Abstract
Plant growth-promoting bacteria (PGPB) are an effective tool for improving nutrients in agricultural systems; however, their efficacy depends on successful colonization in soils. To address this challenge, biochar has been identified as an effective material for enhancing soil ecosystem services and can serve as a protective for PGPB. However, the impact of biochar and PGPB on soil health indicators and plant growth remains poorly understood. This study aimed to evaluate the effects of biochar and PGPB on soil chemical and biological properties in cowpea. We used biochar from bean husk (BHB) and grape fermentation residue (GFB) and Bradyrhizobium elkanii USDA 76 (BRA), Burkholderia cepacia ATCC 25416 (PRB), or Rhizobium altiplani BR10423 (RHI). BHB and PRB stimulated cowpea growth, while GFB and PRB promoted soil phosphatase activity. Overall, different combinations of biochar and PGPR increased soil pH, phosphorus, potassium, organic carbon content, and urease activity, but did not affect microbial biomass carbon and β-glucosidase activities. The biochars inoculated with the BRA showed the highest productivity. For example, plants subjected to the BRA + GFB treatment exhibited a 3.85-fold increase in productivity compared to the additional treatment that involved the use of commercial peat. The study demonstrated a positive effect of biochar and PGPB on soil enzymatic activity, nutrient content, and cowpea growth suggesting a sustainable alternative to chemical fertilizers, especially in poor soils. These findings highlight the potential of biochar as an environmentally sustainable carrier of PGPB while addressing the issue of agricultural waste reuse.
1. Introduction
The expansion of agriculture has been supported by using several inputs, mainly chemical fertilizers which increase the cost of production and can pollute the soil and water [1]. Recently, agricultural systems have emphasized sustainability, particularly from an environmental perspective [2] that includes distinct strategies such as alternative inputs such as biochar and beneficial microorganisms [3]. These actions are in line with the objectives of promoting food security and sustainable production by the United Nations Sustainable Development Goals, as well as with the principles of circular economy [4].
A sustainable strategy to reduce the use of N-fertilizers is the use of plant growth-promoting bacteria (PGPB) that can exhibit multiple mechanisms to enhance plant growth, including biological nitrogen fixation, phosphate solubilization, and/or phytohormone release [5,6,7]. Additionally, PGPB synthesizes siderophores, enhances root development, improves nutrient availability, suppresses pathogenic organisms, and stimulates plant defense mechanisms [8].
However, one of the main challenges encountered in the use of PGPB is their field inoculation and limited survival in the soil [7,9,10,11]. This challenge encompasses aspects of PGPB’s ability to colonize the root system, as well as its capacity to survive within inoculants. Therefore, an effective carrier is necessary to ensure the inoculation, survival, and colonization of PGPB in plants [12]. In this context, biochar has emerged as a promising sustainable carrier to PGPB due to its characteristic of retaining nutrients and being a potential shelter for microorganisms. The use of biochar is advantageous as it provides a sustainable alternative to peat, a finite resource, while also promoting the reuse of agricultural and industrial residues, reducing environmental liabilities [13]. This synergistic mechanism positions biochar-based inoculant carriers as a viable alternative to chemical fertilizers. Previous studies have suggested biochar as an effective bacterial carrier and a promising alternative to peat [3,5,14]. For example, a previous study evaluated the potential of biochar as a carrier for Rhizobium inoculants [15]. Among nine biochars tested, six maintained R. leguminosarum viability for 84 days at 4 °C. These biochars successfully delivered R. leguminosarum to pea plants, enhancing nodulation, biomass production, and nitrogen accumulation, demonstrating biochar’s potential as an effective carrier for microbial inoculants. The combination of biochar with PGPB is reported to enhance soil quality and crop productivity, both under normal and stress conditions [6,14,16]. Among the significant benefits of