Abstract
The soil solution is the compartment where plants uptake nutrients and this phase is in equilibrium with the soil solid phase. Changes in nutrient content and availability in the soil solution can vary among soil types in response to humic acid concentrations, thereby affecting Brachiaria growth. However, there are no studies demonstrating these effects of humic acid application on different soil types and how they affect Brachiaria growth. Thus, the aim of this study was to evaluate the effects of humic acid concentrations (0, 5, 10, 25, and 60 mg kg−1 carbon-humic acid) on Brachiaria brizantha growth and soil solution properties of contrasting tropical soils. Plants were grown for 35 days in greenhouse conditions in pots containing Sandy Entisol, Clayey (Red Oxisol), and Medium Texture (Red-Yellow Oxisol). Soil solution was assessed for pH, electrical conductivity (EC), carbon, and nutrient content. Shoot and root dry matter, as well as macro and micronutrients accumulation in the shoot, were determined. In a soil type-dependent effect, pH, EC, and concentrations of nutrients in solutions changed in response to carbon-humic acid concentration. In the less-buffered soils, Sandy Entisol and Red-Yellow Oxisol, the addition of 30–40 mg kg−1 carbon-humic acid increased root proliferation by 76–89%, while Brachiaria biomass produced in all soils increased by approximately 30%. Levels of carbon in solution were high (>580 mg L−1) and varied depending on the investigated soil type. Though solution carbon contents did not appear to be a driving factor controlling the positive effects of humic acid concentrations on Brachiaria dry matter, there was a direct relationship between other properties and nutrient content in the soil solution, and Brachiaria dry matter production.
1. Introduction
Brazil boasts one of the largest commercial cattle herds globally, estimated to comprise 188 million animals nationally [1]. In 2020, Brazil produced 10.3 million tons of TCE (Ton of carcass equivalent), with 26% of it marketed in over 120 countries, encompassing fresh beef, offal, processed, casings, and salted meat exports [1]. Within the Brazilian grazing area, which accounts for approximately 84% of beef production [1], approximately 47% of grazing systems experience various soil degradation processes, including erosion, soil fertility depletion, compaction, and water scarcity [2]. Brachiaria spp. species dominate Brazilian pasture areas, covering approximately 99 million hectares, with Brachiaria brizantha cultivated across approximately 50 million hectares, making it the largest monoculture in planted areas worldwide [2].
Recovery of pastureland in Brazil is currently focused on reducing fertilizer rate, enhancing the yield and nutritional value of forage crops, alongside improving the efficiency of fertilizer use and the sustainability of grazing systems [2,3,4]. The use of humic acid is one strategy aimed at improving fertilizer use efficiency, forage production and quality [4,5,6,7]. Worldwide, studies investigating the effects of humic acid application on grasses are limited, particularly those concerning Brachiaria spp. grown in tropical soils. Increases in shoot and root dry matter production of Brachiaria treated with foliar spray of humic acid were reported by Pinheiro et al. [4] and Neves et al. [7]. In Brazil, within a Brachiaria decumbens pasture area, the use of 60 mg L−1 carbon-humic acid in foliar spray resulted in a 44% increase in plant height and a 200% increase in forage biomass, although the successive use of humic acid hindered Brachiaria growth [4]. However, in a 15-year-old Brachiaria brizantha pasture area fertilized with both nitrogen (N) and humic substances via foliar sprays, forage yield and digestibility were improved with 200 kg ha−1 N, while humic substances reduced the grass leaf/stem ratio and also impacted positively the Brachiaria growth and nutritional value [7].
Humic acid has direct effects on plant organs and tissues, while also indirectly altering soil properties and nutrient availability [8,9,10].The direct effects of humic substances are attributed to the action of humic acid molecules on plant cells [8,11], stimulating various plant metabolic processes and enhancing nutrient uptake [9,11,12,13,14]. In the soil, depending on the rate, humic acid modifies pH, acidity levels, water retention capacity, and the content of complexed nutrients, thereby improving nutrient availability and plant uptake [10,15,16,17,18,19]. The application of humic acid can block anion adsorption sites such as phosphorus (P), increasing its availability in the soil. Further, humic acid promotes the complexation with cations, mainly iron (Fe), copper (Cu), manganese (Mn), and zinc (Zn), and can enhance their transport to the roots [20,21,22]. These processes together facilitate plant uptake. However, little is known about the effect of humic acid application on nutrient availability in the soil solution, especially when evaluating different soil types [10,15,23]. Nonetheless, in a study on medium-textured soil, it was observed that the application of humic acid at different concentrations could increase the availability of nutrients such as P and Zn in the soil solution [10]. However, this effect is highly dependent on the soil type.
