Soil Amendment with Biochar Reduces the Uptake and Translocation of Perfluoroalkyl Substances by Horticultural Plants Grown in a Polluted Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Site
2.3. Plant Cultivation and Sample Collection
2.3.1. Tomato Plants
2.3.2. Red Chicory Plants
2.4. Soil Chemical Analyses
2.5. PFAS Extraction and Quantification by LC-MS/MS Analysis
2.6. Quality Control and Quality Assurance
2.7. Health Risk Assessment
2.8. Data Analysis
3. Results
3.1. Soil Properties and PFAS Contamination of the Irrigation Water
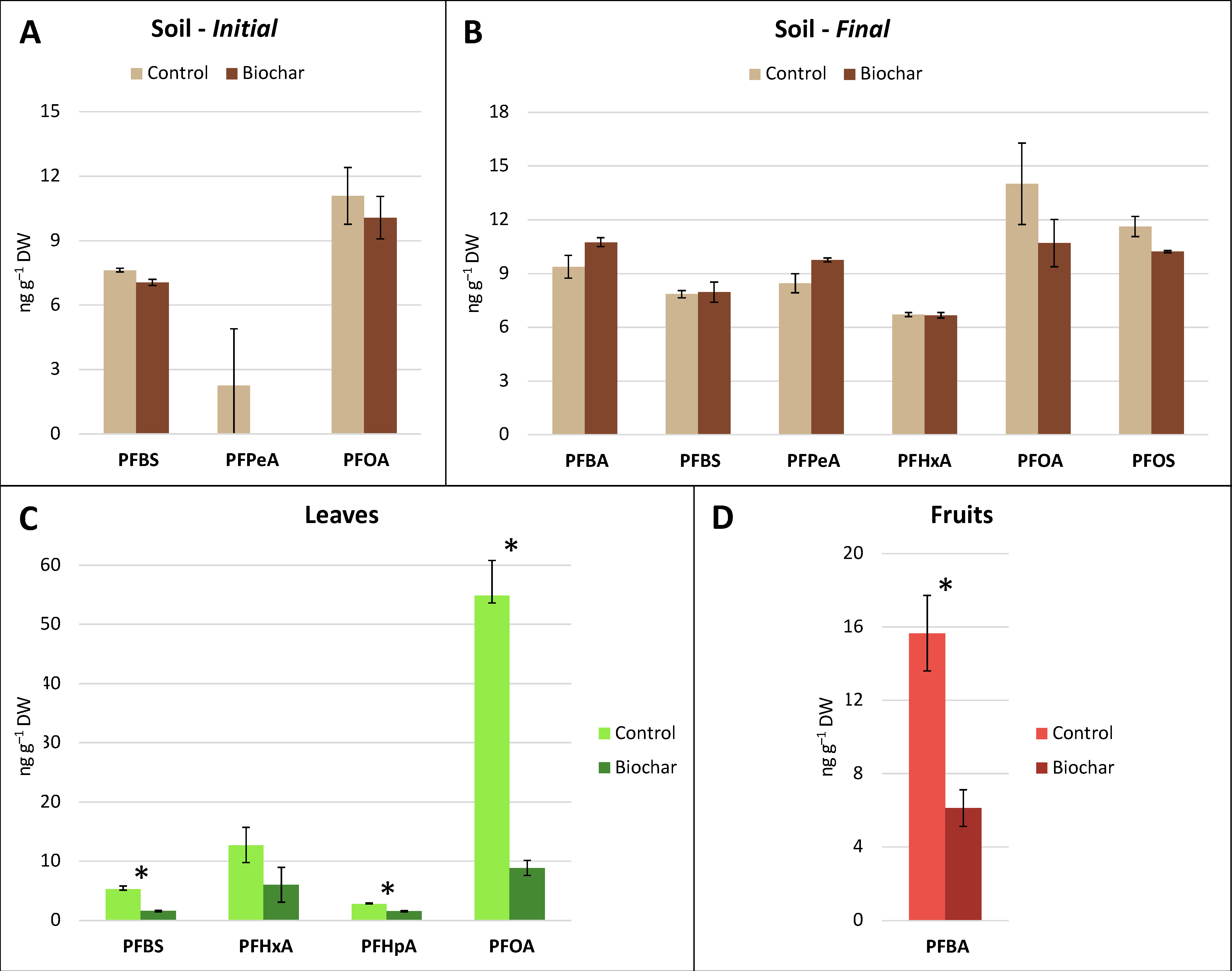
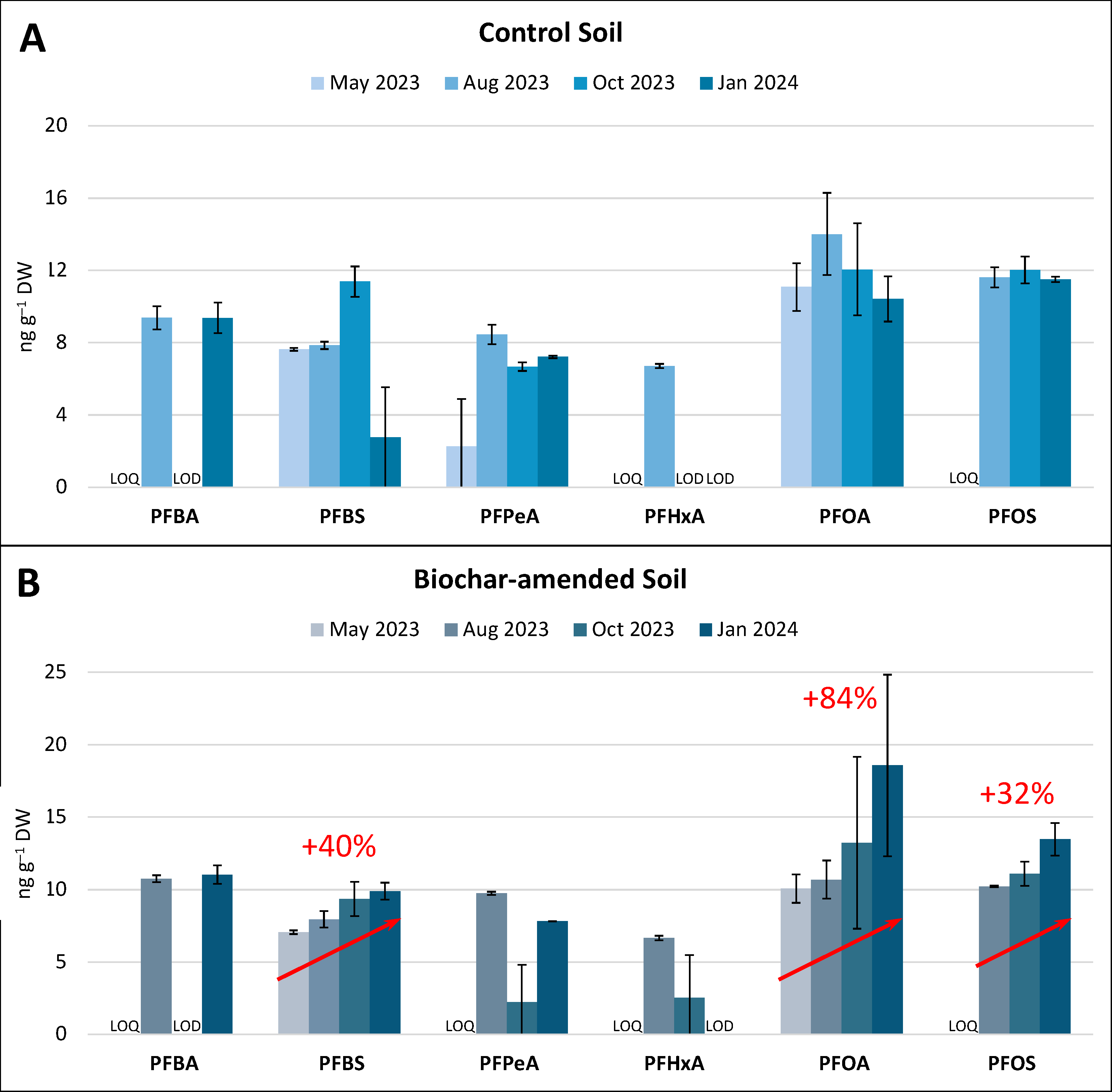
3.2. PFAS Concentration in Soil and Tomato Samples
3.3. PFAS Concentration in Soil and Red Chicory Samples
3.4. Dietary Exposure Assessment
4. Discussion
4.1. PFAS Accumulation in Plant Biomass
4.2. Biochar-Induced PFAS Stabilization in Soil: Is That Good or Bad?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARPAV | Veneto Region Environmental Protection Agency |
| ASE | accelerated solvent extraction |
| BW | body weight |
| C6O4 | ammonium and potassium salts of perfluoro ([5-methoxy-1,3-dioxolan-4-yl]oxy) acetic acid |
| CA | cellulose acetate |
| DI | daily intake |
| DW | dry weight |
| ES | external standard |
| FW | fresh weight |
| HFPO-DA | hexafluoropropylene oxide dimer acid |
| HI | hazard index |
| ICP-OES | inductively coupled plasma optical emission spectrometry |
| IS | internal standard |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| PFAS | poly- and perfluoroalkyl substances |
| PFBA | perfluorobutanoic acid |
| PFBS | perfluorobutane sulfonic acid |
| PFDA | perfluorodecanoic acid |
| PFDoA | perfluorododecanoic acid |
| PFHpA | perfluoroheptanoic acid |