using biochar associated with PGPB are the improvement of plant growth, and the increase in soil nutrient content and enzymatic activities, thereby favoring nutrient cycling and enhancing soil biodiversity [16,17]. The advantages provided by biochar and PGPB in crops represent a sustainable solution to challenges faced by agriculture, such as water scarcity and unpredictable seasonal changes, including droughts. These solutions are particularly relevant in environments with limited access to nitrogen or in agricultural systems with low fertility, as observed in semiarid regions [3]. This biotechnology alternative seems promising for cowpea, an economically important crop in this region, particularly in poor soils, such as those found in semi-arid regions, which could benefit from the combined application of PGPB with biochar
Therefore, it is necessary to evaluate the use of biochar associated with PGPB on semiarid soils and plant growth. We hypothesize that biochar will enhance the positive effects of PGPB on cowpea (Vigna unguiculata (L.) Walp.) growth and soil chemical and biological properties. To address this hypothesis, we investigated the effectiveness of biochar derived from different agro-industrial residues associated with PGPB on cowpea growth and soil chemical and biological properties. This is the first study to evaluate biochar derived from residues with PGPB in semiarid conditions
2. Materials and Methods
2.1. Experimental Materials
Biochar was produced through the pyrolysis process using bean husk (BHB), and grape fermentation residues (GFB) obtained from the local farmers. Both biochars were produced using pyrolysis at 530 °C for 10–12 h under oxygen-limited conditions and the chemical properties are in (Table 1). The commercial peat was used as an additional treatment as recommended by the manufacturer. The properties of soil, peat, and biochars are shown in Table 1 and Table 2 were performed following the methodology described by Teixeira [18]. Different species of PGPB were obtained from GFBN Culture Collection, at the Federal Rural University of Pernambuco. The strains used in the study were Rhizobium altiplani BR10423 strain 44 R1.1 (RHI), Bradyrhizobium elkanii USDA 76 strain 43 R1.1 (BRA), and Burkholderia cepacia ATCC 25416 strain 17A R2.2 (PRB). These strains were isolated from cowpea rhizobial samples obtained from soil in a silvopastoral system in Itambé, Pernambuco, Brazil. A previous study demonstrated that the three isolates were capable of inducing nodule formation and fixing nitrogen in cowpea, which led to their selection for this experiment [19]. Their identification was based on the closest matching sequences found in EzBioCloud [19].

Table 1.
Chemical characterization of bean husk biochar (BHB) end grape fermentation biochar (GFB).
2.2. Field Experiment and Experimental Design
The experiment was conducted at the Federal University of Pernambuco Agreste, Pernambuco State, Brazil (8° 48′ 34.2″ S, 36° 24′ 29.3″ W) at 705 m above sea level. The climate is Humid and classified as “As” (Köppen). The average annual temperature is ~23 °C and the average total annual rainfall is 782 mm. The soil has been classified as Entisol. The chemical attributes of the experimental soil are in Table 2.

Table 2.
Soil and peat chemical properties.
Table 2.
Soil and peat chemical properties.
| OM | N | pH | P | Ca2+ | Mg2+ | K+ | Na+ | S (Bases) | Al3+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | (%) | (H2O) | mg dm−3 | cmolc dm−3 | ||||||
| Soil | 5.5 | 0.13 | 5.37 | 15.5 | 1.04 | 0.60 | 0.14 | 0.02 | 1.80 | 0.08 |
| Peat | 77.25 | - | 5.3 | 0.17 | - | - | 0.36 | - | - | - |
OM—organic matter.
The experiment followed a completely randomized design with a double factorial model plus an additional treatment (3 × 4 +1), with four replicates. The first factor was the type of biochar, which included three levels: (1) no biochar (wB), (2) biochar derived from bean husks (BHB), and (3) biochar derived from grape fermentation residues (GFB). The second factor was the inoculation with plant growth-promoting bacteria (PGPB), consisting of four levels: (1) no inoculation (wI), (2) Rhizobium altiplani BR10423 strain 44 R1.1 (RHI), (3) Bradyrhizobium elkanii USDA 76 strain 43 R1.1 (BRA), and (4) Burkholderia cepacia ATCC 25416 strain 17A R2.2 (PRB). An additional treatment corresponded to the use of commercial peat (Table 2).
