Abiotic Nitrogen Mineralization of Peptone by γ-MnO2: Effects of Dissolved Oxygen and pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Design
2.3. Analysis Methods
3. Results
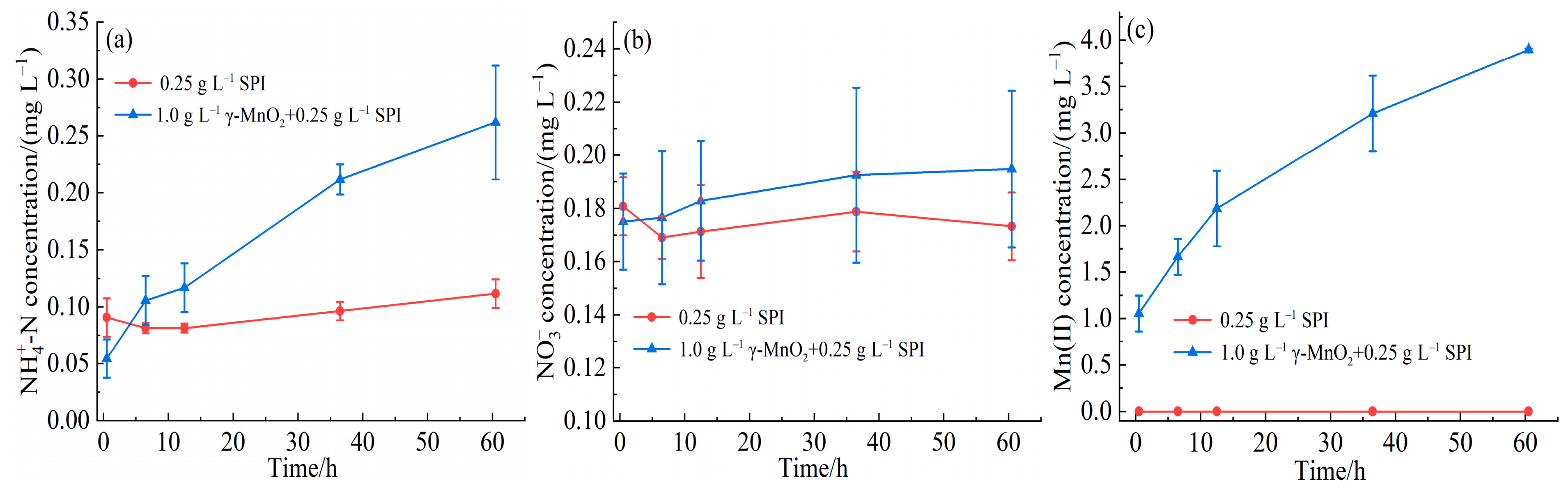
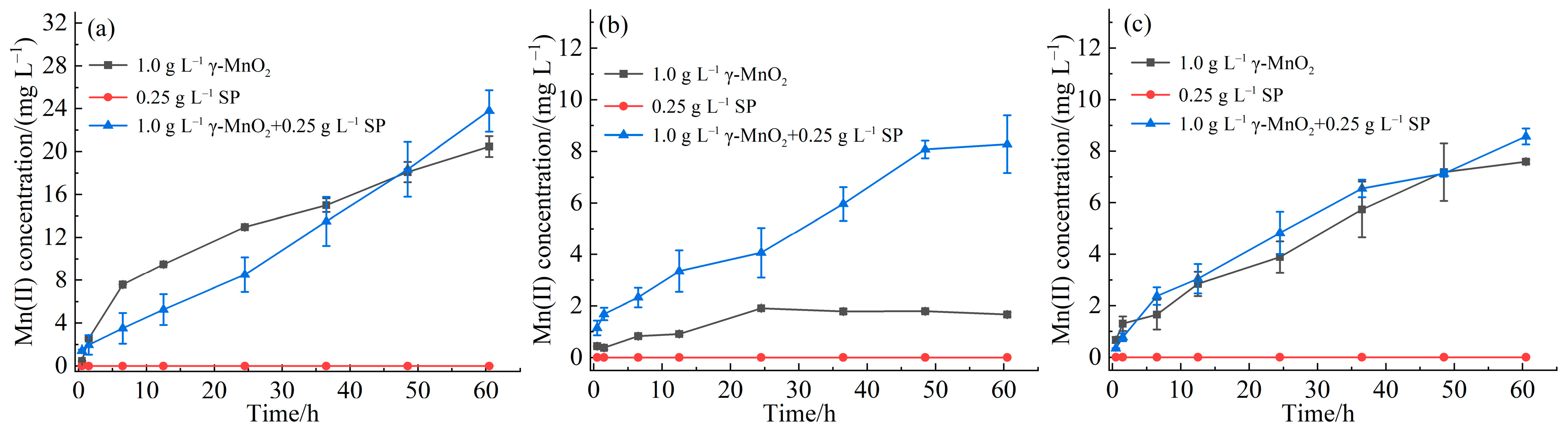
3.1. Interaction Between γ-MnO2 and SPI
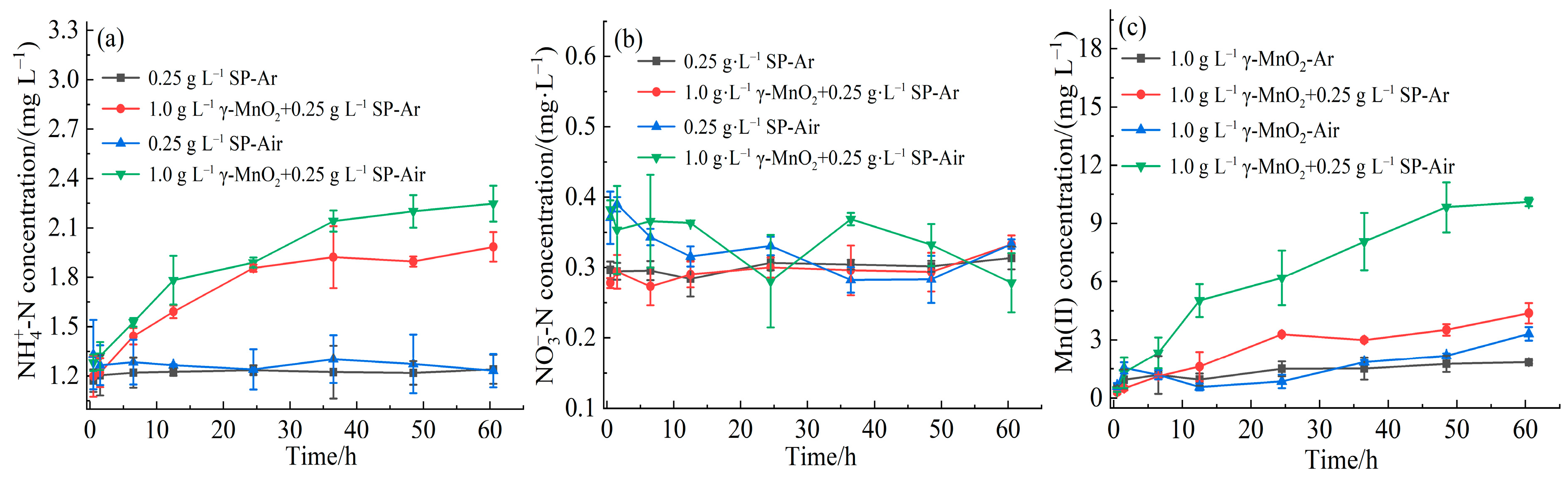
3.2. Effect of Dissolved Oxygen on Abiotic Nitrogen Mineralization
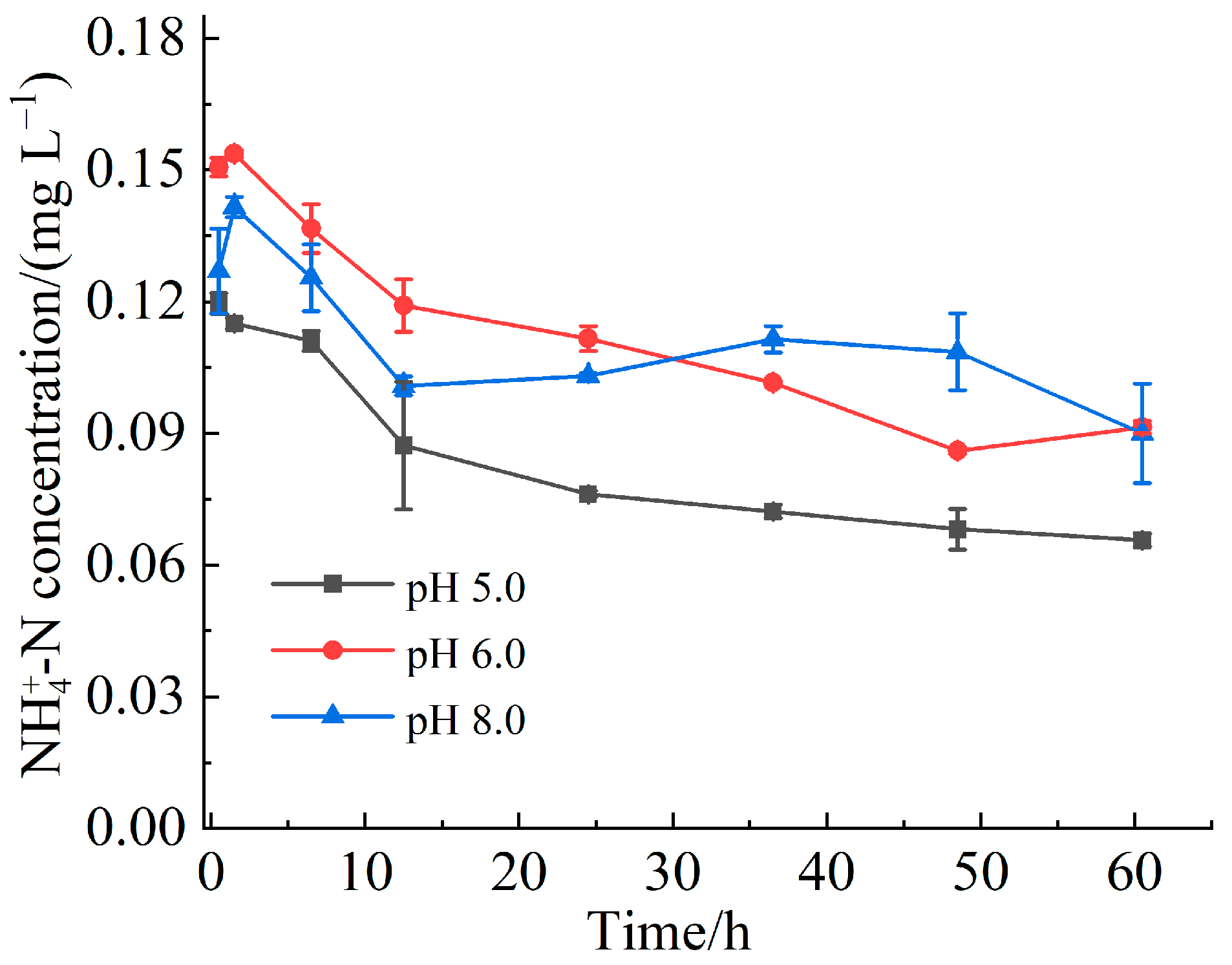
3.3. Effect of pH on Abiotic Nitrogen Mineralization
4. Discussion
4.1. Effect of pH
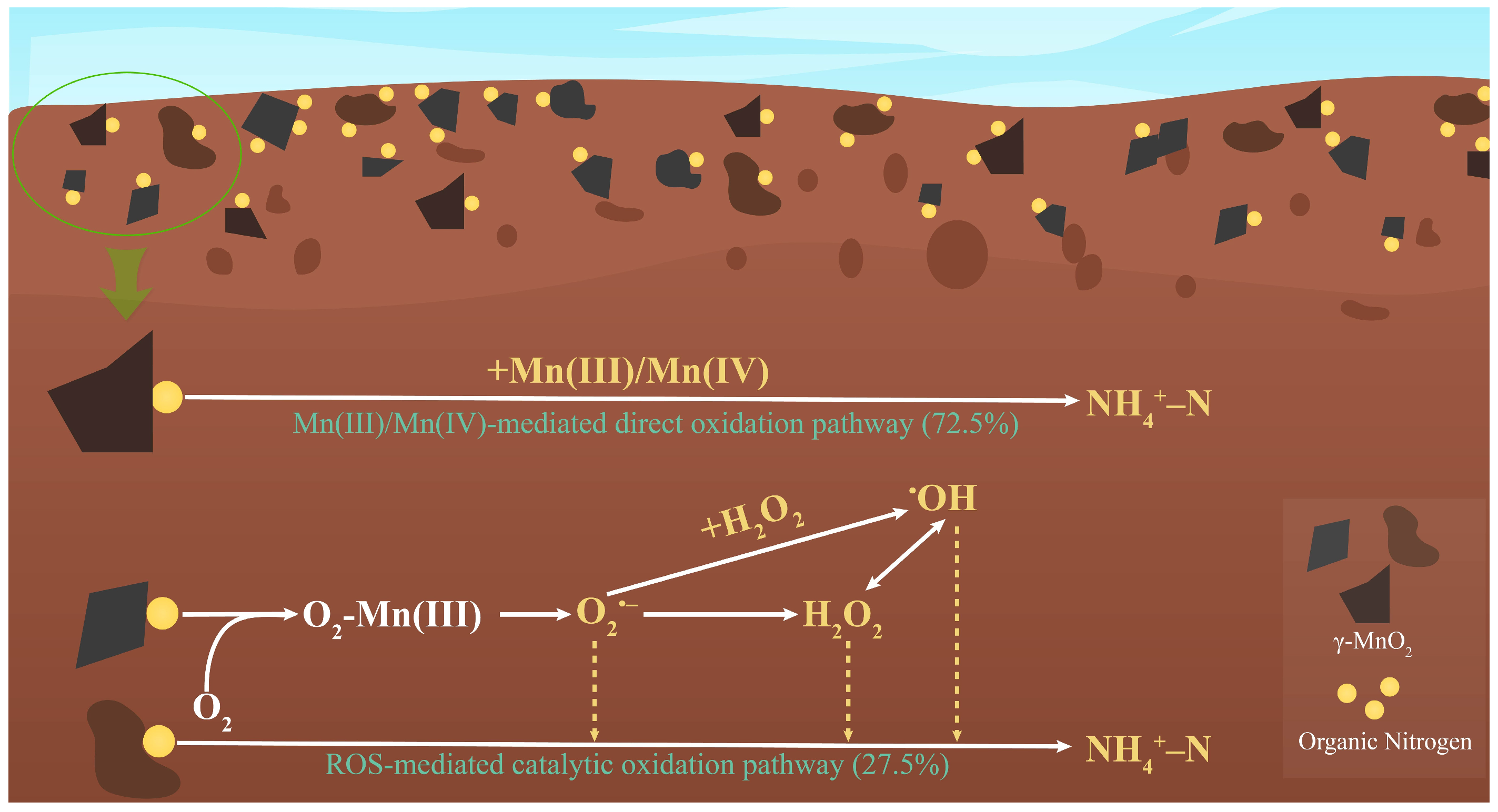
4.2. Contribution of Reactive Intermediates to γ-MnO2-Mediated Peptone Nitrogen Mineralization
4.3. Biogeochemical Significance and Implications for the Nitrogen Cycle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycl. Agroecosys. 2018, 110, 1–5. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Contosta, A.R.; Frey, S.; Schimel, J.; Schnecker, J.; Smith, R.G.; Tiemann, L.; Grandy, A.S. Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 2018, 139, 103–122. [Google Scholar] [CrossRef]
- Chen, Q.H.; Feng, Y.; Zhang, Y.P.; Zhang, Q.C.; Shamsi, I.H.; Zhang, Y.S.; Lin, X.Y. Short-term responses of nitrogen mineralization and microbial community to moisture regimes in greenhouse vegetable soils. Pedosphere 2012, 22, 263–272. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Yang, J.; Brookes, P.C.; Gunina, A. Rhizosphere priming regulates soil organic carbon and nitrogen mineralization: The significance of abiotic mechanisms. Geoderma 2021, 385, 114877. [Google Scholar] [CrossRef]
