Abstract
Plastic mulch is a commonly employed technique in agriculture to enhance crop production. Given the persistence of plastic residues in soil, bioplastics offer a potential alternative. Unfortunately, little is known about the medium-term consequences of both plastic and bioplastic mulches on soil properties. This study aimed to assess the medium-term consequences of plastic and bioplastic mulches and their replacement on soil properties. To this aim, the impact of conventional plastic (polyethylene, CP) and biodegradable plastic (BP) mulches on soil’s abiotic (pH, water content, total and organic carbon and total nitrogen contents) and biotic (microbial biomass, microbial respiration, enzymatic activities and microarthropod communities) properties after 2 years of exposure (T1) and after 3 (T2) and 6 (T3) months of mulch replacement was investigated. Moreover, uncovered soils were assessed as a control. The results highlighted that the samples were more significantly impacted by exposure time to mulches than by the different kinds of mulches. The replacement of both mulches (T2 and T3) decreased the content of C and increased the microbial biomass and activities; moreover, the mulch replacement changed the microarthropod community composition with a decrease of Collembola and an increase of Oribatida and Gamasida, especially in soils covered by biodegradable plastic mulches. Further investigations are needed to better understand the long-term impact of mulches on soil biota in order to prove the potential ecological implications of transitioning to sustainable alternatives.
1. Introduction
Plastic mulches have been widely used in agricultural soils in order to improve crop productivity and to inhibit the growth of undesired plant species [1]. Plastic mulches play a significant role in agricultural systems, encompassing the regulation of soil temperature, the mitigation of erosion and the optimization of nutrient uptake for crop growth [2]. By shielding the soil, plastic mulches protect it from erosive climatic and environmental phenomena and from compaction that could inhibit root growth and delay overall crop development [3]. Plastic mulches also represent a practical solution in reducing weed infestation and water loss, and in improving soil percolation and retention rate.
Unfortunately, their widespread and prolonged use as well as their improper disposal after harvest raise several environmental concerns. For instance, plastic mulches in polyethylene (PE), being difficult to dispose of after harvest [4], persist as residual parts, threatening the soil’s vitality [5] and impacting the soil’s ecosystem by releasing microplastics. Due to their size, microplastics infiltrate soil and interact with various organisms, including bacteria, fungi, nematodes and earthworms [6]. Research has shown that microplastics can alter the composition and function of a soil’s microbial communities, reducing microbial biomass and diversity, and thereby affecting nutrient cycling and soil fertility [7]. Additionally, soil organisms can transport microplastics deeper into the soil, influencing their distribution and potential impact on groundwater systems [8]. Moreover, the physical presence of plastics in the soil can alter its structure and properties, such as porosity and water-holding capacity, leading to negative effects on plant and crop health [9]. However, to date, data about changes in soil properties due to plastic mulches show contrasting effects, as it is highly context-dependent [10]. Some research reports reduction in soil porosity [11], excessive consumption of soil water, acceleration of soil organic carbon mineralization, a loss of carbon pool and a decrease in soil quality [12,13,14]; conversely, other research reports the promotion of soil macro-aggregates [15] and an increase in soil carbon reserve [16]. These findings underscore the complex interplay between plastic mulches and soil properties, highlighting the need for further comprehensive research to optimize the use of plastic mulches in agricultural practices.
Due to the increasing global concern surrounding plastic pollution [17], bioplastic mulches can be used as an alternative as they are composed of fossil-sourced polyesters [18] and are easily and quickly degraded by microorganisms [19]. Bioplastic mulches exhibit surface barrier effects similar to those of plastic ones, but their fate is different [20]. After the growing season, plastic mulches should be removed from the soil’s surface, whereas the bioplastic ones are meant to be tilled in and biodegraded by microorganisms [21]. Actually, the limited recent research conducted in this area highlights significant impacts of bioplastics on soil moisture and on soil’s microbial community composition and diversity [22,23,24,25]. The application of bioplastics involves further investigations and technologies aimed at reducing CO2 emissions from their biodegradation [26]. Biodegradable mulches degrade in soil primarily through microbial activity and hydrolysis, facilitated by enzymes produced by soil bacteria and fungi, which break down the polymer chains into simpler compounds [27,28]. These processes are influenced by environmental conditions and the mulch’s material properties.
According to Liu et al. [29], it is too early to promote bioplastic mulches as their consequences are still scarcely known. So, it is essential to better understand the impact of both plastics and bioplastics on soil and their temporal fate in order to ensure the proper functioning of vital processes such as organic matter decomposition, carbon sequestration and nutrient cycling [30,31]. Within this framework, our research aimed to evaluate the medium-term impact of both conventional and biodegradable plastic mulches on soil biota. To this aim, the impact of conventional (polyethylene, PE) and biodegradable plastic mulches on soil abiotic (pH, water content, total and organic carbon and total nitrogen contents) and biotic (microbial biomass, microbial respiration, enzymatic activities and microarthropod communities) properties after 24 (T1), 29 (T2) and 32 (T3) months of exposure was investigated.
2. Materials and Methods
2.1. Mesocosm Set-Up
The experiment was conducted in mesocosms represented by 14 pots that were 1 m in diameter and filled to a height of approximately 40 cm with limestone debris of different granulometry picked up in a quarry near Caserta (Southern Italy). In addition, fresh soil (0–20 cm in depth) was collected from different agricultural sites from the Napoli district (Southern Italy), which was mixed to obtain a representative sample, and placed in each pot (50 kg each for an approximate height of 30 cm). For the experiment’s aim, 14 pots were built-up according to the Randomized Complete Block Design (RCBD). A mulch made of polyethylene (HDPE) was placed to cover the entire surface in 5 out of the 14 pots for the conventional treatment (CP), whereas a mulch of a commonly used biodegradable plastic (BP) (made of modified starch) was placed on the surface of 5 other pots. No plastic mulches were placed in the last four pots, which were considered as the control (C) of the experiment (Figure 1). The mesocosms were left outdoors on the terrace of the Department of Biology of the University of Naples Federico II so that they were exposed to weather conditions. The terrace is a clean and cemented site, providing a controlled environment while still being subject to natural weather variations. Data on the climatic conditions were obtained from two websites: mean temperature data were obtained from https://www.ilmeteo.it/ (accessed on 30 June 2024) while monthly rainfall data were gathered from https://www.3bmeteo.com/ (accessed on 30 June 2024) (Figure 2).
