Omitting the Application of Nitrogen or Potassium Reduced the Growth of Young Chestnut (Castanea sativa) Trees, While a Lack of Boron Decreased Fruit Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Design
2.3. Management of the Field Trial
2.4. Soil and Leaf Sampling and Laboratory Analysis
2.5. Data Analysis
3. Results
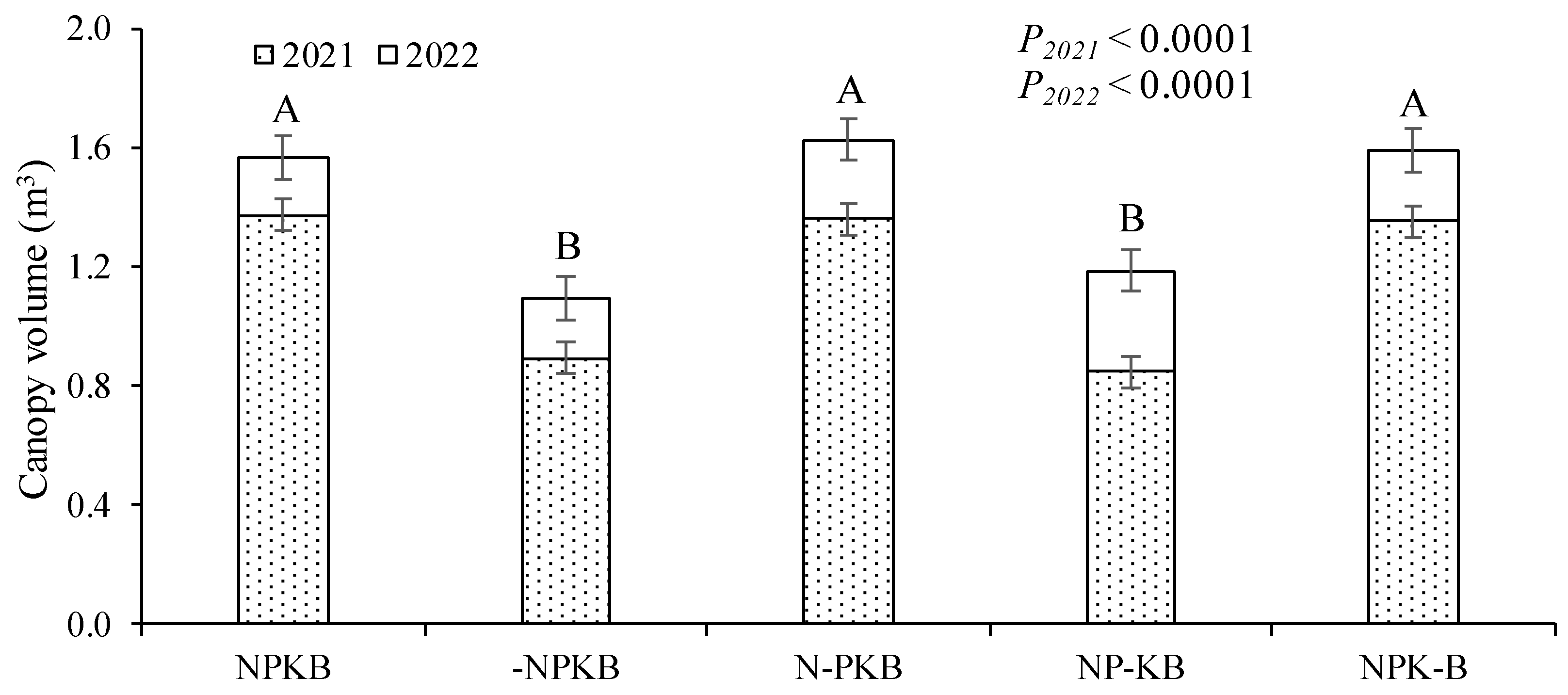
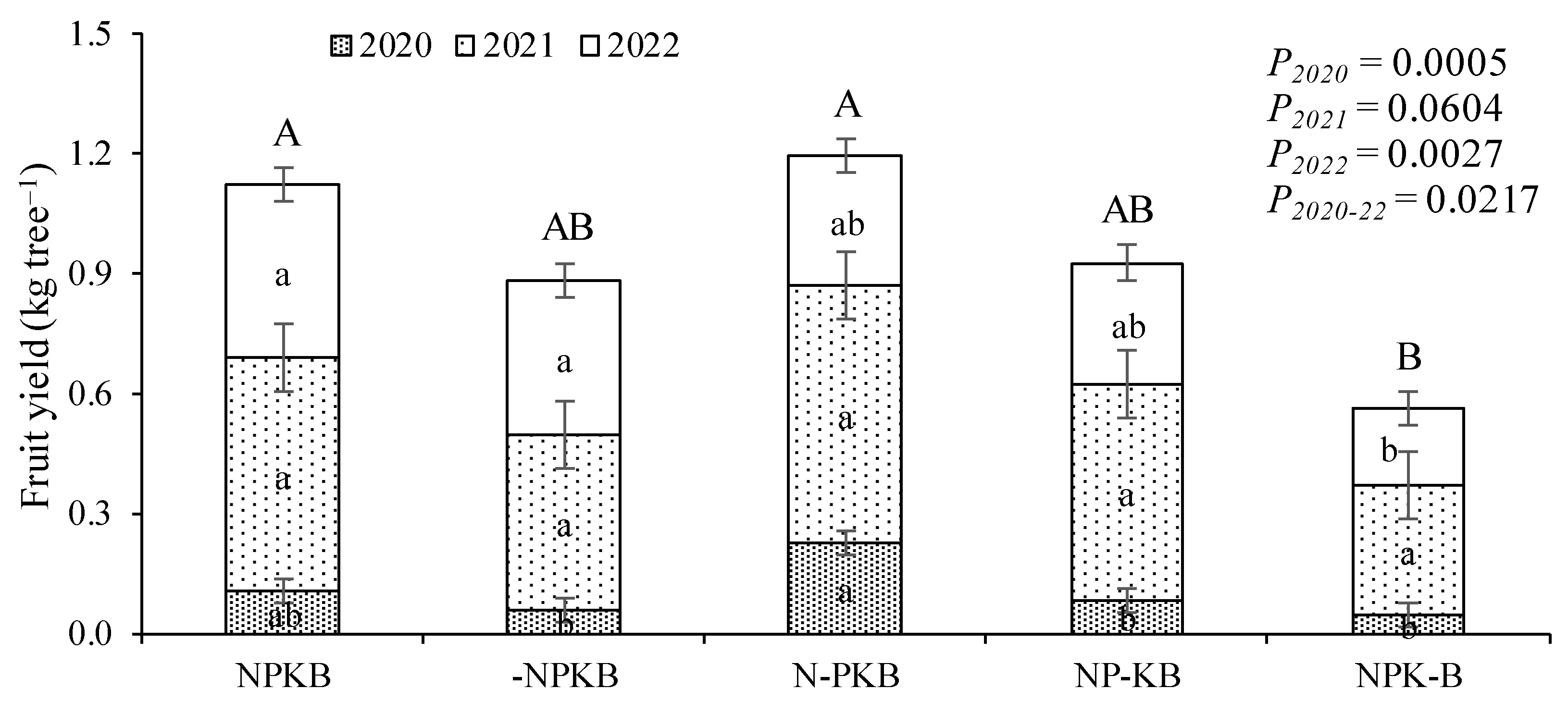
3.1. Tree Growth and Fruit Yield
3.2. Leaf Nutrient Concentration
3.3. Soil Properties and Nutrient Bioavailability
4. Discussion
4.1. The Lack of N and K Negatively Influenced Tree Growth, While the Absence of B Affected Fruit Yield
4.2. The Concentrations of N, K, and B in the Leaves Were Strongly Influenced by Their Applications, While P Had a Modest Effect
4.3. The Variations in Nutrient Application Resulted in Differences in Soil Organic Matter and Nutrient Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krebs, P.; Pezzatti, G.B.; Beffa, G.; Tinner, W.; Conedera, M. Revising the Sweet Chestnut (Castanea sativa Mill.) Refugia History of the Last Glacial Period with Extended Pollen and Macrofossil Evidence. Quat. Sci. Rev. 2019, 206, 111–128. [Google Scholar] [CrossRef]
- Martins, A.; Marques, G.; Borges, O.; Portela, E.; Lousada, J.; Raimundo, F.; Madeira, M. Management of Chestnut Plantations for a Multifunctional Land Use under Mediterranean Conditions: Effects on Productivity and Sustainability. Agrofor. Syst. 2011, 81, 175–189. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Coutinho, J.P.; Peixoto, F.; Alves, J.A. Ecologia do Castanheiro (Castanea sativa Mill.) [Ecology of Chestnut]. In Castanheiros [Chestnuts]; Gomes-Laranjo, J., Ferreira-Cardoso, J., Portela, E., Abreu, C.G., Eds.; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2007; pp. 109–114. (In Portuguese) [Google Scholar]
- Ramalhosa, E.; Pereira, E.L.; Silva, M.F.L. Valorização da castanha. [Fruit valorisation]. In Manual de Boas Práticas do Castanheiro [Handbook of Good Management Practices on Chestnut Orchards]; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 235–244. (In Portuguese) [Google Scholar]
- FAOSTAT. Production: Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 26 February 2024).