- Andersen, A.; Reardon, P.N.; Chacon, S.S.; Qafoku, N.P.; Washton, N.M.; Kleber, M. Protein–mineral interactions: Molecular dynamics simulations capture importance of variations in mineral surface composition and structure. Langmuir 2016, 32, 6194–6209. [Google Scholar] [CrossRef] [PubMed]
- Reardon, P.N.; Chacon, S.S.; Walter, E.D.; Bowden, M.E.; Washton, N.M.; Kleber, M. Abiotic protein fragmentation by manganese oxide: Implications for a mechanism to supply soil biota with oligopeptides. Environ. Sci. technol. 2016, 50, 3486–3493. [Google Scholar] [CrossRef]
- Chacon, S.S.; Reardon, P.N.; Burgess, C.J.; Purvine, S.; Chu, R.K.; Clauss, T.R.; Walter, E.; Myrold, D.D.; Washton, N.; Kleber, M. mineral surfaces as agents of environmental proteolysis: Mechanisms and controls. Environ. Sci Technol. 2019, 53, 3018–3026. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.G.; Chen, Y.F.; Liu, B.; Duan, X.L.; Zhang, M.M.; Nie, X.X.; Yang, L. The mechanism of γ-MnO2-mediated abiotic nitrogen mineralization in peptone and its influencing factors. Acta Pedol. Sin. 2025, 62, 1369–1380. [Google Scholar]
- Marinari, S.; Lagomarsino, A.; Moscatelli, M.C.; Di Tizio, A.; Campiglia, E. Soil carbon and nitrogen mineralization kinetics in organic and conventional three-year cropping systems. Soil Till. Res. 2010, 109, 161–168. [Google Scholar] [CrossRef]
- Colman, B.P.; Schimel, J.P. Drivers of microbial respiration and net N mineralization at the continental scale. Soil Biol. Biochem. 2013, 60, 65–76. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wang, Z.; Tian, Y.; Tian, W.; Liu, Z. Diversity of microbial functional genes promotes soil nitrogen mineralization in boreal forests. Microorganisms 2014, 12, 1577. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; He, N.; Wen, X.; Gao, Y.; Li, S.; Niu, S.; Butterbach-Bahl, K.; Luo, Y.; Yu, G. A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: Latitudinal patterns and mechanisms. Glob. Change Biol. 2017, 23, 455–464. [Google Scholar] [CrossRef]
- Miller, K.S.; Geisseler, D. Temperature sensitivity of nitrogen mineralization in agricultural soils. Biol. Fert. Soils 2018, 54, 853–860. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Change Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Env. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Russo, F.; Johnson, C.J.; Johnson, C.J.; McKenzie, D.; Aiken, J.M.; Pedersen, J.A. Pathogenic prion protein is degraded by a manganese oxide mineral found in soils. J. Gen. Virol. 2009, 90, 275–280. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. BBA Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, H.; Zhao, H.; Zhang, R.; Zhang, C.; Zhu, K.; Guo, X.; Wang, T.; Zhu, L. Oxygen limitation accelerates regeneration of active sites on a MnO2 surface: Promoting transformation of organic matter and carbon preservation. Environ. Sci. Technol. 2022, 56, 9806–9815. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Kuzyakov, Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth Sci. Rev. 2021, 214, 103525. [Google Scholar] [CrossRef]
- Yu, G.H.; Sun, F.S.; Yang, L.; He, X.H.; Polizzotto, M.L. Influence of biodiversity and iron availability on soil peroxide: Implications for soil carbon stabilization and storage. Land Degrad. Dev. 2020, 31, 463–472. [Google Scholar] [CrossRef]