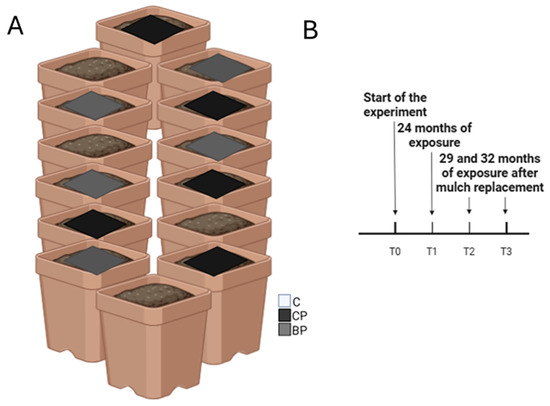
Figure 1.
Scheme of mesocosm setting up: (A) control pots, C; pots covered by conventional plastic mulch, CP; pots covered by bioplastic mulch, BP. Timeline of the performed sampling (B).
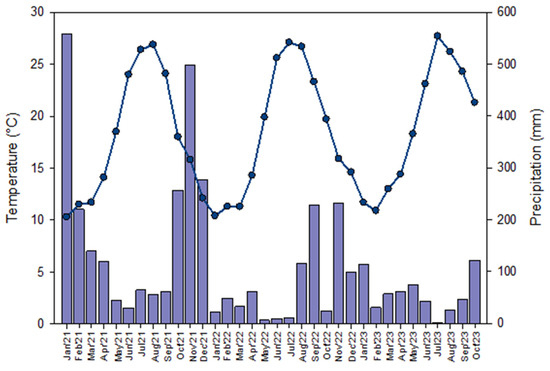
Figure 2.
Mean temperature (line) and rainfall amount (bar chart) in Naples during the experiment’s timeline, from January 2021 to October 2023.
2.2. Sample Collection
The experiment lasted 3 years, from the initial installation up until the final sampling. The mesocosms were built up in November 2020 and the soil was left for 2 months to stabilize its condition. In January 2021, the soil (0–10 cm in depth) was sampled before the placement of plastic mulches (T0). This was then repeated after 2 years of exposure (T1: 24 months of exposure) in February 2023. Successively, in April 2023, mulches were replaced, and soil samples were collected in July 2023 (T2: 29 months of exposure) and October 2023 (T3: 32 months of exposure). Surface soil samples were collected using soil cores (5 cm in diameter and 10 cm deep) from each pot, sieved (mesh: 2 mm) and then stored at 4 °C to proceed with analyses in the laboratory. Further cores (5 cm in diameter and 5 cm deep) were also collected from each pot to perform microarthropod extraction.
2.3. Soil Abiotic Analyses
Soils were characterized for the following parameters: pH, water content (WC), total carbon (C) and nitrogen (N) content and organic carbon (Corg). The soils’ pHs were measured with a water distilled solution 1:2.5 using an electrometric method and measured using a pH-meter. The soils’ WC were determined gravimetrically: 5 g of fresh soil was weighed and then dried at 105 °C until it achieved a consistent weight. The WCs were expressed as the percentage of the dry weight of the samples. The Corg was measured using a CNS Analyzer (Thermo Finnigan, Somerset, NJ, USA) on soil samples previously treated with HCl (10%) to exclude carbonates [32]; the C and N concentrations were evaluated on oven-dried (105 °C) and grounded (Fritsch Analysette Spartan 3 Pulverisette 0) soil samples using a CNS Analyzer [33].
2.4. Soil Biological Analyses
For biotic analyses, soils were preserved at 4 °C and analyzed in 10 days for the following parameters: microbial biomass, microbial respiration and four enzymatic activities (hydrolase, dehydrogenase, β-glucosidase and urease). Total soil microbial biomass (DNA yield) was extracted using a FastDNATM SPIN Kit for Soil (MP Biomedicals, Auckland, New Zealand) and the DNA quality was assessed by agarose gel electrophoresis [34].
Microbial respiration was measured using MicroResp® (Macaulay Scientific Consulting, Aberdeen, UK) assays [35].
Hydrolase activity (HA) was determined by adding fluorescein diacetate (FDA) as a substrate, as reported by Adam and Duncan [36].
Dehydrogenase activity (DHA) was determined using 1.5% 2,3,5-triphenyltetrazolium chloride (TTC) dissolved in 0.1 M TrisHCl buffer (pH 7.5), as reported by Memoli et al. [24].
β-glucosidase activity (β-glu) was determined by adding modified universal buffer (MUB, pH 6), as reported by Tabatabai et al. [37].
Urease activity (U) was determined using 0.1 M urea as a substrate, as reported by Kendeler et al. [38] and Alef et al. [39].
The microarthropod communities were sampled separately with soil corers (0–5 cm depth), carefully transported to the laboratory for extraction, ensuring that the soil structures were not disturbed. The microarthropods were extracted according to the Macfadyen method [40], over a period of 7 days: the temperature above the samples increased during extraction from 35 °C (2 days) to 45 °C (3 days) and then to 60 °C (2 days), whereas the temperature below the samples was kept at 5 °C. After extraction, microarthropods were preserved in 70% ethanol. The microarthropods were observed under a stereomicroscope, identified to order level and described in terms of density (individual number × m−2 of soil), richness (number of taxa at each site) and relative abundance of orders.
2.5. Integrative Biological Response Index (IBR)
The soils’ microbial activities and microarthropod communities were combined into the IBR, according to Beliaeff and Burgeot [41]. In more detail, the successive data-processing steps to the final score were as follows: for each property, the general mean (m) and the standard deviation (s) were calculated to obtain Y:
where X was the mean value of a sole property. Then, Z was calculated as:
or
where Equation (2a) was used in case of inhibiting effects, and Equation (2b) was used in case of stimulating effects. In particular, for this study, hydrolase, dehydrogenase, β-glucosidase, urease, soil respiration, DNA yield and microarthropod density and richness were considered to decrease within adverse conditions.
Y = (X − m)/s
Z = −Y
Z = Y
The minimum value |Min| for each property was then added to Z, resulting in the score (S) calculated as:
where S ≥ 0, and |Min| is the absolute value of the minimum value for Y.
S = Z + |Min|
Then, star plots were created to show the score results (S), and to calculate the IBR as:
Let Si and Si+1 represent two consecutive clockwise scores (radius coordinates) in the star plot, and let n be the total number of radii corresponding to the properties. The area Ai formed between two consecutive scores was calculated as follows:
where β was calculated as:
and α was calculated as:
α = 2π/n
EXCEL software (Microsoft 365) was used for all calculations and SigmaPlot12 15.1.1 was used for the star plots (Figure S1).