- Almeida, A. Instalação da cultura [Crop planting]. In Manual de Boas Práticas do Castanheiro [Handbook of Good Management Practices on Chestnut Orchards]; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 85–92. (In Portuguese) [Google Scholar]
- Patrício, M.A. Sistemas de condução e poda [Training and pruning systems]. In Manual de Boas Práticas do Castanheiro [Handbook of Good Management Practices on Chestnut Orchards]; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 149–170. (In Portuguese) [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Global Edition: London, UK, 2017. [Google Scholar]
- Shorrocks, V.M. The Occurrence and Correction of Boron Deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Gupta, U.C. Boron. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 241–277. [Google Scholar]
- Wimmer, M.A.; Eichert, T. Review: Mechanisms for Boron Deficiency-Mediated Changes in Plant Water Relations. Plant Sci. 2013, 203–204, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bryson, G.; Mills, H.A.; Sasseville, D.N.; Jones, J.B., Jr.; Barker, A.V. Plant Analysis Handobook III. A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing, Inc.: Athens, GA, USA, 2014. [Google Scholar]
- Mulla, D.J.; Strock, J.S. Nitrogen transformation process in soils. In Nitrogen in Agricultural Systems, Agrono-My Monograph n.49; Schepers, J.S., Raun, W.R., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 2008; pp. 361–400. [Google Scholar] [CrossRef]
- Poikane, S.; Phillips, G.; Birk, S.; Free, G.; Kelly, M.G.; Willby, N.J. Deriving nutrient criteria to support ‘good’ ecological status in European lakes: An empirically based approach to linking ecology and management. Sci. Total Environ. 2019, 650, 2074–2084. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Function of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Sepehr, E.; Rengel, Z.; Fateh, E.; Sadaghiani, M.R. Differential Capacity of Wheat Cultivars and White Lupin to Acquire Phosphorus from Rock Phosphate, Phytate and Soluble Phosphorus Sources. J. Plant Nutr. 2012, 35, 1180–1191. [Google Scholar] [CrossRef]
- Li, G.; Huang, G.; Li, H.; van Ittersum, M.K.; Leffelaar, P.A.; Zhang, F. Identifying Potential Strategies in the Key Sectors of China’s Food Chain to Implement Sustainable Phosphorus Management: A Review. Nutr. Cycl. Agroecosyst. 2016, 104, 341–359. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.A.; Moutinho-Pereira, J.M.; Correia, C.; Arrobas, M. Olive Tree Response to Applied Phosphorus in Field and Pot Experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of Potassium Transport in Plants under Hostile Conditions: Implications for Abiotic and Biotic Stress Tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.S.; Peiter, E. Potassium in Agriculture: Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Qiu, S.; Xie, J.; Zhao, S.; Xu, X.; Hou, Y.; Wang, X.; Zhou, W.; He, P.; Johnston, A.M.; Christie, P.; et al. Long-Term Effects of Potassium Fertilization on Yield, Efficiency, and Soil Fertility Status in a Rain-Fed Maize System in Northeast China. Field Crops Res. 2014, 163, 1–9. [Google Scholar] [CrossRef]
- Zhao, S.; He, P.; Qiu, S.; Jia, L.; Liu, M.; Jin, J.; Johnston, A.M. Long-Term Effects of Potassium Fertilization and Straw Return on Soil Potassium Levels and Crop Yields in Northcentral China. Field Crops Res. 2014, 169, 116–122. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R. The Potassium Paradox: Implications for Soil Fertility, Crop Production and Human Health. Renew. Agric. Food Syst. 2013, 29, 3–27. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Soyergin, S. Effects of Soil and Leaf Treatments to Eliminate Boron Deficiency in Olives. Commun. Soil Sci. Plant Anal. 2010, 41, 2004–2010. [Google Scholar] [CrossRef]
- Thapa, U.; Prasad, P.H.; Rai, R. Studies on Growth, Yield and Quality of Broccoli (Brassica oleracea L. var italica Plenck) as Influenced by Boron and Molybdenum. J. Plant Nutr. 2016, 39, 261–267. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.A.; Arrobas, M. Soil and Foliar Applied Boron in Olive: Tree Crop Growth and Yield, and Boron Remobilization within Plant Tissue. Span. J. Agric. Res. 2019, 17, e0901. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Raimundo, S.; Pereira, A.; Arrobas, M. Large Chestnut Trees (Castanea sativa) Respond Poorly to Liming and Fertilizer Application. J. Soil Sci. Plant Nutr. 2020, 20, 1261–1270. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Grade, V.; Barroso, V.; Pereira, A.; Cassol, L.C.; Arrobas, M. Chestnut Response to Organo-Mineral and Controlled-Release Fertilizers in Rainfed Growing Conditions. J. Soil Sci. Plant Nutr. 2020, 20, 390–391. [Google Scholar] [CrossRef]
- IPMA (Instituto Português do Mar e da Atmosfera) [Portuguese Institute of the Sea and the Atmosphere]. Normais Climatológicas [Climate Normals]. 2024. Available online: https://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 15 April 2024). (In Portuguese).
- WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Temminghoff, E.E.; Houba, V.J. Plant Analysis Procedures, 2nd ed.; Kluwer Academic Publishers: London, UK, 2004. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Technical Paper 9; ISRIC (International Soil Reference Information Center); FAO (Food and Agriculture Organization of the United Nations): Wageningen, The Netherlands, 2002; ISBN 90-6672-044-1. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Available Micronutrients (Cu, Fe, Mn, Zn) and Heavy Metals (Ni, Pb, Cd), DTPA Extraction Method; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/3/cc0048en/cc0048en.pdf (accessed on 15 April 2024).
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Nitrate by ultraviolet spectrophotometric method. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Portela, E.; Martins, A.; Pires, A.L.; Raimundo, F.; Marques, G. Práticas Culturais no Souto: O Manejo do Solo [Soil Management Practices in Chestnut Orchards]. In Castanheiros [Chestnuts]; Gomes-Laranjo, J., Ferreira-Cardoso, J., Portela, E., Abreu, C.G., Eds.; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2007; pp. 207–264. (In Portuguese) [Google Scholar]
- Silva, E.; Arrobas, M.; Gonçalves, A.; Martins, S.; Raimundo, S.; Pinto, L.; Brito, C.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.A.A. Controlled-Release Fertilizer Improved Soil Fertility but not Olive Tree Performance. Nutr. Cycl. Agroecosyst. 2021, 120, 1–15. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.; Rodrigues, M.A. Olive Response to Potassium Applications under Different Water Regimes and Cultivars. Nutr. Cycl. Agroecosyst. 2018, 112, 387–401. [Google Scholar] [CrossRef]
- Portela, E.A.C.; Ferreira-Cardoso, J.V.; Louzada, J.L. Boron Application on a Chestnut Orchard: Effect on Yield and Quality of Nuts. J. Plant Nutr. 2011, 34, 1245–1253. [Google Scholar] [CrossRef]
- Portela, E.; Ferreira-Cardoso, J.; Louzada, J.; Gomes-Laranjo, J. Assessment of Boron Application in Chestnuts: Nut Yield and Quality. J. Plant Nutr. 2015, 38, 973–987. [Google Scholar] [CrossRef]
- Arrobas, M.; Decker, J.V.; Feix, B.L.; Godoy, W.I.; Casali, C.A.; Correia, C.M.; Rodrigues, M.A. Biochar and Zeolites Did Not Improve Phosphorus Uptake or Crop Productivity in a Field Trial Performed in an Irrigated Intensive Farming System. Soil. Use Manag. 2022, 38, 564–575. [Google Scholar] [CrossRef]
- Arrobas, M.; Silva, J.; Busato, M.R.; Ferreira, A.C.; Raimundo, S.; Pereira, A.; Finatto, T.; de Mello, N.A.; Correia, C.M.; Rodrigues, M.Â. Large Chestnut Trees Did Not Respond to Annual Fertiliser Applications, Requiring a Long-Term Approach to Establishing Effective Fertilisation Plans. Soil Syst. 2023, 7, 2. [Google Scholar] [CrossRef]
- Pereira, E.; Coelho, V.; Tavares, R.M.; Lino-Neto, T.; Baptista, P. Effect of competitive interactions between ectomycorrhizal and saprotrophic fungi on Castanea sativa performance. Mycorrhiza 2012, 22, 41–49. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The mutualistic interaction between plants and arbuscular mycorrhizal fungi. Microbiol. Spectr. 2016, 4, 10–1128. [Google Scholar] [CrossRef]