| PFHxA | perfluorohexanoic acid |
| PFHxS | perfluorohexane sulfonic acid |
| PFNA | perfluorononanoic acid |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctane sulfonic acid |
| PFPeA | perfluoropentanoic acid |
| PFTeDA | perfluorotetradecanoic acid |
| PFUnA | perfluoroundecanoic acid |
| RfD | oral reference dose |
| SRM | selected reaction monitoring |
| TC | total carbon |
| TFA | trifluoroacetate |
| THQ | target hazard quotient |
| TOC | total organic carbon |
| TWI | tolerable weekly intake |
| UHPLC | ultra-high performance liquid chromatography |
References
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.B.; Goel, R. Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Giglioli, S.; Colombo, L.; Azzellino, A. Cluster and multivariate analysis to study the diffuse contamination of emerging per- and polyfluoroalkyl substances (PFAS) in the Veneto Region plain (North-eastern Italy). Chemosphere 2023, 319, 137916. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Health Organization. Keeping Our Water Clean: The Case of Water Contamination in the Veneto Region, Italy. 2017. Available online: https://www.who.int/europe/publications/i/item/9789289052467 (accessed on 24 April 2024).
- Mastrantonio, M.; Bai, E.; Uccelli, R.; Cordiano, V.; Screpanti, A.; Crosignani, P. Drinking water contamination from perfluoroalkyl substances (PFAS): An ecological mortality study in the Veneto Region, Italy. Eur. J. Public Health 2018, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- EEA—European Environmental Agency. 2024. Available online: https://www.eea.europa.eu/publications/zero-pollution/cross-cutting-stories/pfas (accessed on 24 April 2024).
