J. Chromatog. Sep. Tech. 2017, 8, 348. [Google Scholar] [CrossRef]
- Bond, G.; Eccles, H.; Kavi, P.C.; Holdsworth, A.F.; Rowbotham, D.; Mao, R. Removal of Cesium from Simulated Spent Fuel Dissolver Liquor. J. Chromatog. Separ. Tech. 2019, 10, 417. [Google Scholar] [CrossRef]
- Ding, D. Removal of Cesium from Aqueous Solution by Using Newly Developed Adsorbents and Comparative Study. Ph.D. Thesis, University of Tsukuba, Tsukuba, Japan, April 2014. [Google Scholar]
- Pawde, S.M.; Deskmukh, K. Influence of γ irradiation on the properties of polyacrylonitrile films. J. Appl. Polym. Sci. 2008, 2569–2578. [Google Scholar] [CrossRef]
- Papaconstantinou, E.; Hoffman, M.Z. One-Electron Reduction of 18-Molybdodiphosphate and 18-Tungstodiphosphate Ions in Aqueous Solution. A Pulse Radiolysis Study. Inorg. Chem. 1982, 21, 2087–2089. [Google Scholar] [CrossRef]
- Troupis, A.; Hiskia, A.; Papaconstantinou, E. Synthesis of Metal Nanoparticles by Using Polyoxometalates as Photocatalysts and Stabilizers. Angew. Chem. Int. Ed. 2002, 41, 1911–1914. [Google Scholar] [CrossRef]
- Sebesta, F.; John, J.; Motl, A.; Stamberg, K. Evaluation of Polyacrylonitrile (PAN) as a Binding Polymer for Absorbers Used to Treat Liquid Radioactive Wastes; Report SAND-95-2729; Sandia National Labs: Livermore, CA, USA, 1995. [Google Scholar] [CrossRef]
- Rao, K.L.N.; Mathew, C.; Deshpande, R.S.; Jadhav, A.V.; Pande, B.M.; Shulka, J.P. Effects of electron beam irradiation on inorganic exchanger AMP. Radiat. Phys. Chem. 1997, 49, 85–87. [Google Scholar] [CrossRef]
- Narasimharao, K.L.; Sarma, K.S.; Mathew, C.; Jadhav, A.V.; Shulka, J.P.; Natarajan, V.; Seshagiri, T.K.; Sali, S.K.; Dhiwar, V.I.; Pande, B.; et al. Physico-chemical and ion exchange characteristics of ammonium molybdophosphate irradiated with electrons. J. Chem. Soc. Faraday Trans. 1998, 94, 1641–1647. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Eccles, H.; Rowbotham, D.; Bond, G.; Kavi, P.C.; Edge, R. The Effect of Gamma Irradiation on the Ion Exchange Properties of Caesium-Selective Ammonium Phosphomolybdate-Polyacrylonitrile (AMP-PAN) Composites under Spent Fuel Recycling Conditions. Separations 2019, 6, 23. [Google Scholar] [CrossRef]
- Bykhovskii, D.N.; Kol’tsova, T.I.; Kuz’mina, M.A. Phases of variable composition in crystallization of cesium phosphomolybdate. Radiochemistry 2006, 48, 429–433. [Google Scholar] [CrossRef]
- Broadbank, R.W.C.; Dhabanandana, S.; Harding, R.D. A possible use of ammonium 12-molybdophosphate for assaying certain radioactive fission products in water. Analyst 1960, 85, 365–370. [Google Scholar] [CrossRef]
- Paul, N.; Hammond, R.B.; Hunter, T.N.; Edmondson, M.; Maxwell, L.; Biggs, S. Synthesis of nuclear waste simulants by reaction precipitation: Formation of caesium phosphomolybdate, zirconium molybdate and morphology modification with citratomolybdate complex. Polyhedron 2015, 89, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Dash, A.; Varma, R.N.; Ram, R.; Saxena, S.K.; Mathakar, A.R.; Avhad, B.G.; Sastry, K.V.S.; Sangurdekar, P.R.; Venkatesh, M. Fabrication of Cesium-137 Brachytherapy Sources Using Vitrification Technology. Canc. Biother. Radiopharm. 2009, 24, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, Y.C.; Shin, W.S.; Choi, S.J. Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate–polyacrylonitrile (AMP–PAN). Chem. Eng. J. 2010, 162, 685–695. [Google Scholar] [CrossRef]