- Noll, L.; Zhang, S.; Zheng, Q.; Hu, Y.; Wanek, W. Wide-spread limitation of soil organic nitrogen transformations by substrate availability and not by extracellular enzyme content. Soil Biol. Biochem. 2019, 133, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, T.; Tunlid, A.; Persson, P. Proteolysis of iron oxide-associated bovine serum albumin. Environ. Sci. Technol. 2020, 54, 5121–5130. [Google Scholar] [CrossRef]
- Utomo, W.P.; Wu, H.; Ng, Y.H. Quantification methodology of ammonia produced from electrocatalytic and photocatalytic nitrogen/nitrate reduction. Energies 2022, 16, 27. [Google Scholar] [CrossRef]
- García-Robledo, E.; Corzo, A.; Papaspyrou, S. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Mar. Chem. 2014, 162, 30–36. [Google Scholar] [CrossRef]
- Ng, T.B. Soybean: Applications and Technology; Intech: Rijeka, Croatia, 2021; pp. 341–364. ISBN 978-953-307-207-4. [Google Scholar]
- Remucal, C.K.; Ginder-Vogel, M. A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci. Proc. Imp. 2014, 16, 1247–1266. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.H. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ. Sci. Technol. 2003, 37, 2421–2430. [Google Scholar] [CrossRef]
- Serpone, N. Aquatic chemistry: Chemical equilibria and rates in natural waters. J. Chem. Educ. 1996, 73, A277. [Google Scholar]
- Zhang, H.; Chen, W.R.; Huang, C.H. Kinetic modeling of oxidation of antibacterial agents by manganese oxide. Environ. Sci. Technol. 2008, 42, 5548–5554. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, C.; Chen, J.; Chen, X. Oxidative transformation of 17β-estradiol by MnO2 in aqueous solution. Arch. Environ. Con. Tox. 2009, 57, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hedman, C.; Liu, C.; Guo, T.; Pedersen, J.A. Transformation of sulfamethazine by manganese oxide in aqueous solution. Environ. Sci. Technol. 2012, 46, 2642–2651. [Google Scholar] [CrossRef]