- Ortas, I.; Bykova, A. The effect of mycorrhiza inoculation and phosphorus application on phosphorus efficiency of wheat plants. Commun. Soil Sci. Plant Anal. 2018, 49, 1199–1207. [Google Scholar] [CrossRef]
- Valentine, A.J.; Mortimer, P.E.; Kleinert, A.; Kang, Y.; Benedito, V.A. Carbon metabolism and costs of arbuscular mycorrhizal associations to host roots. In Symbiotic Endophytes; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 233–252. [Google Scholar]
- Lopez-Lefebre, L.R.; Rivero, R.M.; Garcia, P.C.; Sánchez, E.; Ruiz, J.M.; Romero, L. Boron Effect on Mineral Nutrients of Tobacco. J. Plant Nutr. 2002, 25, 509–522. [Google Scholar] [CrossRef]
- Long, Y.; Peng, J. Interaction between Boron and Other Elements in Plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Godbold, D.L.; Hoosbeek, M.R.; Lukac, M.; Cotrufo, M.F.; Janssens, I.A.; Ceulemans, R.; Polle, A.; Velthorst, E.J.; Scarascia Mugnozza, G.; Angelis, P.; et al. Mycorrhizal Hyphal Turnover as a Dominant Process for Carbon Input into Soil Organic Matter. Plant Soil 2006, 281, 15–24. [Google Scholar] [CrossRef]
- Márquez-García, F.; Sánchez, E.J.G.; Castro-Garcia, S.; Ordóñez-Fernández, R. Improvement of Soil Carbon Sink by Cover Crops in Olive Orchards under Semiarid Conditions. Influence of the Type of Soil and Weed. Span. J. Agric. Res. 2013, 11, 335. [Google Scholar] [CrossRef]
- Torres, M.R.-R.; Ordóñez-Fernández, R.; Giráldez, J.V.; Márquez-García, J.; Laguna, A.; Carbonell-Bojollo, R. Efficiency of Four Different Seeded Plants and Native Vegetation as Cover Crops in the Control of Soil and Carbon Losses by Water Erosion in Olive Orchards. Land Degrad. Dev. 2018, 29, 2278–2290. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Rhizosphere in relation to plant nutrition. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 347–368. [Google Scholar] [CrossRef]
- Römheld, V. Diagnosis of deficiency and toxicity of nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 299–312. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of Plants to Adverse Chemical Soil Conditions. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 409–472. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Uren, N.C. Manganese Oxidation and Reduction in Soils: Effects of Temperature, Water Potential, pH and Their Interactions. Soil Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]



| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Soil Properties | NPKB | -NPKB | N-PKB | NP-KB | NPK-B | Prob. | SE |
| 1 OC (g kg−1) | 8.1 a | 5.3 b | 8.5 a | 9.0 a | 8.8 a | <0.0001 | 0.34 |
| 2 pH (H2O) | 5.2 a | 5.5 a | 5.3 a | 5.2 a | 5.2 a | 0.2834 | 0.12 |
| 2 pH (KCl) | 4.0 a | 4.2 a | 4.1 a | 4.0 a | 4.1 a | 0.1472 | 0.07 |
| Extractable macro- and micronutrients (mg kg−1) | |||||||
| 3 Phosphorus (P2O5) | 212.5 a | 206.8 a | 41.7 c | 184.5 ab | 152.1 b | <0.0001 | 11.70 |
| 3 Potassium (K2O) | 313.0 a | 395.7 a | 386.0 a | 58.0 b | 370.0 a | <0.0001 | 26.06 |
| 4 Boron | 5.2 a | 5.7 a | 4.4 a | 4.3 a | 0.9 b | 0.0002 | 0.45 |
| 5 Iron | 26.2 a | 26.6 a | 33.9 a | 26.1 a | 25.2 a | 0.2084 | 2.66 |