The bacterial strain inoculums were cultured in 125 mL Erlenmeyer flasks containing nutrient broth (Nutrient Broth K25-1216, KASVI), composed of meat extract and gelatin peptone. The bacterial culture was incubated at 28 °C under constant agitation (150 rpm) until it reached the logarithmic phase. The optical density (OD) at 600 nm (A600) was measured to monitor bacterial growth, ensuring concentration before inoculum preparation (106 colony-forming units mL−1). Here, we inoculated the bacteria into the biochar 4 h before the experiment and applied it both to the seeds and to the soil near the seeds. The viability assessment of the inoculum added to the biochar was conducted using the serial dilution method [20]. The procedure involved using test tubes containing 9 mL of 0.85% saline solution, which were shaken for 1 min before performing serial dilutions. Dilutions ranging from 10−2 to 10−5 were plated on PDA (potato dextrose agar) plates. After inoculation, the plates were incubated in a BOD chamber at 28 °C. The colonies were then compared with a control plate containing the original bacterial culture. The results confirmed that the bacteria remained viable throughout the evaluation period.
For the experiment, cowpea seeds of the cultivar ‘Miranda IPA 207’ were used as planting materials. The management practices commonly employed for cowpea cultivation in the region were implemented. The experiment was conducted in pots for 100 days after sowing with 7 kg of soil, in a controlled environment greenhouse (Temperature = ~26 °C; Humidity = 70%; Light/dark cycle duration = 11.5 to 12.5 h of daylight; Irrigation frequency = once per day).
We evaluated the shoot dry matter (SDM), root dry matter (RDM), and the estimate of grain yield per hectare (Prod), which was estimated based on the total grain yield per plant and extrapolated to yield per hectare. Soil samples were collected at ’the 0–10 cm layer’of each pot. Soil pH was determined in H2O (1:2.5). Total organic carbon (TOC) was extracted using potassium dichromate (K2Cr2O7) and determined by colorimetry. K+ and available P were extracted using ion exchange resin and pH was measured in a CaCl2 solution (0.01 mol L−1). Al3+ was extracted using a KCl solution (1 mol L− 1), while Na+ was extracted by Mehlich−1 method [21]. The following parameters were determined: total organic carbon (TOC), K, P, and total N. The enzyme activities, i.e., β-glucosidase (Beta, EC 3.2.1.21) [22], urease (Ure, EC 3.5.1.5) [23], and acid phosphatase (Aci.P) (EC 3.1.3.2) [24].
2.3. Statistical Analyses
All statistical analyses and exploratory graphical analyses were performed in the R computational environment with build version 4.3.1. After testing the assumptions of normality (Shapiro–Wilk), data were evaluated by a two-way analysis of variance (ANOVA), and when significant (α = 0.05), means comparison Fisher’s LSD test with Bonferroni’s adjustment. Furthermore, the factorial treatments were compared with the additional treatment by Dunnett’s test. The ANOVA was performed with the data transformed into ranks, considered a robust procedure for non-normal errors, resistant to outliers, and highly efficient for many other distributions. Principal component analysis (PCA) was used to identify main plant and soil variables influenced by different levels of treatments and combinations, using the factoextra package (version 1.0.7).
3. Results
The soil chemical and biological attributes differed between the bacteria and biochar applied (Figure 1). For example, the application of biochar increased the availability of P and K, although no differences were observed comparing biochar with and without PGPB (Figure 1b,c).
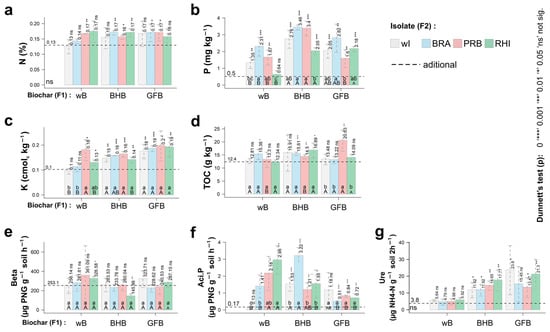
Figure 1.