- USGS—United States Geological Survey. 2024. Available online: https://geonarrative.usgs.gov/pfasustapwater/ (accessed on 24 April 2024).
- Ramos, P.; Ashworth, D.J. Per- and poly-fluoroalkyl substances in agricultural contexts and mitigation of their impacts using biochar: A review. Sci. Total. Environ. 2024, 927, 172275. [Google Scholar] [CrossRef]
- Ingelido, A.M.; Abballe, A.; Gemma, S.; Dellatte, E.; Iacovella, N.; De Angelis, G.; Zampaglioni, F.; Marra, V.; Miniero, R.; Valentini, S.; et al. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ. Int. 2018, 110, 149–159. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Eens, M.; Bervoets, L. Dynamic spatiotemporal changes of per- and polyfluoroalkyl substances (PFAS) in soil and eggs of private gardens at different distances from a fluorochemical plant. Environ. Pollut. 2024, 346, 123613. [Google Scholar] [CrossRef]
- Weidemann, E.; Lämmer, R.; Stahl, T.; Göckener, B.; Bücking, M.; Breuer, J.; Kowalczyk, J.; Just, H.; Boeddinghaus, R.S.; Gassmann, M. Leaching and Transformation of Perfluoroalkyl Acids and Polyfluoroalkyl Phosphate Diesters in Unsaturated Soil Column Studies. Environ. Toxicol. Chem. 2022, 41, 2065–2077. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Saugo, M.; Ioverno, E.; Olivieri, A.; Bertola, F.; Pasinato, A.; Ducatman, A. PFOA and testis cancer in the Veneto Region (Italy). Environ. Health 2024, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Polesello, S.; Valsecchi, S. Rischio Associato Alla Presenza di Sostanze Perfluoro-Alchiliche (PFAS) Nelle Acque Potabili e Nei corpi Idrici Recettori di Aree Industriali Nella Provincia di Vicenza e Aree Limitrofe. IRSA-CNR. 2013. Available online: https://www.regione.veneto.it/web/sanita/archivio-dati-pfas-popolazione (accessed on 15 February 2025).