Soil type and soil organic matter (SOM) content and the chemical nature of organic compounds significantly influence plant nutritional status, growth, and yield by regulating the availability of nutrients, water, soluble carbon compounds, soil pH, electrical conductivity (EC), and the efficacy of various agricultural inputs, including humates [10,15,24,25]. Nutrients present in the soil solution are in equilibrium with those associated with the soil solid phase and with the nutrient adsorbed onto tropical soil colloids [10]. The availability of nutrients can be determined either by analyzing the entire soil or by assessing the concentration of readily available nutrients in the soil solution that can meet the crop’s nutritional requirements [10,18,26]. Despite being a significant nutrient pool for plants, the capacity of soil solution in humic acid-treated soils to reflect soil fertility status and supply nutrients to pasture crops is still insufficiently understood.
In addition to soil texture, native SOM soil mineralogy is a key factor that influences the magnitude, function, and optimal application rate of humic acid for different crops [4,10,15,17,18,27]. The interaction between soil and humic acid plays a crucial role in regulating the effectiveness of humic fertilization on crop nutritional status and growth [10,15,19,28,29]. Upon addition to soils, humic acid becomes adsorbed to clay minerals and is prone to adsorption onto soil colloids [20,30]. The exogenous carbon (C) introduced by humic acid into soils may interact with soil components, leading to variations in the content of bioactive carbon pools in the soil solution. These bioactive carbon pools can influence soil processes and biochemical and physiological processes that ultimately affect crop growth [10,11,20].
In soil, the rate of humic acid significantly influences the crop’s response to humic substances [10,11,15,18]. The association between plant biomass and humic substances rate is often characterized by quadratic functions, indicating a threshold concentration beyond which plant growth is either reduced or completely inhibited [11,15,18,20,31]. Consistent with the findings of Rose et al. [15], an initial peak in plant growth response to humic substances is observed within the range of 5–40 mg kg−1. Beyond this optimal concentration, there is a negative impact on plant growth attributed to deleterious effects of humic bioactive fractions on plant cells [32,33]. The complexation and availability of nutrients in less-buffered growth media, such as sandy soils, are additional factors influencing plant response to humic fertilization [8,15]. The rate of humic acid application varies depending on the soil and crop treated, or the mode of humic acid application (via foliar or soil application). Therefore, it is crucial to establish the optimum humic acid rate for grass plants as it varies based on plant species, soil type, and the source of humic substances [10,18,20].
According to the limited literature supporting the effects of the application of humic acid on the soil solution in different soil types related to plant growth, we hypothesize that: (i) in a concentration-dependent manner, humic acid would enhance Brachiaria growth more significantly in sandy soil (Sandy Entisol) compared to Oxisols with higher clay and soil organic matter (SOM) contents; (ii) humic acid would have little impact on shoot growth but play a significant role in increasing Brachiaria root biomass; and (iii) the response of Brachiaria to humic acid rates would not be affected by the native concentration of carbon available in whole soil and soil solution. The aims of this study were to: (i) evaluate the chemical composition of the solution of highly fertilized tropical soils treated with increasing concentrations of humic acid; (ii) identify the optimum concentration of humic acid to improve the nutritional status and biomass of Brachiaria grown in contrasting tropical soils; and (iii) investigate whether the background carbon concentrations in soil solution can inhibit the response of Brachiaria to exogenous carbon added by humic acid.
2. Materials and Methods
2.1. Soils Characterization
Three experiments were conducted simultaneously using different soils cultivated with Brachiaria brizantha cv. BRS Paiaguás, under greenhouse conditions. Sandy Entisol, Red-Yellow Oxisol, and Red-Oxisol samples, according to the Soil Taxonomy, from the surface layer (0.0–0.2 m) were collected under native forest vegetation in Lavras, state of Minas Gerais, Brazil. The primary attributes of the soils studied are shown in Table 1.

Table 1.