Effect of biochar and PGPB on soil properties cultivated with cowpea. (a) N, (b) P, (c) K, (d) Total organic carbon (TOC), (e) beta-glucosidase, (f) Acid phosphatase, (g) urease. Bars: SE (n = 4 experimental repetitions). Significant differences among treatments are indicated by different lowercase letters referring to the route of inoculation and uppercase letters to the inoculums (p < 0.05). Means followed by one or more asterisks (*) differed significantly from additional treatment according to Dunnett’s test. Variables with ’ns’ close to the origin of the coordinates did not show significant variability by the F-test. Factor 1 (F1): wB—without biochar; BHB—bean husk biochar; GFB—grape fermentation biochar. Factor 2 (F2): wI—without inoculum; BRA— Bradyrhizobium elkanii USDA 76; PRB— Burkholderia cepacia ATCC 25416; RHI— Rhizobium altiplani BR10423.
On the other hand, the application of biochar regardless of the inoculation with PGPB did not increase the content of N (Figure 1a). The application of GFB inoculated with PRB increased the TOC content as compared to uninoculated biochar (Figure 1d).
Importantly, the responses of soil enzymes were different compared to the application of biochar with and without PGPB (Figure 1). The application of biochar with or without PGPB did not affect the activity of beta-glucosidase (Figure 1e). The activity of urease was stimulated by both biochar regardless of the presence of PGPB (Figure 1g).
On the other hand, the activity of soil acid phosphatase was higher when biochar (BHB) was associated with Bradyrhizobium, while the inoculation of Rhizobium without biochar also increased the acid phosphatase (Figure 1f).
The shoot and root biomasses were significantly influenced by the interaction between biochar and PGPB (Figure 2). Comparing biochar without bacteria, the application of BHB inoculated with PGPB increased the shoot biomass of cowpea, while this biochar inoculated with Burkholderia increased the root biomass (Figure 2a). No significant differences were observed to shoot and root biomass with the application of GFB inoculated with PGPB as compared to uninoculated biochar.
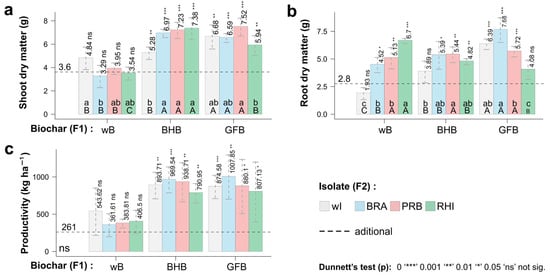
Figure 2.
Effect of biochar and PGPB on cowpea growth. (a) dry matter of the aerial part, (b) dry matter of the root, (c) productivity. Bars: SE (n = 4 experimental replicates). Significant differences among treatments are indicated by different lowercase letters referring to the route of inoculation and uppercase letters to the inoculums (p < 0.05). Means followed by one or more asterisks (*) differed significantly from additional treatment according to Dunnett’s test. Variables with ’ns’ close to the origin of the coordinates did not show significant variability by the F-test. Factor 1 (F1): wB—without biochar; BHB—bean husk biochar; GFB—grape fermentation biochar. Factor 2 (F2): wI—without inoculum; BRA— Bradyrhizobium elkanii USDA 76; PRB— Burkholderia cepacia ATCC 25416; RHI— Rhizobium altiplani BR10423.
Notably, the cowpea productivity increased with the application of both biochar regardless of association with PGPB (Figure 2c). Importantly, productivity was more than twice as high compared to commercial peat when both types of biochar were applied.In addition, the biochars inoculated with the BRA strain were the treatments that resulted in the highest productivity. For example, plants subjected to the BRA + GFB treatment exhibited a 3.85-fold increase in productivity compared to the additional treatment that involved the use of commercial peat.