- ARPAV—Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto. Monitoraggio Delle Sostanze per- e Polifluoroalchiliche (PFAS) Nella Rete di Sorveglianza Delle Acque Sotterranee. 2024. Available online: https://www.arpa.veneto.it/temi-ambientali/acque-interne/rapporti-pfas (accessed on 15 February 2025).
- ARPAV—Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto. Concentrazione di Sostanze Perfluoroalchiliche (PFAS) Nelle Acque Prelevate da ARPAV; Anni 2013–2024. Available online: https://www.arpa.veneto.it/dati-ambientali/open-data/idrosfera/concentrazione-di-sostanze-perfluoroalchiliche-pfas-nelle-acque-prelevate-da-arpav (accessed on 15 February 2025).
- Smalling, K.L.; Romanok, K.M.; Bradley, P.M.; Morriss, M.C.; Gray, J.L.; Kanagy, L.K.; Gordon, S.E.; Williams, B.M.; Breitmeyer, S.E.; Jones, D.K.; et al. Per- and polyfluoroalkyl substances (PFAS) in United States tapwater: Comparison of underserved private-well and public-supply exposures and associated health implications. Environ. Int. 2023, 178, 108033. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Revisiting the ‘forever chemicals’; PFOA and PFOS exposure in drinking water. npj Clean Water 2023, 6, 57. [Google Scholar] [CrossRef]
- European Food Safety Agency. CONTAM Panel Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef]
- European Food Safety Agency. CONTAM Panel Scientific Opinion on the risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [Google Scholar] [CrossRef]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef]
- Schilling Costello, M.C.; Lee, L.S. Sources, Fate, and Plant Uptake in Agricultural Systems of Per- and Polyfluoroalkyl Substances. Curr. Pollut. Rep. 2020, 10, 799–819. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Wang, M.; He, Q.; Niu, X.; Liang, Y. Distribution of perfluoroalkyl substances (PFASs) in aquatic plant-based systems: From soil adsorption and plant uptake to effects on microbial community. Environ. Pollut. 2020, 257, 113575. [Google Scholar] [CrossRef] [PubMed]
- Adu, O.; Ma, X.; Sharma, V.K. Bioavailability, phytotoxicity and plant uptake of per-and polyfluoroalkyl substances (PFAS): A review. J. Hazard. Mater. 2023, 447, 130805. [Google Scholar] [CrossRef]
- Xu, B.; Qiu, W.; Du, J.; Wan, Z.; Zhou, J.L.; Chen, H.; Liu, R.; Magnuson, J.T.; Zheng, C. Translocation, bioaccumulation, and distribution of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in plants. iScience 2022, 25, 104061. [Google Scholar] [CrossRef]
- Scearce, A.E.; Goossen, C.P.; Schattman, R.E.; Mallory, E.B.; MaCrae, J.D. Linking drivers of plant per- and polyfluoroalkyl substance (PFAS) uptake to agricultural land management decisions. Biointerphases 2023, 18, 040801. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Zulfiqar, U.; Farooq, M.; Hussain, S.; Mehmood, T.; Naveed, M.; Li, Y.; Liqun, C.; Saeed, Q.; et al. Biochar application for remediation of organic toxic pollutants in contaminated soils, an update. Ecotoxicol. Environ. Saf. 2022, 248, 114322. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, C.; Chen, H.; Sørmo, E.; Cornelissen, G.; Gao, Y.; Reguyal, F.; Sarmah, A.; Ippolito, J.; Kammann, C.; et al. A critical review of biochar for the remediation of PFAS-contaminated soil and water. Sci. Total Environ. 2024, 951, 174962. [Google Scholar] [CrossRef] [PubMed]