Texture, chemical and physicochemical properties of the non-fertilized soils before the addition of humic acid.
2.2. Humic Acid Characterization
The humic acid sample was extracted from leonardite using a 0.5 mol L−1 KOH solution at the ratio of 1:10 (w/v). The chemical properties of the humic acid are described in Table 2. Humic acid sample was scanned, and the main peaks in BBFs spectra were identified in the mid-infrared region at wavenumbers ranging from 4000 to 800 cm−1 (Figure 1). For each spectrum, 64 scans with a resolution of 2 cm−1 were collected using the Attenuated Total Reflectance Fourier Transform Infrared (FTIR) spectroscopy technique in an Agilent® Cary 630 spectrometer.

Table 2.
pH, electrical conductivity (EC) and total nutrient content of humic acid used.
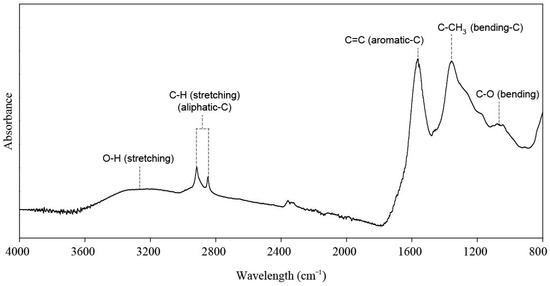
Figure 1.
Mid-infrared spectral signatures and the main bands assigned to organic functional groups present in the leonardite-derived humic acid.
2.3. Experimental Design and Treatments
In the three experiments conducted with different soils, the treatments consisted of five humic acid concentrations based on carbon content as follows: 0, 5, 10, 25, and 60 mg kg−1 carbon-humic acid. The concentrations of carbon-humic acid was chosen based on previous studies where it was shown that the effects of humic acid application on soils under conditions similar to the study were concentrated in the range of 5–50 mg kg−1 [10,18,31]. A randomized block experimental design was utilized with three replicates. Soil samples were dried, ground, passed through a 2 mm sieve, and incubated for 21 days with CaCO3 and MgCO3 at a 3:1 ratio to neutralize acidity and increase soil base saturation to 60%. Soil water-holding capacity was maintained close to 70%.
After acidity correction, the soil was dried again, ground, and passed through a 2 mm sieve, and then the pots filled with 1.5 kg soil. Humic acid was uniformly mixed with the whole soil in pots. Subsequently, phosphorus (P) was added to the soils at the following concentrations: 200 mg kg (Sandy Entisol), 350 mg kg−1 (Red-Yellow Oxisol), and 500 mg kg−1 (Red Oxisol). Phosphorus added to Oxisols was based on soil texture, remaining P, and P rates recommended for crop grown in greenhouse condition in closed small pots, so that the dose was increased according to the higher clay content in the soils, as per previously published studies [10,35]. Soluble sources of macro and micronutrients were added to soil samples to meet the nutritional requirements of Brachiaria brizantha plants grown in pots, with the following concentrations: 100 mg kg−1 of N (NH4H2PO4), 100 mg kg−1 of potassium (K) and 41 mg kg−1 of sulfur (S) (K2SO4), 0.81 mg kg−1 of boron (B) (H3BO3), 1.33 mg kg−1 of Cu (CuSO4.4H2O), 3.66 mg kg−1 of Mn (MnCl2.4H2O), 1.55 mg kg−1 of Fe (FeCl3.6H2O), 0.15 mg kg−1 of molybdenum (Mo) ((NH4)6Mo7O24.4H2O), and 6 mg kg−1 of Zn (ZnSO4.7H2O).
Following this, ten seeds of Brachiaria were sown per pot. After five days, thinning was performed, and two grass plants were cultivated per pot. Topdressing fertilization was applied by adding 100 mg kg−1 of N and 100 mg kg−1 of K (NH4NO3 and KNO3) at 15 and 32 days after sowing (DAS).
2.4. Soil Solution Sampling and Analysis
Two days after sowing Brachiaria, soil solution was collected from each experimental unit (pot) using the Suolo Acqua® sampler [25]. The solution sampler was inserted into the middle third portion of the soil packed in the pot simultaneously with the addition of soil and humic acid concentrations. After maintaining soil moisture close to 100% water-holding capacity for 12 h to achieve equilibrium between soil liquid and solid phases, soil solution samples were collected from each soil experimental unit using tubes conditioned to approximately ~70 kPa in a vacuum pump. A needle placed at the outer end of each Suolo Acqua® sampler was inserted into the rubber-sealing cap of the soil solution sampling tube.