The multivariate analysis explained 43.7% of the total variation and showed a clear separation between treatments with biochar associated with PGPB and those without biochar and commercial peat (Figure 3). The result showed that TOC, urease, acid phosphatase, P and K content, and cowpea productivity were associated with biochar and PGPB.
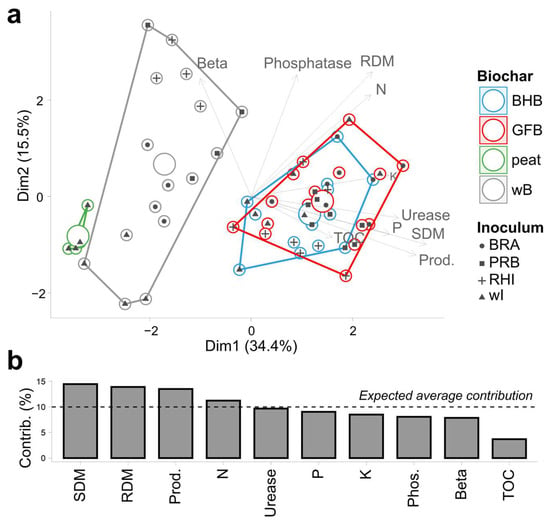
Figure 3.
Principal component analysis (PCA). (a) Biplot demonstrating that the biochar source was the main clustering factor (different colors) while the inoculum had a secondary influence (different shapes). (b) Analysis of the percentage of variance explanation for the ten variables analyzed in descending order. The dashed horizontal line indicates the expected percentage of explanation for variables set. wB—without biochar; BHB—bean husk biochar; GFB—grape fermentation biochar. F2 (Inoculum): wI—without inoculum; BRA— Bradyrhizobium elkanii USDA 76; PRB— Burkholderia cepacia ATCC 25416; RHI— Rhizobium altiplani BR10423. Variables: SDM—shoot dry matter; RDM—root dry matter; Prod—productivity; TOC—total organic carbon; Beta—beta-glucosidase; ure—urease; Pho.aci—acid phosphatase.
4. Discussion
Here, we analyzed the efficiency of biochar produced by bean husk or grape fermentation residue combined with BRA, PRB, or RHI to restore soil indicators (chemical and biological properties) and cowpea development in sandy soil from drylands. The results of this study showed positive effects of biochar inoculated with PGPB on cowpea growth, availability of P and K, TOC content, and the activity of acid phosphatase. Indeed, the multivariate analysis showed a clear association of these soil and plant parameters with both biochar inoculated with PGPB. On the other hand, we observed positive effect of applying only biochar on cowpea productivity and the activity of urease. Thus, these findings support partly the hypothesis that biochar associated with PGPB could effectively enhance plant growth and productivity, increase nutrients content, and stimulate enzymatic activity. Regarding the positive effect of biochar, mainly BHB, and PGPB, this could be due to the characteristics of porosity and water retention capacity found in the biochar which create a moist environment and favors bacterial survival and activity [5,9]. This suggests that both biochar, particularly BHB, could be potentially used as microbial carriers [25,26].
The results showed an increased plant growth, as observed by higher shoot and root biomass, when the biochar BHB was inoculated with Burkholderia. This suggests that the application of BHB could have stimulated the interaction of Burkholderia and plant roots [17] which potentialize the root growth, and consequently increase shoot biomass [6,12]. Previous studies have reported positive effects of Burkholderia stimulating the growth of sorghum [27] and soybean [28]. Comparing biochar, the positive effect of biochar BHB on cowpea growth could be related to bean residues which present characteristics to stimulate the Burkholderia activity, such as functional groups effective for microbial adhesion and proliferation [26]. The PGPB can express different mechanisms such as biological nitrogen fixation, phytohormone production, phosphate solubilization, production of siderophores and/or phytohormone release [6,7,19]. These mechanisms likely contributed to the observed increase in biomass and productivity. Here, we selected bacterial strains that were previously tested and proven to fix nitrogen, ensuring their effectiveness in promoting plant growth [19]. Furthermore, PGPBs enhanced root system development, improving water and nutrient uptake, which is particularly important in nutrient-limited soils, as used here. Additionally, biochars inoculated with the BRA strain resulted in the highest productivity. Notably, plants subjected to the BRA+GFB exhibited a 3.85-fold increase in productivity compared to the commercial peat that reinforces the potential of biochar as an effective carrier for PGPB [15].