- Sørmo, E.; Silvani, L.; Bjerkli, N.; Hagemann, N.; Zimmerman, A.R.; Hale, S.E.; Hansen, C.B.; Hartnik, T.; Cornelissen, G. Stabilization of PFAS-contaminated soil with activated biochar. Sci. Total Environ. 2021, 763, 144034. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, L.; Chen, G. A mechanical investigation of perfluorooctane acid adsorption by engineered biochar. J. Clean. Prod. 2022, 340, 130742. [Google Scholar] [CrossRef]
- Silvani, L.; Cornelissen, G.; Botnen Smebye, A.; Zhang, Y.; Okkenhaug, G.; Zimmerman, A.R.; Thune, G.; Sævarsson, H.; Hale, S.E. Can biochar and designer biochar be used to remediate per- and polyfluorinated alkyl substances (PFAS) and lead and antimony contaminated soils? Sci. Total Environ. 2019, 694, 133693. [Google Scholar] [CrossRef]
- Krahn, K.M.; Cornelissen, G.; Castro, G.; Arp, H.P.H.; Asimakopoulos, A.G.; Wolf, R.; Holmstad, R.; Zimmerman, A.R.; Sørmo, E. Sewage sludge biochars as effective PFAS-sorbents. J. Hazard. Mater. 2023, 445, 130449. [Google Scholar] [CrossRef]
- Holly, M.A.; Gunn, K.M.; Keymer, D.; Sanford, J.R. Evaluation of per- and polyfluoroalkyl substances leaching from biosolids and mitigation potential of biochar through undisturbed soil columns. ACS ES&T Water 2024, 4, 413–426. [Google Scholar] [CrossRef]
- Sørmo, E.; Lade, C.B.M.; Zhang, J.; Asimakopoulos, A.G.; Åsli, G.W.; Hubert, M.; Goranov, A.I.; Arp, H.P.H.; Cornelissen, G. Stabilization of PFAS-contaminated soil with sewage sludge- and wood-based biochar sorbents. Sci. Total Environ. 2024, 922, 170971. [Google Scholar] [CrossRef]
- Battisti, I.; Trentin, A.R.; Franzolin, E.; Nicoletto, C.; Masi, A.; Renella, G. Uptake and distribution of perfluoroalkyl substances by grafted tomato plants cultivated in a contaminated site in northern Italy. Sci. Total Environ. 2024, 915, 170032. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle Size Analysis by Hydrometer: A Simplified Method for Routine Textural Analysis and a Sensitivity Test of Measurement Parameters. Soil Sci. Soc. Am. J. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter; and a proposed modification of the chromic acid titration method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954.
- Normandin, V.; Kotuby-Amacher, J.; Miller, R.O. Modification of the ammonium acetate extraction for the determination of exchangeable cations in calcareous soil. Commun. Soil Sci. Plant Anal. 1998, 29, 1785–1791. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. American Society of Agronomy. Soil Sci. Soc. Am. J. 1982, 1159. [Google Scholar] [CrossRef]
- Sharma, N.; Barion, G.; Shrestha, I.; Ebinezer, L.B.; Trentin, A.R.; Vamerali, T.; Mezzalira, G.; Masi, A.; Ghisi, R. Accumulation and effects of perfluoroalkyl substances in three hydroponically grown Salix L. species. Ecotoxicol. Environ. Saf. 2020, 191, 110150. [Google Scholar] [CrossRef]
- Adams, K.J.; Pratt, B.; Bose, N.; Dubois, L.G.; St John-Williams, L.; Perrott, K.M.; Ky, K.; Kapahi, P.; Sharma, V.; MacCoss, M.J.; et al. Alzheimer’s Disease Metabolomics Consortium. Skyline for Small Molecules: A Unifying Software Package for Quantitative Metabolomics. J. Proteome Res. 2020, 19, 1447–1458. [Google Scholar] [CrossRef]
- ITRC—Interstate Technology & Regulatory Council. PFAS Technical and Regulatory Guidance Document, September 2023. Available online: https://pfas-1.itrcweb.org/ (accessed on 14 August 2025).