2.6. Soil Quality Index (SQI)
The soil quality index (SQI) was calculated considering the soils’ chemical and biological properties, which were ranked from 0 to 1 (score), respectively, reflecting low and high quality, according to [42]. The selected soil parameters for the index were the following: pH, water content, hydrolase, dehydrogenase, β-glucosidase, urease and DNA yield. For each site, the SQI was calculated, summing the parameter scores, and dividing for the number of parameters, as reported by [43]:
where S is the score assigned to each parameter and n is the number of the investigated parameters. Under the proposed framework, an ideal soil would have an SQI value of 1, while a severely degraded soil would have a value of 0.
2.7. Statistical Analyses
As the investigated soil properties did not match the basic assumptions of normality and homoscedasticity required for parametric statistics (Wilk–Shapiro test for α = 0.05), the data were normalized applying the Log10 function to perform statistical analyses. The two-way Anova repeated measures (Two-way RM-Anova, for α = 0.05; n = 14) were performed to assess the differences of each investigated soil property at different sampling times (T0, T1, T2 and T3) and for each treatment (C, CP and BP).
The non-parametric Spearman correlation rank methodology was used to avoid the bias of correlation between variables with non-normal distribution, in order to explore the relationship between abiotic and biotic soil properties.
The pairwise PERMANOVA analysis (pairwise.perm.anova function) was performed on the basis of all the abiotic and biotic properties, in order to highlight the significant (at least p < 0.05) separation of samples according to exposure time (T0 and T1 considered together as the pre-mulch replacement and T1 and T2 considered together as the post-mulch replacement) and treatment (C, CP and BP) and their interactions.
The statistical analyses and box plots were performed by the R 4.3.1 programming environment with vegan and gg plot 2 packages, while the graphs about climate conditions and microarthropod abundances were generated using the SigmaPlot12 software (Jandel Scientific, San Rafael, CA, USA).
3. Results
3.1. Soil Chemical and Physical Properties
The results from the statistical analyses showed that all soil abiotic properties were affected by the experiment time. Only the pH was affected by the treatments (C, CP and BP), and the interaction between time and treatment influenced both the pH and the nitrogen content (N) (Table 1).

Table 1.
F-values of the Two-way RM-Anova performed to evaluate the impact of experiment time, the treatments used and the interaction between these factors on soil abiotic properties.
In particular, the mean value of soil pH was 7.36 at T0 and, after a decrease at T1, it increased for all the treatments (C, CP and BP) with the statistically highest values at T3 (C: 7.61; CP: 7.69; BP: 7.73). The comparison among treatments at each sampling time highlighted that the pH was statistically higher in BP soil at T1 and in C soil at T2, whereas there were no statistically differences among treatments at T3 (Figure 3; Table 1).
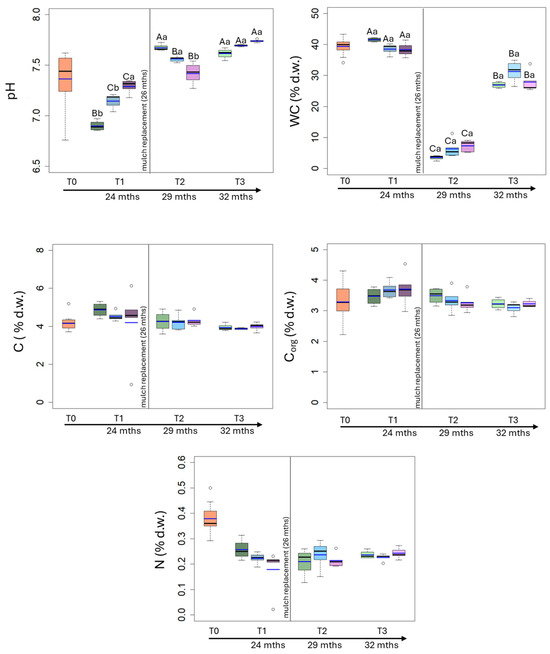
Figure 3.
Box plot reporting median (black dash), mean (blue dash), minimum and maximum of pH, water content, carbon, nitrogen and organic carbon contents in uncovered soils (green plots) and soils covered by conventional (light blue plots) and biodegradable (dark rose plots) plastic mulches. The soils were collected at the beginning of the mesocosm setting up (T0), after 24 (T1), 29 (T2) and 32 (T3) months of exposure. The vertical line indicates the separation between the first application of plastic mulches and their replacement after 26 months. The capital and small letters indicate significant differences among exposure times within each treatment and among treatments within each exposure time, respectively, for p values < 0.05 (Two-way RM-Anova).
The mean value of soil water content was 39.4% d.w. at T0, it remained almost constant till T1 (C: 41.5% d.w.; CP: 38.5% d.w.; BP: 38.3% d.w.), then it statistically decreased at T2 (C: 3.56% d.w.; CP: 6.37% d.w.; BP: 7.33% d.w.) and increased at T3 (C: 30.0% d.w.; CP: 31.3% d.w.; BP: 28.4% d.w.). The comparison among treatments at each sampling time highlighted statistically significant differences only at T1, when C soils showed the highest water content (Figure 3; Table 1).
Carbon content was 4.15% d.w. at T0; it was slightly higher at T1 (C: 4.87% d.w.; CP: 4.52% d.w.; BP: 4.63% d.w.) and lower at T2 (C: 4.25% d.w.; CP: 4.21% d.w.; BP: 4.31% d.w.) and T3 (C: 3.93% d.w.; CP: 3.87% d.w.; BP: 3.98% d.w.) (Figure 3; Table 1).
The mean value of organic carbon content was 3.29% d.w. at T0 and it slightly increased at T1 (C: 3.29% d.w.; CP: 3.47% d.w.; BP: 3.68% d.w.) and T2 (C: 3.49% d.w.; CP: 3.33% d.w.; BP: 3.26% d.w.), then it repristinated to its original values at T3 (C: 3.22% d.w.; CP: 3.10% d.w.; BP: 3.23% d.w.). The organic carbon content did not statistically differ among treatments or over time (Figure 3; Table 1).
3.2. Soil Microbial Biomass and Activities
The results from the statistical analyses showed that all the soils’ biotic properties, with the exception of the dehydrogenase activity, were affected by the experiment time. Specifically, only soil microbial respiration was affected by the treatments (C, CP and BP), and the interaction between time and treatment influenced β-glucosidase and Urease activities (Table 2).