| 5 Zinc | 0.4 a | 0.4 a | 0.3 a | 0.6 a | 0.3 a | 0.1244 | 0.07 |
| 5 Copper | 0.6 b | 0.4 b | 0.9 a | 0.5 b | 0.3 b | 0.0057 | 0.25 |
| 5 Manganese | 8.5 a | 6.7 a | 7.6 a | 6.4 a | 6.7 a | 0.2923 | 0.72 |
| Exchangeable complex (cmolc kg−1) | |||||||
| 6 Calcium | 6.6 a | 5.4 a | 5.1 a | 4.9 a | 5.0 a | 0.4448 | 0.68 |
| 6 Magnesium | 1.8 a | 1.7 a | 1.5 a | 1.2 a | 1.5 a | 0.4679 | 0.21 |
| 6 Potassium | 0.9 ab | 1.2 a | 1.1 ab | 0.1 c | 0.9 b | < 0.0001 | 0.06 |
| 6 Sodium | 0.3 a | 0.3 a | 0.2 a | 0.2 a | 0.2 a | 0.1868 | 0.02 |
| 7 Aluminum | 1.3 a | 0.9 a | 1.2 a | 1.4 a | 1.3 a | 0.1172 | 0.12 |
| 7 Acidity | 3.5 b | 1.6 c | 3.1 b | 3.9 a | 4.0 a | <0.0001 | 0.08 |
| 8 CEC | 13.0 a | 10.2 a | 11.1 a | 10.4 a | 11.7 a | 0.1023 | 0.70 |
| Inorganic nitrogen (mg kg−1) | |||||||
| 9 N-NH4+ | 48.1 a | 22.3 b | 60.8 a | 63.7 a | 44.3 ab | 0.0013 | 5.04 |
| 10 N-NO3− | 9.0 a | 1.2 b | 8.7 a | 7.6 a | 6.2 a | <0.0001 | 0.69 |
| DMY | Nitrogen | Phosphorus | Potassium | Calcium | Magnesium | Boron | Iron | Manganese | Zinc | Copper | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g pot–1 | g kg–1 | mg kg–1 | |||||||||
| NPKB | 6.1 a | 12.1 a | 2.8 a | 25.3 a | 5.2 bc | 2.6 bc | 124.2 a | 143.1 ab | 355.9 b | 17.3 a | 5.9 a |
| -NPKB | 2.5 c | 11.0 b | 3.0 a | 26.7 a | 5.4 bc | 2.7 abc | 129.2 a | 221.8 a | 492.4 a | 18.7 a | 6.0 a |
| N-PKB | 5.2 b | 11.1 ab | 2.4 a | 24.3 a | 3.9 c | 2.3 c | 119.7 a | 143.3 ab | 301.3 b | 16.3 b | 4.3 a |
| NP-KB | 4.5 b | 11.5 ab | 2.8 a | 8.4 b | 7.9 a | 3.1 a | 127.9 a | 110.6 b | 298.6 b | 18.9 a | 4.8 a |
| NPK-B | 4.7 b | 12.0 ab | 2.7 a | 25.3 a | 6.4 b | 2.9 ab | 11.0 b | 117.7 b | 344.3 b | 21.1 a | 5.4 a |
| Prob. | <0.0001 | 0.044 | 0.1183 | 0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.0168 | <0.0001 | 0.0650 | 0.0981 |
| SE | 0.16 | 0.29 | 0.15 | 2.06 | 0.44 | 0.12 | 8.84 | 16.2 | 17.62 | 0.76 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrobas, M.; Raimundo, S.; Correia, C.M.; Rodrigues, M.Â. Omitting the Application of Nitrogen or Potassium Reduced the Growth of Young Chestnut (Castanea sativa) Trees, While a Lack of Boron Decreased Fruit Yield. Soil Syst. 2024, 8, 104. https://doi.org/10.3390/soilsystems8040104
Arrobas M, Raimundo S, Correia CM, Rodrigues MÂ. Omitting the Application of Nitrogen or Potassium Reduced the Growth of Young Chestnut (Castanea sativa) Trees, While a Lack of Boron Decreased Fruit Yield. Soil Systems. 2024; 8(4):104. https://doi.org/10.3390/soilsystems8040104
Chicago/Turabian StyleArrobas, Margarida, Soraia Raimundo, Carlos Manuel Correia, and Manuel Ângelo Rodrigues. 2024. "Omitting the Application of Nitrogen or Potassium Reduced the Growth of Young Chestnut (Castanea sativa) Trees, While a Lack of Boron Decreased Fruit Yield" Soil Systems 8, no. 4: 104. https://doi.org/10.3390/soilsystems8040104
APA StyleArrobas, M., Raimundo, S., Correia, C. M., & Rodrigues, M. Â. (2024). Omitting the Application of Nitrogen or Potassium Reduced the Growth of Young Chestnut (Castanea sativa) Trees, While a Lack of Boron Decreased Fruit Yield. Soil Systems, 8(4), 104. https://doi.org/10.3390/soilsystems8040104