- Kavcar, P.; Sofuoglu, A.; Sofuoglu, S.C. A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int. J. Hyg. Environ. Health 2009, 212, 216–227. [Google Scholar] [CrossRef]
- Baldi, A.; Truschi, S.; Bruschi, P.; Lenzi, A. Preliminary Assessment of Four Wild Leafy Species to Be Used as Baby Salads. Horticulturae 2023, 9, 650. [Google Scholar] [CrossRef]
- Alsafra, Z.; Scholl, G.; De Meulenaer, B.; Eppe, G.; Saegerman, C. Hazard Ratio and Hazard Index as Preliminary Estimators Associated to the Presence of Furans and Alkylfurans in Belgian Foodstuffs. Foods 2022, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R, RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Helsel, D.R. Statistics for Censored Environmental Data Using MINITAB® and R, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Brusseau, M.L.; Anderson, R.H.; Guo, B. PFAS concentrations in soils: Background levels versus contaminated sites. Sci. Total Environ. 2020, 740, 140017. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M.; Eljarrat, E.; Díaz-Cruz, S.; Rodríguez-Mozaz, S.; Wunderlin, D.; Barcelo, D. Review of emerging contaminants in aquatic biota from Latin America: 2002–2016. Environ. Toxicol. Chem. 2017, 36, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Naile, J.E.; Khim, J.S.; Jones, P.D.; Newsted, J.L. Aquatic toxicology of perfluorinated chemicals. Rev. Environ. Contam. Toxicol. 2010, 202, 1–52. [Google Scholar] [CrossRef]
- Bondada, B.R.; Tu, S.; Ma, L.Q. Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Sci. Total Environ. 2004, 332, 61–70. [Google Scholar] [CrossRef]
- Stolpe, C.; Krämer, U.; Müller, C. Heavy metal (hyper)accumulation in leaves of Arabidopsis halleri is accompanied by a reduced performance of herbivores and shifts in leaf glucosinolate and element concentrations. Environ. Exp. Bot. 2017, 133, 78–85. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Rankin, K.; Mabury, S.A.; Jenkins, T.M.; Washington, J.W. A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 2016, 161, 333–341. [Google Scholar] [CrossRef]
- Jones, K.C. Persistent Organic Pollutants (POPs) and Related Chemicals in the Global Environment: Some Personal Reflections. Environ. Sci. Technol. 2021, 55, 9400–9412. [Google Scholar] [CrossRef]
- Etz, B.D.; Shukla, M.K. Per- and polyfluoroalkyl substances chemical degradation strategies: Insights into the underlying reaction mechanisms. Curr. Opin. Chem. Eng. 2023, 42, 100956. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Bhhatarai, B.; Gramatica, P. Prediction of aqueous solubility; vapor pressure and critical micelle concentration for aquatic partitioning of perfluorinated chemicals. Environ. Sci. Technol. 2011, 45, 8120–8128. [Google Scholar] [CrossRef]
- Burkhardt, J.B.; Cadwallader, A.; Pressman, J.G.; Magnuson, M.L.; Williams, A.J.; Sinclair, G.; Speth, T.F. Polanyi adsorption potential theory for estimating PFAS treatment with granular activated carbon. J. Water Process Eng. 2023, 53, 103691. [Google Scholar] [CrossRef]
- Anderson, R.H.; Adamson, D.T.; Stroo, H.F. Partitioning of poly- and perfluoroalkyl substances from soil to groundwater within aqueous film-forming foam source zones. J. Contam. Hydrol. 2019, 220, 59–65. [Google Scholar] [CrossRef]
- Gredelj, A.; Nicoletto, C.; Valsecchi, S.; Ferrario, C.; Polesello, S.; Lava, R.; Zanon, F.; Barausse, A.; Palmeri, L.; Guidolin, L.; et al. Uptake and translocation of perfluoroalkyl acids (PFAA) in red chicory (Cichorium intybus L.) under various treatments with pre-contaminated soil and irrigation water. Sci. Total Environ. 2020, 708, 134766. [Google Scholar] [CrossRef]