Table 2.
F-values of the Two-way RM-Anova performed to evaluate the impact of experiment time, the treatments used and the interaction between these factors on soil biotic properties.
The microbial biomass, namely DNA yield, was initially (T0) 13,047 ng g−1 d.w. and it statistically increased over the time, with the highest values at T3 (C: 28,457; CP: 28,574; BP: 21,849 ng g−1 d.w.) (Figure 4). The DNA yield did not statistically differ among the treatments at each sampling time (Table 2).
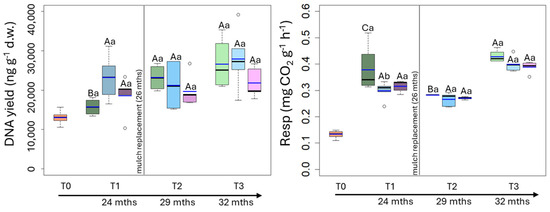
Figure 4.
Box plot reporting median (black dash), mean (blue dash), minimum and maximum of microbial biomass and microbial respiration in uncovered soils (green plots) and soils covered by conventional (light blue plots) and biodegradable (dark rose plots) plastic mulches. The soils were collected at the beginning of the mesocosm setting up (T0), after 24 (T1), 29 (T2) and 32 (T3) months of exposure. The vertical line indicates the separation between the first application of plastic mulches and their replacement after 26 months. The capital and small letters indicate significant differences among exposure times within each treatment and among treatments within each exposure time, respectively, for p values < 0.05 (Two-way RM-Anova).
Microbial respiration (Resp) was 0.13 mg CO2 g−1 h−1 at T0, increased at T1 reaching values ranging from 0.29 and 0.37 mg CO2 g−1 h−1 and remained almost constant till T3 (Figure 4). Except for T1, when the respiration was statistically higher in C (0.37 mg CO2 g−1 h−1) than in CP and BP soils (0.29 mg CO2 g−1 h−1 and 0.31 mg CO2 g−1 h−1, respectively), it did not statistically vary among treatments at the other sampling times (Figure 4; Table 2).
The temporal trends of the enzymatic activities for each treatment are reported in Figure 5.
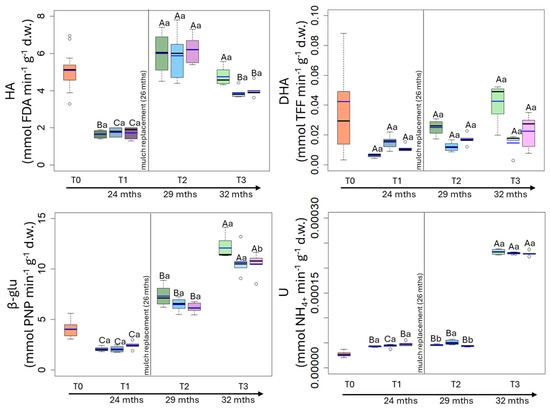
Figure 5.
Box plot reporting median (black dash), mean (blue dash), minimum and maximum of hydrolase (HA), dehydrogenase (DHA), β-glucosidase (β-glu) and urease (U) in uncovered soils (green plots) and soils covered by conventional (light blue plots) and biodegradable (dark rose plots) plastic mulches. The soils were collected at the beginning of the mesocosm setting up (T0), after 24 (T1), 29 (T2) and 32 (T3) months of exposure. The vertical line indicates the separation between the first application of plastic mulches and their replacement after 26 months. The capital and small letters indicate significant differences among exposure times within each treatment and among treatments within each exposure time, respectively, for p values < 0.05 (Two-way RM-Anova).
The hydrolase activity (HA) was, on average, 5.06 mmol FDA min−1 g−1 h−1 at T0, it decreased at T1 reaching values ranging between 1.65 and 1.72 mmol FDA min−1 g−1 h−1, then it increased till the initial value (Figure 5). No differences in HA were observed among treatments at each sampling time (Figure 5).
The dehydrogenase activity (DHA) was, on average, 0.032 mmol TFF min−1 g−1 h−1 at T0, it decreased at T1 and subsequently increased at T3, reaching values similar to the initial one (Figure 5). No differences in DHA were observed among treatments at each sampling time (Figure 5).
The β-glucosidase activity (β-glu) was, on average, 4.05 mmol PNP min−1 g−1 h−1 at T0, it remained almost constant till T1 and then suddenly increased, reaching the highest values at T3 (C: 12.1 mmol PNP min−1 g−1 h−1; PE: 10.8 mmol PNP min−1 g−1 h−1; M: 10.5 mmol PNP min−1 g−1 h−1). The only differences among treatments were observed at T3 when β-glu in C soil was statistically higher than in BP and CP soils (Figure 5).
The urease activity (U) was 2.7 × 10−5 mmol NH4+ min−1 g−1 h−1 at T0 and remained almost constant till T2, then statistically increased, reaching values ranging between 2.32 × 10−4 mmol NH4+ min−1 g−1 h−1 and 2.29 × 10−4 mmol NH4+ min−1 g−1 h−1 (Figure 5). The only differences among treatments were observed at T3 when U in C soil (2.32 × 10−4 mmol NH4+ min−1 g−1 h−1) was statistically higher than in BP and CP soils (2.293 × 10−4 mmol NH4+ min−1 g−1 h−1 and 2.290 × 10−4 mmol NH4+ min−1 g−1 h−1, respectively (Figure 5).
3.3. Microarthropod Community Analyses
The results from the statistical analyses showed that the microarthropod community and the taxonomical indices did not vary with time, treatment, or their interactions, except for the Prostigmata order, which was impacted by interactions between time and treatment (Table 3).

Table 3.
F-values of the Two-way RM-Anova performed to evaluate the impact of experiment time, the treatments used, and the interaction between these factors on soil microarthropod community.
Notably, the mean value of microarthropod density was 1172 organisms m−2 at T0 and remained almost constant over the time. No statistical differences occurred among treatments at each sampling time, except for T1 when the microarthropod density in the BP soil (1783 organisms m−2) was higher than that of the C and CP soils (509 organisms m−2 and 679 organisms m−2, respectively) (Figure 6; Table 3).
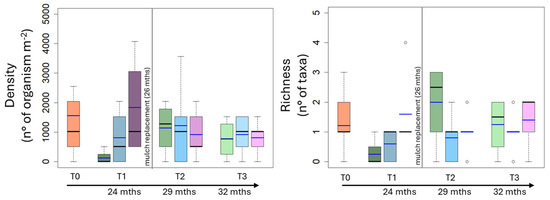
Figure 6.