The utilization of biochar was already demonstrated as positive to soil extracellular enzyme activities, fostering biological processes and enhancing nutrient availability [16]. Our study revealed that both biochar increased the activity of urease and phosphatase, while beta-glucosidase did not show significant changes. Interestingly, the activity of phosphatase was higher when biochar (BHB) was inoculated with Bradyrhizobium. This suggests a synergistic effect of biochar and Bradyrhizobium in stimulating phosphatase activity. Bradyrhizobium is a well-known PGPB acting on N fixation in cowpea, but a previous study has reported Bradyrhizobium increasing the activity of phosphatase in the rhizosphere of soybean [29].
Our results showed a positive effect of biochar increasing the availability of P and K. A previous meta-analysis study showed biochar having a great potential alternative to chemical P fertilizers promoting the availability of P to plants in soil amended with biochar [30]. Regarding the availability of K, [31] observed that biochar increased by 125% the availability of K in the soil. On the other hand, the application of GFB inoculated with Burkholderia increased the TOC content in soil. Previous studies have reported Burkholderia as an efficient degrader of organic residues which can contribute to C cycling and formation of organic C [32,33].
In addition to the effect of biochar, inoculation with PGPB also results in increased nutrient availability in the soil, particularly P and N, due to the metabolic modifications induced by these rhizobacteria. Overall, inoculation with PGPB was effective in increasing the availability of N in the soil, regardless of the presence of biochar. This increase in N content can be attributed to the biological nitrogen fixation activity performed by these strains [9,19]. Additionally, there are reports of increased phosphorus dissolution and available phosphorus content in the soil after inoculation with PGPB [29]. Therefore, it is possible to infer that the increase in plant production is related to this increase in the available nutrient content in the soil.
The multivariate analysis showed that biochar inoculated with PGPB can improve soil health and crop performance (Figure 3). This indicates a positive effect of this interaction, as it combines the benefits of bacteria with those of biochar. Inoculation with biochar and PGPB was responsible for increasing the activities of enzymes and nutrients in the soil, mainly N, P, and K, which is related to the increase in plant productivity. Biochar is known as effective soil chemical conditioners, due to their increase in cation exchange capacity, soil acidity neutralization, water retention, soil stability, and long-term soil health maintenance, resulting particularly in increased crop yield. Our findings are in line with previous studies, highlighting the potential benefits of biochar in agriculture, mainly as potential carrier for PGPB [9,14]. Importantly, this study suggests the possibility of using biochar and PGPB to improve crop growth in sandy soil.
5. Conclusions
In this study, we demonstrated the positive effect of biochar and plant growth-promoting bacteria in improving soil properties and cowpea growth. The application of biochar derived from bean husk and inoculated with Burkholderia stimulated cowpea growth, whereas biochar derived from grape fermentation residues and inoculated with Bradyrhizobium promoted higher phosphatase activity. Both types of biochar showed high potential in stimulating cowpea growth and improving nutrient availability in soil. This finding is particularly important for sandy and poor soils, as it offers the possibility of enhancing plant growth with lower rates of chemical fertilizers. This is the first study to evaluate biochar derived from bean husk and grape fermentation residue with PGPB in semiarid conditions. However, further studies are needed to assess different combinations in field-scale experiments, investigate long-term impacts on soil health, and evaluate economic feasibility.