- Felizeter, S.; Jürling, H.; Kotthoff, M.; De Voogt, P.; McLachlan, M.S. Uptake of perfluorinated alkyl acids by crops: Results from a field study. Environ. Sci. Process Impacts 2021, 23, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Stigliani, W.M. Changes in valued “capacities” of soils and sediments as indicators of nonlinear and time-delayed environmental effects. Environ. Monit. Asses. 1988, 10, 245–307. [Google Scholar] [CrossRef]
- Gale, E.F. The Chemical Activities of Bacteria, 3rd ed.; Academic Press: New York, NY, USA, 1951. [Google Scholar]
- Tang, Z.; Vogel, T.M.; Wang, Q.; Wei, C.; Ali, M.; Song, X. Microbial defluorination of TFA, PFOA, and HFPO-DA by a native microbial consortium under anoxic conditions. J. Haz. Mater. 2024, 465, 133217. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Da, Y.; Yu, J.; Long, B.; Zhang, P.; Bakker, C.; McCarl, B.A.; Yuan, J.S.; Dai, S.Y. Sustainable environmental remediation via biomimetic multifunctional lignocellulosic nano-framework. Nat. Commun. 2022, 13, 4368. [Google Scholar] [CrossRef]

| Property (Unit of Measure) | Control Soil | Biochar-Amended Soil |
|---|---|---|
| Sand (%) | 43.8 | 45.8 |
| Silt (%) | 35.8 | 34.4 |
| Clay (%) | 20.4 | 19.8 |
| pH (H2O) | 7.58 ± 0.08 | 7.7 ± 0.2 |
| CTOT (g kg−1) | 1.56 ± 0.02 | 4.93 * ± 0.16 |
| CORG (%) | 1.49 ± 0.02 | 4.77 * ± 0.17 |
| NTOT (%) | 0.176 ± 0.001 | 0.195 * ± 0.004 |
| NORG (%) | 0.176 ± 0.002 | 0.181 ± 0.005 |
| PTOT (%) | 0.119 ± 0.002 | 0.122 ± 0.002 |
| Pavailable (%) | 0.026 ± 0.001 | 0.027 ± 0.002 |
| NO3−-N (mg kg−1) | 20.5 ± 3.8 | 29.5 ± 2.6 |
| NH4+-N (mg kg−1) | 10.1 ± 1.2 | 15.6 ± 1.2 |
| SO42− (mg kg−1) | 40.8 ± 4.2 | 44.6 ± 11.2 |
| Caexchangeable (mg kg−1) | 2747 ± 42 | 2884 ± 23 |
| Kexchangeable (mg kg−1) | 433 ± 9 | 649 ± 82 |
| Mgexchangeable (mg kg−1) | 308 ± 7 | 346 ± 14 |
| Naexchangeable (mg kg−1) | 40 ± 7 | 25 ± 1 |
| Tomato Experiment | Red Chicory Experiment | ||
|---|---|---|---|
| Molecule | May 2023 | August 2023 | October 2023 |
| PFBA | 1.83 | 1.09 | 2.17 |
| PFBS | 0.87 | 2.22 | 0.85 |
| PFPeA | 1.15 | 0.72 | 1.24 |
| PFHxA | 1.26 | 0.69 | 0.55 |
| PFHxS | <LOQ | 0.13 | <LOQ |
| PFHpA | 0.39 | 0.19 | 0.20 |
| PFOA | 7.04 | 4.63 | 3.01 |
| PFOS | <LOQ | <LOQ | <LOQ |
| PFNA | <LOD | <LOQ | <LOD |
| PFDA | <LOD | <LOD | <LOD |
| PFUnA | <LOD | <LOD | <LOD |
| PFDoA | <LOD | <LOD | <LOD |
| PFTeDA | <LOD | <LOD | <LOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battisti, I.; Trentin, A.R.; Sabia, A.; Masi, A.; Renella, G. Soil Amendment with Biochar Reduces the Uptake and Translocation of Perfluoroalkyl Substances by Horticultural Plants Grown in a Polluted Area. Soil Syst. 2025, 9, 100. https://doi.org/10.3390/soilsystems9030100
Battisti I, Trentin AR, Sabia A, Masi A, Renella G. Soil Amendment with Biochar Reduces the Uptake and Translocation of Perfluoroalkyl Substances by Horticultural Plants Grown in a Polluted Area. Soil Systems. 2025; 9(3):100. https://doi.org/10.3390/soilsystems9030100
Chicago/Turabian StyleBattisti, Ilaria, Anna Rita Trentin, Andrea Sabia, Antonio Masi, and Giancarlo Renella. 2025. "Soil Amendment with Biochar Reduces the Uptake and Translocation of Perfluoroalkyl Substances by Horticultural Plants Grown in a Polluted Area" Soil Systems 9, no. 3: 100. https://doi.org/10.3390/soilsystems9030100
APA StyleBattisti, I., Trentin, A. R., Sabia, A., Masi, A., & Renella, G. (2025). Soil Amendment with Biochar Reduces the Uptake and Translocation of Perfluoroalkyl Substances by Horticultural Plants Grown in a Polluted Area. Soil Systems, 9(3), 100. https://doi.org/10.3390/soilsystems9030100