Box plot reporting median (black dash), mean (blue dash), minimum and maximum of density and richness in uncovered soils (green plots) and soils covered by conventional (light blue plots) and biodegradable (dark rose plots) plastic mulches. The soils were collected at the beginning of the mesocosm setting up (T0) and then after 24 (T1), 29 (T2) and 32 (T3) months of exposure. The vertical line indicates the separation between the first application of plastic mulches and their replacement after 26 months.
In total, seven orders of microarthropods (Figure S2; Tables S1 and S2; Figure 7) were identified: Gamasida, Oribatida, Prostigmata (belonging to Acarina), Collembola, Coleoptera (larva form), Diptera (larva and adult forms) and Hemiptera (larva form). Among them, Collembola were ubiquitous, except in BP soils at T3, and were the most abundant at T0 and in all treated soils at T1 (Figure 7; Table 4). Moreover, at T0, only Collembola, Oribatida and Coleoptera were present, whereas the other orders appeared over the time and in the different treated soils (Figure 7; Table 4). Over time, the microarthropods’ relative abundances showed different trends according to the treatment (Figure 7). Notably, in C soil, the relative abundance of Collembola decreased in T2 and T3 (T1: 100%; T2: 25%; T3: 36%); by contrast, Diptera larva increased in T2 and T3 (T1: 0%; T2: 22%; T3: 16%). The other orders were found exclusively at certain times (Gamasida at T3 with a relative abundance of 50%; Oribatida at T2 with a relative abundance of 33% and Prostigmata at T2 with a relative abundance of 22%). In CP soils, the relative abundance of Collembola decreased in T2 and T3 (T0: 87%; T1: 72%; T2: 22%; T3: 36%), those of Oribatida and Diptera increased over the time (T0: 10% T1: 11%, T2: 22% for Oribatida; T0: 0%; T1: 5.5%, T2: 11%, T3: 12.5% for Diptera). Gamasida appeared at T2 and then decreased (T2: 44%, T3: 25%), and Prostigmata were present only at T3, with a relative abundance of 25% (Figure 7). In BP soils, the relative abundance of Collembola rapidly decreased (T1: 100%; T2: 23%; T3: 0%). Oribatida were present only at T0 and T2, with relative abundances of 10% and 58%, respectively; Coleoptera were present only at T0 and T3 with relative abundances of 2% and 22%, respectively; Gamasida were present only at T2 and T3 with relative abundances of 17% and 67%, respectively; Hemiptera with a relative abundance of 11% (Figure 7; Table 3). Moreover, statistical differences among treatments were observed only for the Prostigmata order, which was found exclusively in C soils at T2 and in CP soils at T3 (Table 5).
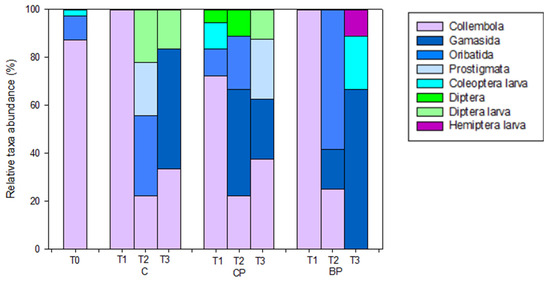
Figure 7.
Relative abundances of microarthropod orders (%) in uncovered (C) and covered soils by conventional (CP) and biodegradable (BP) plastic mulches collected at the beginning of the mesocosm setting up (T0) and then after 24 (T1), 29 (T2) and 32 (T3) months of exposure.

Table 4.
T-values of the Two-way RM-Anova performed on the soil microarthropods community within the same treatment (C: uncovered soils; CP: soils covered by conventional plastic mulches, BP: soils covered by biodegradable plastic mulches) at different sampling time (T1: after 24 months of exposure, T2: after 29 months of exposure, T3: after 32 months of exposure).

Table 5.
T-values of the Two-way RM-Anova test performed on the soil microarthropod community to evaluate differences between treatments (C: uncovered soils; CP: soils covered by conventional plastic mulches, BP: soils covered by biodegradable plastic mulches) within the same sampling time (T1: after 24 months of exposure, T2: after 29 months of exposure, T3: after 32 months of exposure).
The mean value of microarthropod richness was 1.4 taxa at T0 and remained almost constant over time. The microarthropod richness did not statistically vary over time or among treatments at each sampling time (Figure 6), except at T2 for C soil where the highest values (2.6 taxa) were calculated as compared to BP and CP soils (1.2 and 1.3 taxa, respectively) (Figure 6; Table 3).
3.4. Soil Biological Indices
The results from the statistical analyses showed that neither time and treatment nor their interaction impacted the soil biological indices (Table 6).

Table 6.
F-values of the Two-way RM-Anova performed to evaluate the impact of experiment time, the treatments used, and the interaction between these factors on soil abiotic and biotic properties.
The temporal trends of the calculated biological indices are reported in Figure 8. The mean value of the SQI index was 0.45 at T0 and ranged from 0.45 to 0.57 over time (Figure 8); finally, that of the IBR index was 1.22 at T0 and ranged from 0.78 and 2.05 over time (Figure 8), with higher values at T1. No statistical differences of the calculated biological indices were observed among treatments at each sampling time.
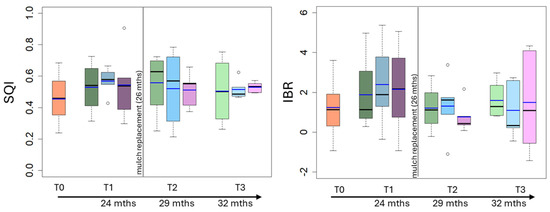
Figure 8.
Box plot reporting median (black dash), mean (blue dash), minimum and maximum of soil quality index (SQI) and integrative biological response (IBR) indices in uncovered soils (green plots) and soils covered by conventional (light blue plots) and biodegradable (dark rose plots) plastic mulches. The soils were collected at the beginning of the mesocosm setting up (T0) and then after 24 months (T1), 29 (T2) and 32 (T3) months of exposure. The vertical line indicates the separation between the first application of plastic mulches and their replacement after 26 months).