Author Contributions
Data curation, D.P.d.C.; funding acquisition, E.V.d.M.; investigation, R.F.d.F.; methodology, M.d.A.L.J., G.P.D., J.R.d.S.L. and C.H.; project administration, E.V.d.M.; resources, I.d.S.A., A.P.M.F. and M.M.d.S.; visualization, C.H.; writing—review and editing, A.O.S., A.S.F.A. and E.V.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (313421/2021-8, 313174/2018-0; 426497/2018-0; 307335/2017-8; 304107/2020-4; ONDACBC:465764/2014-2 and NEXUS: 441305/2017-2), and Fundação de Amparo a Ciência e Tecnologia de Pernambuco (FACEPE) (APQ-1747-5.01/22; APQ-1464-5.01/22; APQ-0223-5.01/15; APQ-0419-5.01/15; APQ-0431-5.01/17; APQ-0498-3.07/17). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES 88887.736369/2017-00 and Finance Code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no competing interests.
References
- Zheng, S.; Yin, K.; Yu, L. Factors influencing the farmer’s chemical fertilizer reduction behavior from the perspective of farmer differentiation. Heliyon 2022, 8, e11918. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.M.; Costa, M.K.L.; Rocha, S.M.B.; Leite, M.R.L.; de Alcantara Neto, F.; de Souza, H.A.; Pereira, A.P.A.; Melo, V.M.M.; de Medeiros, E.V.M.; Mendes, L.W.; et al. Soil management shapes bacterial and archaeal communities in soybean rhizosphere: Comparison of no-tillage and integrated crop-livestock systems. Rhizosphere 2024, 30, 100886. [Google Scholar] [CrossRef]
- Medeiros, E.V.; Costa, D.P.; Silva, E.L.D.; França, A.F.; Lima, J.R.S.; Hammecker, C.; Mendes, L.W.; Pereira, A.P.A.; Araujo, A.S.F. Biochar and Trichoderma as an eco-friendly and low-cost alternative to improve soil chemical and biological properties. Waste Biomass Valoriz. 2023, 15, 1439–1450. [Google Scholar] [CrossRef]
- Xiong, X.; He, M.; Dutta, S.; Tsang, D.C. Biochar and sustainable development goals. In Biochar in Agriculture for Achieving Sustainable Development Goals; Academic Press: Cambridge, MA, USA, 2022; pp. 15–22. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C. Biochar-Bacillus consortium for a sustainable agriculture: Physicochemical and soil stability analyses. Biochar 2023, 5, 17. [Google Scholar] [CrossRef]
- Anbuganesan, V.; Vishnupradeep, R.; Mehnaz, N.; Kumar, A.; Freitas, H.; Rajkumar, M. Synergistic effect of biochar and plant growth promoting bacteria improve the growth and phytostabilization potential of Sorghum bicolor in Cd and Zn contaminated soils. Rhizosphere 2024, 29, 100844. [Google Scholar] [CrossRef]
- Wang, Y.; Wenqing, L.I.; Binghai, D.U.; Hanhao, L.I. Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Babalola, O.O.; Hassen, A.I. Rhizosphere competence and applications of plant growth-promoting rhizobacteria in food production-a review. Sci. Afr. 2024, 23, e02081. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Z.; Liu, C.; Zhang, Z.; Liu, X. Biochar combined with Bacillus subtilis SL-44 as an eco-friendly strategy to improve soil fertility, reduce Fusarium wilt, and promote radish growth. Ecotoxicol. Environ. Saf. 2023, 251, 114509. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Manchanda, G.; Maurya, I.K.; Maheshwari, N.K.; Tiwari, P.K.; Rai, A.R. Streptomyces from rotten wheat straw endowed the high plant growth potential traits and agro-active compounds. Biocatal. Agric. Biotechnol. 2019, 17, 507–513. [Google Scholar] [CrossRef]
- Prajakta, B.M.; Suvarna, P.P.; Raghvendra, S.P.; Alok, R.R. Potential biocontrol and superlative plant growth promoting activity of indigenous Bacillus mojavensis PB-35 (R11) of soybean (Glycine max) rhizosphere. SN Appl. Sci. 2019, 1, 1143. [Google Scholar] [CrossRef]
- Gou, Z.; Zheng, H.; He, Z.; Su, Y.; Chen, S.; Chen, H.; Sun, Y. The combined action of biochar and nitrogen-fixing bacteria on microbial and enzymatic activities of soil N cycling. Environ. Pollut. 2023, 317, 120790. [Google Scholar] [CrossRef]