The soil solution samples were then filtered through a membrane with a 0.45 μm pore diameter, and C in the soil solution was determined using an automatic (dry combustion) carbon analyzer in the liquid mode (Elementar, model Vario TOC Cube, Langenselbold, Germany). Soil solution pH and EC were determined using a bench Mettler Toledo digital conductivity and pH meter. Soil solution concentrations of P, K, Ca, Mg, S, Cu, Mn, Fe and Zn were determined using the inductively coupled plasma technique with an optical emission spectrometer (ICP-OES).
2.5. Plant Analysis
Brachiaria was harvested 35 days after sowing, separated into shoot and root parts, which were then dried at 60 °C until a constant weight was achieved. After drying, the biomasses were weighed to determine shoot and root dry matter. Total dry matter was calculated by adding shoot dry matter to root dry matter. The root:shoot ratio was calculated as the ratio of root dry matter to shoot dry matter. Brachiaria shoot biomass was ground and passed through a 1 mm sieve for determination of macro and micronutrients. Brachiaria shoot biomass was digested in a mixture of nitric and perchloric acids at a ratio of 4:1 (v/v) and concentrations of P, K, Ca, Mg, S, Fe, Mn, Cu and Zn in extracts were determined in ICP-OES [36]. The total nitrogen (N) content was determined by digesting plant tissue with sulfuric acid followed by distillation of the digested plant material and titration [34,36]. Nutrient accumulation in the shoot was calculated by multiplying the nutrient concentration in the shoot (mg kg−1) by the respective shoot dry matter [37].
2.6. Statistical Analysis
For each soil, the dataset underwent analysis of variance (ANOVA). When the assumptions of ANOVA were met and the means of treatments (humic acid concentrations) were significantly different (p < 0.05), linear mathematical models were fitted to carbon-humic acid concentrations with soil solution attributes, Brachiaria dry matter, and nutrients accumulated in shoots. All statistical analyses were performed using the R software ExpDes package [38]. The regression model that best fit the dataset was selected based on the significance of the mathematical equation parameters (p < 0.05), the lowest sum of squared errors, and the equation with the highest adjusted coefficient of determination (R2).
For each soil type, Pearson’s correlation matrix test was conducted to assess the degree of association among shoot and root dry matter and soil solution attributes. To examine the effects of carbon-humic acid concentrations on the solution properties of the three soils investigated, a cluster analysis through principal component analysis (PCA) was performed. In the cluster analysis, the dataset was normalized to remove the general effect of each soil type on the solution and plant attributes by subtracting the mean of the control (no humic acid addition) from the mean of each analyzed variable. This allowed for accurate evaluation of the humic acid effect on the analyzed attributes regardless of the soil type investigated. The cluster analysis was based on the matrix of Euclidean distances among means, using the Ward’s algorithm hierarchical clustering procedure [39]. Each dendrogram branch was calculated using the bootstrap support approach and the pvclust package [40]. These statistical routines were performed using the R software 4.3.1 [41].
3. Results
3.1. Soil Solution
Carbon in soil solution increased over carbon-humic acid concentrations added to Red Oxisol, Sandy Entisol and Red-Yellow Oxisol (Figure 2a). Carbon in solution was influenced by humic acid concentration added to soils, varying from 580 to 733 mg L−1 in the Sandy Entisol, 990–1250 mg L−1 in Red Oxisol, and from 800 to 1605 mg L−1 in the Red-Yellow Oxisol. Use of humic acid did not alter the solution pH of Red-Yellow Oxisol and Red Oxisol (Figure 2b), though the pH of Sandy Entisol solution linearly increased over humic acid concentrations, reaching values near 6.5. The use of carbon-humic acid concentrations in the Sandy Entisol and Red-Yellow Oxisol increased solution EC to values close to 3 dS m−1, thus, to levels, at least in closed growth systems, high enough to hamper Brachiaria growth. In the clayey-buffered Oxisol (Red Oxisol), solution EC was low (~1.7 dS m−1) and not affected by humic acid concentrations (Figure 2c).