- Moon, J.-K.; Kim, K.-W.; Jung, C.-H.; Shui, Y.-G.; Lee, E.-H. Preparation of Organic-Inorganic Composite Adsorbent Beads for Removal of Radionuclides and Heavy Metal Ions. J. Radioanal. Nucl. Chem. 2000, 246, 299–307. [Google Scholar] [CrossRef]
- Travaglini, G.; Wachter, P.; Marcus, J.; Schlenker, C. The blue bronze K0.3MoO3: A new one-dimensional conductor. Solid State Comm. 1981, 37, 599–603. [Google Scholar] [CrossRef]
- Grassie, N.; Hay, J.N. Thermal coloration and insolubilization in polyacrylonitrile. J. Polym. Sci. 1962, 56, 163, 189–202. [Google Scholar] [CrossRef]
- Aguilera, L.; Montero, I.; Davila, M.E.; Ruiz, A.; Galan, L.; Nistor, V.; Raboso, D.; Palomares, J.; Soria, F. CuO nanowires for inhibiting secondary electron emission. J. Phys. D Appl. Phys. 2013, 46, 165104. [Google Scholar] [CrossRef]
- Gonzalez, P.J.; Rivas, M.G.; Brondino, C.D.; Bursakov, S.A.; Moura, I.; Moura, J.J.G. EPR and redox properties of periplasmic nitrate reductase from Desulfovibrio desulfuricans ATCC 27774. J. Biol. Inorg. Chem. 2006, 11, 609–616. [Google Scholar] [CrossRef]
- Pascaru, I.; Constantinescu, O.; Constantinescu, M.; Arizan, O.J. L’étude des centres paramagnétiques produits dans le Mo 7 O 24 (NH 4 ) 6.4H 2 O polycristallin irradié. Chim. Phys. 1965, 62, 1283. [Google Scholar]
- Gehringer, P. IAEA Lecture—Application of Electron Beams in Industry and Environmental Protection. In Proceedings of the IAEA’s Interregional Training Course on Developments in the Application of Electron Beams in Industry and Environmental Protection, Warsaw, Poland, 6–17 October 1997. [Google Scholar]
- Zhaoyi, T.; Zhaoya, H.; Dong, Z.; Xiaolin, W. Structural characterization of ammonium molybdophosphate with different amount of cesium adsorption. J. Radioanal. Nucl. Chem. 2014, 299, 1165–1169. [Google Scholar] [CrossRef]
- Coleman, M.M.; Sivy, G.T. Polymer Characterization; Craver, C.D., Ed.; ACS: Washington, DC, USA, 1983; pp. 559–570. [Google Scholar]
- Liu, W.; Wang, M.; Xing, Z.; Wu, G. The free radical species in polyacrylonitrile fibers induced by γ-radiation and their decay behaviors. Radiat. Phys. Chem. 2012, 81, 835–839. [Google Scholar] [CrossRef]
- Ilhan, S.; Kahruman, C.; Yusufoglu, I. Characterization of the thermal decomposition products of ammonium phosphomolybdate hydrate. J. Anal. Appl. Pyrol. 2007, 78, 363–370. [Google Scholar] [CrossRef]
- Misono, M. Heterogeneous Catalysis by Heteropoly Compounds of Molybdenum and Tungsten. Catal. Rev. Sci. Eng. 1987, 29, 269–321. [Google Scholar] [CrossRef]
- Tsigdinos, G.A. Heteropoly compounds of molybdenum and tungsten. Top. Curr. Chem. 1977, 76, 1–64. [Google Scholar] [CrossRef]
- Ilhan, S.; Kalpakli, A.O.; Kahruman, C.; Yusufoglu, I. The investigation of thermal decomposition mechanism of ammonium phosphotungstate hydrate in inert gas atmosphere. Thermochim. Acta 2012, 546, 1–7. [Google Scholar] [CrossRef]
- Borkel, C. Understanding the Mobility of Caesium, Nickel and Selenium Released from Waste Disposal: Chemical Retention Mechanisms of Degraded Cement. Ph.D. Thesis, Polytechnic University of Catalonia, Barcelona, Spain, 11 November 2015. [Google Scholar]
- Tranter, T.J.; Vereshchagina, T.A.; Utgikar, V. An Inorganic Microsphere Composite for the Selective Removal of 137Cesium from Acidic Nuclear Waste Solutions. 1: Equilibrium Capacity and Kinetic Properties of the Sorbent. Solv. Extr. Ion Exch. 2009, 27, 199–218. [Google Scholar] [CrossRef]