The correlation between soil properties was assessed by distinguishing the applied treatment of soils and are reported in Table S3. Particularly, in C soils, microbial activities were negatively correlated to water content and carbon and nitrogen concentrations; in CP soils, β-glucosidase was positively correlated to pH and negatively to water content and organic carbon concentration; in BP soils, β-glucosidase and microbial respiration were negatively correlated to nitrogen and organic carbon concentrations. Moreover, in C soils, the abundance of Collembola was negatively correlated to carbon concentration, that of Gamasida was negatively correlated to total and organic carbon concentrations; and Prostigmata were only negatively correlated to water content. In CP soils, the abundance of Collembola was positively correlated to water content, whereas that of Gamasida was negative. In BP soils, Gamasida were positively correlated to pH levels and negatively correlated to organic carbon concentrations.
3.5. Effects of Treatment and Time on Soil Properties
According to PERMANOVA analysis, the samples were significantly impacted (p < 0.001) by exposure time, but not by treatment. Moreover, the interactions between exposure time and treatment significantly impacted the samples. Each treatment (C, CP and BP) significantly differed between the pre-mulch (T0-T1) and post-mulch (T2-T3) replacement (p < 0.05). Additionally, at T0 and T1, C significantly differed from BP and CP (p < 0.05). After mulch replacements (T2 and T3), C significantly differed from BP (p< 0.05).
4. Discussion
The presence of plastic and bioplastic mulches, their subsequent replacement and their exposure time affected soil properties, as underlined by the PERMANOVA analysis. In the present research, the two kinds of plastics did not differently impact soil abiotic and biotic properties, according to several studies performed in the medium-term [16,17]. The benefit of bioplastic mulches on soil fertility would seem to occur in the long-term, when the total C content increases, due to their degradation, improving soil structure [44]. Additionally, the use of bioplastics has been associated with increased microbial biomass and activity due to the biodegradable nature of the material, which provides a carbon source for soil microorganisms. Moreover, the gradual breakdown of bioplastics contributes to the slow release of nutrients, improving and ensuring sustained soil fertility [14]. Collectively, these findings suggest that bioplastic could be a more sustainable option in agricultural practices [16].
In the present research, after 24 months since the first placement of plastic mulches, for both the conventional and the bioplastic varieties, only soil pH exhibited significant variations among the investigated abiotic properties. As the pH increased over the time in all soils (those uncovered and covered by both plastic and bioplastic mulches), it can be supposed that the experiment’s duration and the overall environmental conditions were the main factors responsible [44,45]. However, the role of the presence of plastic mulches cannot be excluded, as pH levels were significantly higher in the covered soils [46], with a major impact induced by bioplastic mulches as further increases in pH levels were observed in soils covered by this kind of mulch [17]. After 24 months since the first placement of plastic mulches, the overall decrease of enzymatic activities in the soils was likely due to the higher nitrogen content as compared to the initial value, which could have slowed down the nutrient request and then the microbial activity [47].
The replacement of both the plastic mulches did not significantly affect the soils’ microbial communities, as no differences were detected for microbial biomass, respiration and enzymatic activities. The slight reduction of both the microbial biomass and respiration observed after 29 months of exposure was suddenly recovered [48]. As the temporal trend of microbial biomass, respiration and enzymatic activity in soils covered by plastic mulches was comparable to that in uncovered soils, it can be supposed that the overall environmental conditions, rather than the presence of plastic mulches, were the main factors responsible for variations observed in the soils’ microbial communities [49]. The lack of differences in the enzymatic activities in covered soils as compared to the uncovered ones would suggest that the presence of plastics influence microbial biomass and activity. In particular, the bioplastic mulches result in a resource for microorganisms [21]; whereas the increases in β-glucosidase and dehydrogenase activities over time in soils covered by plastic mulches, rather than in the uncovered ones, indicate an effectiveness in the metabolisms of their microbial communities [50]. The increase of the urease activity in both uncovered and covered soil, could be due to various factors. Indeed, the increase of urease in uncovered soils, which is associated with a decrease in both carbon and nitrogen contents, would suggest a conspicuous use of organic matter that stimulated microbial activity [51]. These findings agree with Bandopadhyay et al. [21], who reported high urease activity, which resulted in an elevated consumption of nutrients, such as carbon and nitrogen, that in turn decreased over time [52]. By contrast, plastic mulches seem to exert negative impacts on organic carbon utilization by soil microorganisms [31].
Although the replacement of plastic mulches did not significantly change microarthropod density and richness, it did prompt a shift in the composition of the microarthropod communities. Notably, the replacement of bioplastic mulches seemed to cause a more noticeable impact on microarthropods, promoting the dominance of predators (mainly belonging to Gamasida, Coleoptera, Diptera and Hemiptera), presenting piercing/sucking mouth parts. Moreover, the presence of certain larvae could likely have been favored by the elevated temperature that occurred during the summertime when the bioplastic mulches were replaced. It can be supposed that the bioplastic mulches had protected them and their development [53,54]. The subsequent decrease in Collembola was likely due to their sensitivity to soil moisture [55], whereas the increase in Oribatida and Gamasida confirms the hypothesis that their show of resilience is attributable to environmental conditions [56,57].
Although some of the investigated soil properties changed according to the experiment’s duration and the treatments, SQI and IBR did not provide similar information. This discrepancy may be because these indices reflect overall soil conditions rather than individual soil properties. The use of mulch can pose a risk to soil health due to the release of contaminants. Studies such as those by Santini et al. [58] have highlighted that plastic mulch can function as vectors for chemical contaminants, such as heavy metals, which can accumulate in the soil and alter its chemical and biological properties. Furthermore, the release of microplastics (MP) into the soil can interfere with soil structure, reducing porosity, and negatively affecting microbial activity [9]. The continuous release of microplastics not only compromises soil quality but can also enter the food chain, posing a potential risk to the health of higher organisms, including humans [59]. However, the stability of the presented biological indices suggested that the variations in specific soil properties were not significant enough to impact the overall soil quality, regardless of the treatments applied [60]. Similarly, the fluctuations in microbial activities observed after the mulch was replaced may represent only transient responses to disturbance events that microorganisms quickly re-establish [48].
5. Conclusions
The obtained results highlighted that the exposure time to plastic mulches, but not the kind, significantly influenced soil biota over the 3-year mesocosm study. Notably, the replacement of plastic mulches caused an increase in microbial biomasses and enzymatic activities, which caused a decrease in the total of C content. In addition, the presence and replacement of plastic mulches did prompt a noticeable shift in microarthropod communities, with an observed decrease in Collembola abundance and an increase in predators, such as Gamasida, Coleoptera, Diptera and Hemiptera.