- Petrie, R.A.; Grossl, P.R.; Sims, R.C. Oxidation of pentachlorophenol in manganese oxide suspensions under controlled Eh and pH environments. Environ. Sci. Technol. 2002, 36, 3744–3748. [Google Scholar] [CrossRef]
- Sheng, D.G.; Xu, C.; Xu, L.; Qiu, Y.; Zhou, H. Abiotic oxidation of 17beta-estradiol by soil manganese oxides. Environ. Pollut. 2009, 157, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Mizuno, T.; Oku, J.I.; Tanaka, T. pH-induced conformational change in an α-helical coiled-coil is controlled by his residues in the hydrophobic core. Protein Peptide Lett. 2003, 10, 27–33. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, H.J.; Na, K.; Bae, Y.H. Poly (l-histidine)–PEG block copolymer micelles and pH-induced destabilization. J. Control. Release 2003, 90, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Ruttala, H.B.; Chitrapriya, N.; Poudal, B.K.; Choi, J.Y.; Kim, S.T.; Youn, Y.S.; Ku, S.K.; Choi, H.G.; Yong, C.S.; et al. Engineering of cell microenvironment-responsive polypeptide nanovehicle co-encapsulating a synergistic combination of small molecules for effective chemotherapy in solid tumors. Acta Biomater. 2017, 48, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Xu, W.; Wang, Y.; Yang, J.; Huang, Y.; Hu, L.; He, C.; Shu, D.; Leung, D.Y.C.; Pang, Z. Enhanced performance and conversion pathway for catalytic ozonation of methyl mercaptan on single-atom Ag deposited three-dimensional ordered mesoporous MnO2. Environ. Sci. Technol. 