- Horowitz, E.P.; Dietz, M.L.; Diamond, H.; Rogers, R.D.; Leonard, R.A. Advanced chemical separations in support of the clean option strategy. Global ‘93—Future Nuclear Systems: Emerging Fuel Cycles and Waste Disposal Options, Seattle, WA, USA, 12–17 September 1993. [Google Scholar]
- Egorov, E.V.; Novikov, P.D. Action of Ionizing Radiation on Ion Exchange Materials; Israel Program for Scientific Translations: Jerusalem, Israel, 1967; pp. 74–77. [Google Scholar]
- Zhao, W.; Yamamoto, Y.; Tagawa, S. Regulation of the Thermal Reactions of Polyacrylonitrile by γ-Irradiation. Chem. Mater. 1999, 11, 1030–1034. [Google Scholar] [CrossRef]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The Potential of Metal Oxalates as Novel Flame Retardants and Synergists for Engineering Polymers. Polym. Degrad. Stab. 2014, 110, 290–297. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Synthesis and thermal analytical screening of metal complexes as potential novel fire retardants in polyamide 6.6. Polym. Degrad. Stab. 2017, 144, 420–433. [Google Scholar] [CrossRef]
- Holdsworth, A.F. Novel Metal Complex Fire Retardants for Engineering Polymers. Ph.D. Thesis, University of Bolton, Bolton, UK, 2015. [Google Scholar]
- Salles, V.; Bernard, S.; Brioude, A.; Cornu, D.; Miele, P. A new class of boron nitride fibers with tunable properties by combining an electrospinning process and the polymer-derived ceramics route. Nanoscale 2010, 2, 215–217. [Google Scholar] [CrossRef]
- Edmonds, I.; Love, C.E.; Harvey, T. Effects of Gamma Irradiation on the Molecular and Physical Detection Properties of Bacillus Spores. J. Bioterror. Biodef. 2018, 9, 158. [Google Scholar] [CrossRef]
- Nagaishi, R.; Jiang, P.Y.; Katsumura, Y.; Domae, M.; Ishigure, K. Radiolysis of Concentrated Nitric Acid Solutions. In Proceedings of the 6th Japan-China Bilateral Symphosium on Radiation Chemistry, Waseda University, Tokyo, Japan, 6–11 November 1994. [Google Scholar]
- Nagaishi, R. A model for radiolysis of nitric acid and its application to the radiation chemistry of uranium ion in nitric acid medium. Radiat. Phys. Chem. 2001, 60, 369–375. [Google Scholar] [CrossRef]
- IAEA Nuclear Data. Available online: https://www-nds.iaea.org/wimsd/fpyield.htm (accessed on 15 April 2018).









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holdsworth, A.F.; Eccles, H.; Rowbotham, D.; Brookfield, A.; Collison, D.; Bond, G.; Kavi, P.C.; Edge, R. The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites. Clean Technol. 2019, 1, 294-310. https://doi.org/10.3390/cleantechnol1010020
Holdsworth AF, Eccles H, Rowbotham D, Brookfield A, Collison D, Bond G, Kavi PC, Edge R. The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites. Clean Technologies. 2019; 1(1):294-310. https://doi.org/10.3390/cleantechnol1010020
Chicago/Turabian StyleHoldsworth, Alistair F., Harry Eccles, Daniel Rowbotham, Adam Brookfield, David Collison, Gary Bond, Parthiv C. Kavi, and Ruth Edge. 2019. "The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites" Clean Technologies 1, no. 1: 294-310. https://doi.org/10.3390/cleantechnol1010020
APA StyleHoldsworth, A. F., Eccles, H., Rowbotham, D., Brookfield, A., Collison, D., Bond, G., Kavi, P. C., & Edge, R. (2019). The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites. Clean Technologies, 1(1), 294-310. https://doi.org/10.3390/cleantechnol1010020