- da França, R.F.; de Medeiros, E.V.; Silva, R.O.; Fausto, R.A.d.S.; de Souza, C.A.F.; de Oliveira, J.B.; Lima, J.R.d.S.; Araújo, A.P. Perspectives for Biochar as a vehicle for inoculation of phosphate solubilizing bacteria: A review. Res. Soc. Dev. 2022, 11, e36211124885. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Siddique, M.H.; Anwar, F.; Shahzad, T. Unlocking the potential of co-applied biochar and plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture under stress conditions. Chem. Biol. Technol. Agric. 2022, 9, 58. [Google Scholar] [CrossRef]
- Hardy, K.; Knight, J.D. Evaluation of biochars as carriers for Rhizobium leguminosarum. Can. J. Microbiol. 2021, 67, 53–63. [Google Scholar] [CrossRef]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-inoculation of rhizobacteria and biochar application improves growth and nutrients in soybean and enriches soil nutrients and enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Fekih, I.B.; Ditta, A.; Xing, S. Biochar has the potential to alter the soil pH which can affect microbial biomass in soil. Role of biochar and plant growth promoting rhizobacteria to enhance soil carbon sequestration-a review. Environ. Monit. Assess. 2019, 191, 251. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Santos, A.B.D.; Fracetto, G.G.M.; Fracetto, F.J.C.; Lira Junior, M.A. Rhizobial diversity in shrub-tree legume-based silvopastoral systems. Bragantia 2019, 81, e2622. [Google Scholar] [CrossRef]
- Wollum, A.G. Cultural methods for soil microorganisms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 781–802. [Google Scholar]
- Silva, F.D. (Ed.) Análises Químicas para Avaliação da Fertilidade do solo, Manual de Análise Química de Solos, Plantas e Fertilizantes; Embrapa: Brasília, Brazil, 1999; pp. 75–166. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.S. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2024, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Kuramae, E.E.; Derksen, S.; Schlemper, T.R.; Dimitrov, M.R.; Costa, O.Y.A.; Silveira, A.P.D.D. Sorghum Growth Promotion by Paraburkholderia tropica and Herbaspirillum frisingense: Putative Mechanisms Revealed by Genomics and Metagenomics. Microorganisms 2020, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.D.A.; España, M.; Aguirre, C.; Kojima, K.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyama, T. Burkholderia and Paraburkholderia are predominant soybean rhizobial genera in Venezuelan soils in different climatic and topographical regions. Microbes Environ. 2019, 34, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Zhao, Y.; Guan, D.; Li, L.; Zhao, B.; Ma, M.; Jiang, X.; Tian, C.; Cao, F.; Li, J. Effects of Bradyrhizobium Co-Inoculated with Bacillus and Paenibacillus on the Structure and Functional Genes of Soybean Rhizobacteria Community. Genes 2022, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Martins, C.C.; Araújo, T.C.; Marciano, C.R.; Barcelos, J.G.; Ribeiro, R.M.S.; Silva, M.G.; Barroso, D.G. Biochar decreases nutrient leaching in KCl-fertilized Podzols grown with black Mucuna. Rev. Bras. Ciênc. Solo 2023, 47, e0220086. [Google Scholar] [CrossRef]
- Cyle, K.T.; Klein, A.R.; Aristilde, L.; Martínez, C.E. Ecophysiological Study of Paraburkholderia sp. Strain 1N under Soil Solution Conditions: Dynamic Substrate Preferences and Characterization of Carbon Use Efficiency. Appl. Environ. Microbiol. 2020, 86, e01851-20. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.C.; DeRito, C.M.; Shapleigh, J.P.; Madsen, E.L.; Buckley, D.H. Phenolic acid-degrading Paraburkholderia prime decomposition in forest soil. ISME Commun. 2021, 1, 4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).