2018, 52, 13399–13409. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Zheng, T.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Promoted catalytic transformation of polycyclic aromatic hydrocarbons by MnO2 polymorphs: Synergistic effects of Mn3+ and oxygen vacancies. Appl. Catal. B Environ. Energy. 2020, 272, 119030. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, J.; Han, R.; Wang, Z.; Christie, P.; Zhang, S. Sustained production of superoxide radicals by manganese oxides under ambient dark conditions. Water Res. 2021, 196, 117034. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Zhang, H.; Xiao, M.; Duan, X.; Zhang, M.; Yang, L.; Fan, H.; Liu, B. Abiotic Nitrogen Mineralization of Peptone by γ-MnO2: Effects of Dissolved Oxygen and pH. Soil Syst. 2025, 9, 123. https://doi.org/10.3390/soilsystems9040123
Hong J, Zhang H, Xiao M, Duan X, Zhang M, Yang L, Fan H, Liu B. Abiotic Nitrogen Mineralization of Peptone by γ-MnO2: Effects of Dissolved Oxygen and pH. Soil Systems. 2025; 9(4):123. https://doi.org/10.3390/soilsystems9040123
Chicago/Turabian StyleHong, Jun, Hang Zhang, Manli Xiao, Xiaoli Duan, Minmin Zhang, Li Yang, Hao Fan, and Bo Liu. 2025. "Abiotic Nitrogen Mineralization of Peptone by γ-MnO2: Effects of Dissolved Oxygen and pH" Soil Systems 9, no. 4: 123. https://doi.org/10.3390/soilsystems9040123
APA StyleHong, J., Zhang, H., Xiao, M., Duan, X., Zhang, M., Yang, L., Fan, H., & Liu, B. (2025). Abiotic Nitrogen Mineralization of Peptone by γ-MnO2: Effects of Dissolved Oxygen and pH. Soil Systems, 9(4), 123. https://doi.org/10.3390/soilsystems9040123