The role of both conventional and bioplastic mulches is not clear. However, the present research has provided valuable insights into the dynamics of soil abiotic and biotic properties, highlighting the need for comprehensive risk assessments to understand the long-term ecological implications of mulching. The findings from the present research highlight the necessity for investigations into the long-term consequences of mulching and the need for effective risk management strategies to better understand these consequences on soil health and to promote sustainable agricultural practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/soilsystems8030092/s1, Figure S1: Biological response score star plot; Figure S2: Total abundance of microarthropod community taxa. Tables S1 and S2: T-values of the Two-Way-RM-Anova test performed on the total abundance of microarthropod. Table S3: Coefficients of the Spearman test performed to evaluate the correlations.
Author Contributions
Conceptualization, G.S. and G.M.; methodology, M.Z. and G.S.; software, M.Z. and G.S.; validation, G.S., M.Z., L.S., V.M., R.D. and G.M.; formal analysis, M.Z. and R.D.; investigation, G.S. and M.Z.; resources, G.M.; data curation, M.Z., L.S. and R.D.; writing—original draft preparation, M.Z. and L.S.; writing—review and editing, M.Z., L.S., R.D. and G.M.; visualization, G.S., M.Z., L.S., V.M., R.D. and G.M.; supervision, G.M.; project administration, G.M.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Biology Department of University Federico II of Naples.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that has been used is confidential.
Acknowledgments
This study was conducted within the Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, P.; Wei, T.; Han, Q.; Ren, X.; Jia, Z. Effects of different film mulching methods on soil water productivity and maize yield in a semiarid area of China. Agr. Water Manag. 2020, 241, 106382. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Toleikiene, M.; Haider, I.; Aslam, M.U.; Nazar, M.A. Potential agricultural and environmental benefits of mulches—A review. Bull. Natl. Res. Cent. 2020, 44, 1–16. [Google Scholar] [CrossRef]
- Nachimuthu, G.; Halpin, N.V.; Bell, M.J. Productivity benefits from plastic mulch in vegetable production likely to limit adoption of alternate practices that deliver water quality benefits: An on-farm case study. Horticulturae 2017, 3, 42. [Google Scholar] [CrossRef]
- Changrong, Y.; Wenqing, H.; Neil, C. Plastic-film mulch in Chinese agriculture: Importance and problems. World Agric. 2014, 4, 32–36. [Google Scholar]
- Astner, A.F.; Hayes, D.G.; O’Neill, H.; Evans, B.R.; Pingali, S.V.; Urban, V.S.; Young, T.M. Mechanical formation of micro- and nano-plastic materials for environmental studies in agricultural ecosystems. Sci. Total Environ. 2019, 685, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between microplastics and soil fauna: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Judy, J.D.; Williams, M.; Gregg, A.; Oliver, D.; Kumar, A.; Kookana, R.; Kirby, J.K. Microplastics in municipal mixed-waste organic outputs induce minimal short to long-term toxicity in key terrestrial biota. Environ. Pollut. 2019, 252, 522–531. [Google Scholar] [CrossRef]
- Almeida, M.P.D.; Gaylarde, C.; Pompermayer, F.C.; Lima, L.D.S.; Delgado, J.D.F.; Scott, D.; Neves, C.V.; Vieira, K.S.; Baptista Neto, J.A.; Fonseca, E.M. The complex dynamics of microplastic migration through different aquatic environments: Subsidies for a better understanding of its environmental dispersion. Microplastics 2023, 2, 62–77. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Šimůnek, J.; Shi, H.; Hu, Q.; Zhang, Y. Evaluating the effects of biodegradable and plastic film mulching on soil temperature in a drip-irrigated field. Soil Tillage Res. 2021, 213, 105116. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.X.; Xue, Q.; Hui, X.M.N. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ’White revolution’ to ’white pollution’-agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, B.; Li, Z.; Zhao, C.; Qian, R.; Huang, F.; Zhang, P.; Li, H.; Jia, Z. Ameliorating C and N balance without loss of productivity by applying mulching measures in rainfed areas. Agr. Ecosyst Environ. 2023, 343, 108267. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Liang, B.; Liu, J.; Zong, H.; Guo, X.; Wang, X.; Song, N. Variations in soil aggregate distribution and associated organic carbon and nitrogen fractions in long-term continuous vegetable rotation soil by nitrogen fertilization and plastic film mulching. Sci. Total Environ. 2022, 835, 155420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xue, Y.; Jin, T.; Zhang, K.; Li, Z.; Sun, C.; Mi, Q.; Li, Q. Effect of long-term biodegradable film mulch on soil physicochemical and microbial properties. Toxics 2022, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Beriot, N.; Gort, G.; Lwanga, E.H.; Gooren, H.; Yang, X.; Geissen, V. Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Ghimire, S.; Flury, M.; Scheenstra, E.J.; Miles, C.A. Sampling and degradation of biodegradable plastic and paper mulches in field after tillage incorporation. Sci. Total Environ. 2020, 703, 135577. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xie, D.; Yang, C. Effects of a PLA/PBAT biodegradable film mulch as a replacement of polyethylene film and their residues on crop and soil environment. Agric. Water Manag. 2021, 255, 107053. [Google Scholar] [CrossRef]
- Mazzon, M.; Gioacchini, P.; Montecchio, D.; Rapisarda, S.; Ciavatta, C.; Marzadori, C. Biodegradable plastics: Effects on functionality and fertility of two different soils. Appl. Soil Ecol. 2022, 169, 104216. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Kumaresan, S.M.; Muthuraman, M.S.; Park, S.U. An update on polyethylene and biodegradable plastic mulch films and their impact on the environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Inubushi, K.; Kakiuchi, Y.; Suzuki, C.; Sato, M.; Ushiwata, S.Y.; Matsushima, M.Y. Effects of biodegradable plastics on soil properties and greenhouse gas production. Soil Sci. Plant Nutr. 2022, 68, 183–188. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 1–31. [Google Scholar] [CrossRef]
- Liu, L.; Zou, G.; Zuo, Q.; Li, S.; Bao, Z.; Jin, T.; Liu, D.; Du, L. It is still too early to promote biodegradable mulch film on a large scale: A bibliometric analysis. Environ. Technol. Innovat. 2022, 27, 102487. [Google Scholar] [CrossRef]
- Mo, F.; Yu, K.L.; Crowther, T.W.; Wang, J.Y.; Zhao, H.; Xiong, Y.C.; Liao, Y.C. How plastic mulching affects net primary productivity soil C fluxes and organic carbon balance in dry agroecosystems in China. J. Clean. Prod. 2020, 263, 121470. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, W.; Dai, Z.; Li, J.; Mao, W.; Yu, F.; Ma, J.; Wang, S.; Zeng, X. Comparative analysis of the effects of plastic mulch films on soil nutrient yields and soil microbiome in three vegetable fields. Agronomy 2022, 12, 506. [Google Scholar] [CrossRef]
- Pribyl, D.W. A Critical Review of the Conventional SOC to SOM Conversion Factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Panico, S.C.; De Marco, A.; Barile, R.; Maisto, G. Soil element fractions affect phytotoxicity, microbial biomass and activity in volcanic areas. Sci. Total. Environ. 2018, 636, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Chemidlin Prévost-Bouré, N.; Christen, R.; Dequiedt, S.; Mougel, C.; Lelievre, M.; Jolivet, C.; Shahbazkia, H.R.; Guillou, L.; Arrouays, D.; Ranjard, L. Validation and application of a PCR primer set to quantify fungal communities in the soil environment by real-time quantitative PCR. PLoS ONE 2011, 6, e24166. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A Rapid Microtiter Plate Method to Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis, Part 2—Chemical and Microbiological Properties; Page, A.L., Ed.; Soil Science Society of America (SSSA): Madison, WI, USA, 1982; pp. 903–947. [Google Scholar]
- Kendeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Enzyme Activities. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 1995; pp. 311–373. [Google Scholar]
- Macfadyen, A. Improved Funnel-Type Extractors for Soil Arthropods. J. Anim. Ecol. 1961, 30, 171–184. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Leitgib, L.; Kálmán, J.; Gruiz, K. Comparison of bioassays by testing whole soil and their water extract from contaminated sites. Chemosphere 2007, 66, 428–434. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1942–1962. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bandopadhyay, S.; English, M.E.; Bary, A.I.; DeBruyn, J.M.; Schaeffer, S.M.; Miles, C.A.; Reganold, J.P.; Flury, M. Impacts of biodegradable plastic mulches on soil health. Agric. Ecosyst. Environ. 2019, 273, 36–49. [Google Scholar] [CrossRef]
- Pedra, F.; Inácio, M.L.; Fareleira, P.; Oliveira, P.; Pereira, P.; Carranca, C. Long-Term Effects of Plastic Mulch in a Sandy Loam Soil Used to Cultivate Blueberry in Southern Portugal. Pollutants 2024, 4, 16–25. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Guo, T.; Xing, Y.; Mo, F.; Wang, H.; Fan, J.; Zhang, F. Effects of plastic mulch and nitrogen fertilizer on the soil microbial community, enzymatic activity and yield performance in a dryland maize cropping system. Eur. J. Soil Sci. 2021, 72, 400–412. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J.; Xing, Y.; Xu, G.; Wang, H.; Deng, J.; Wang, Y.; Zhang, F.; Li, P.; Li, Z. The effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 2019, 153, 121–173. [Google Scholar]
- Huang, F.; Liu, Z.; Mou, H.; Li, J.; Zhang, P.; Jia, Z. Impact of farmland mulching practices on the soil bacterial community structure in the semiarid area of the loess plateau in China. Eur. J. Soil Biol. 2019, 92, 8–15. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Dong, Q.; Feng, H.; Siddique, K.H. Plastic mulching significantly improves soil enzyme and microbial activities without mitigating gaseous N emissions in winter wheat-summer maize rotations. Field Crop. Res. 2022, 286, 108630. [Google Scholar] [CrossRef]
- Liu, X.; Dong, W.; Si, P.; Zhang, Z.; Chen, B.; Yan, C.; Zhang, Y.; Liu, E. Linkage between soil organic carbon and the utilization of soil microbial carbon under plastic film mulching in a semi-arid agroecosystem in China. Arch. Agron. Soil Sci. 2019, 65, 1788–1801. [Google Scholar] [CrossRef]
- Nazir, A.; Laila, U.-E.; Bareen, F.-E.; Hameed, E.; Shafiq, M. Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant. Sustainability 2021, 13, 13796. [Google Scholar] [CrossRef]
- Siepel, H. Life-history tactics of soil microarthropods. Biol. Fertil. Soils 1994, 18, 263–278. [Google Scholar] [CrossRef]
- Coulson, S.J.; Convey, P.; Schuuring, S.; Lang, S.I. Interactions between winter temperatures and duration of exposure may structure Arctic microarthropod communities. J. Therm. Biol. 2023, 114, 103499. [Google Scholar] [CrossRef] [PubMed]
- Aupic-Samain, A.; Baldy, V.; Delcourt, N.; Krogh, P.H.; Gauquelin, T.; Fernandez, C.; Santonja, M. Water availability rather than temperature control soil fauna community structure and prey–predator interactions. Funct. Ecol. 2021, 35, 1550–1559. [Google Scholar] [CrossRef]
- Santorufo, L.; Van Gestel, C.A.; Maisto, G. Sampling season affects conclusions on soil arthropod community structure responses to metal pollution in Mediterranean urban soils. Geoderma 2014, 226, 47–53. [Google Scholar] [CrossRef]
- Lakshmi, G.; Okafor, B.N.; Visconti, D. Soil microarthropods and nutrient cycling. In Environment, Climate, Plant and Vegetation Growth; Springer: Berlin/Heidelberg, Germany, 2020; pp. 453–472. [Google Scholar]
- Santini, G.; Maisto, G.; Memoli, V.; Di Natale, G.; Trifuoggi, M.; Santorufo, L. Does the element availability change in soils exposed to bioplastics and plastics for six months? Int. J. Environ. Res. Public Health 2022, 19, 9610. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Yan, C.; Mancl, K.; Gong, D.; He, J.; Mei, X. Macro-and/or microplastics as an emerging threat effect crop growth and soil health. Resour. Conserv. Recycl. 2022, 186, 106549. [Google Scholar] [CrossRef]
- Santorufo, L.; Memoli, V.; Panico, S.C.; Santini, G.; Barile, R.; Giarra, A.; Di Natale, G.; Trifuoggi, M.; De Marco, A.; Maisto, G. Combined Effects of Wildfire and Vegetation Cover Type on Volcanic Soil (Functions and Properties) in a Mediterranean Region: Comparison of Two Soil Quality Indices. Int. J. Environ. Res. Public Health 2021, 18, 5926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).