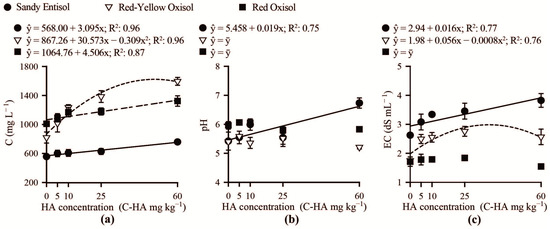
Figure 2.
The content of carbon (C) (a), pH (b), and electrical conductivity (EC) (c) in the soil solution of contrasting weathered soils as related to increasing humic acid concentrations (C-HA).
In the solutions of Red-Yellow Oxisol and Red Oxisol, the levels of Ca and Mg remained unchanged with the use of humic acid (Figure 3a,b). However, in the Sandy Entisol solution, Ca levels sharply increased while Mg levels decreased as the concentration of humic acid increased. Across all soil types, K exhibited a linear increase in solution with increasing carbon-humic acid concentration (Figure 3c). High and variable levels (ranging from 105 to 180 mg L−1) of available K were determined in the solution of Sandy Entisol sandy samples treated with increasing humic acid concentrations. Iron in the solution varied across soil types and depended on humic acid concentration (Figure 3d). In the Sandy Entisol and Red-Yellow Oxisol, the availability of Fe in the solution is limited and slightly increased in response to humic acid concentrations. Conversely, in the Red Oxisol, the use of humic acid sharply increased Fe availability in the soil solution. In fact, in the Red Oxisol, humic acid plays a crucial role in improving Fe solubility, which varies from 0.5 mg L−1 at the lowest concentration of carbon-humic acid to 17.5 mg L−1 at carbon-humic acid concentrations close to 35–40 mg kg−1. Overall, Zn and Cu (Figure 3e,f) in the soil solution increased with increasing humic acid concentrations, while Mn in the soil solution is less responsive to changes in humic acid concentrations, increasing only in the Red-Yellow Oxisol-humic acid treated soil (Figure 3g). Phosphorus in the Red Oxisol soil solution shows slight changes, sharply increased (55–120 mg L−1) in the solution of Red-Yellow Oxisol, while its availability is reduced in the Sandy Entisol solution as carbon-humic acid concentration increased (Figure 3h). In the Sandy Entisol and Red Oxisol, the use of humic acid did not change S in the soil solution, while in the Red-Yellow Oxisol, the S increased as the carbon-humic acid concentration reaches concentrations up to 32 mg kg−1 (Figure 3i).
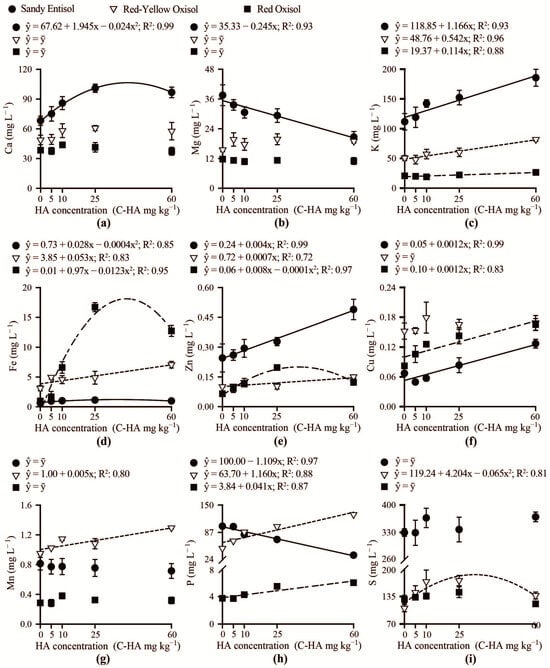
Figure 3.
The dissolved contents of calcium (Ca) (a), magnesium (Mg) (b), potassium (K) (c), iron (Fe) (d), zinc (Zn) (e), copper (Cu) (f), manganese (Mn) (g), phosphorus (P) (h), and sulfur (S) (i) in the soil solution of contrasting weathered soils as related to increasing humic acid concentrations (C-HA).
3.2. Brachiaria Nutrition and Growth
The growth of Brachiaria in response to humic acid use depended on soil type (Figure 4). The Brachiaria shoot dry matter was not affected by humic acid concentrations for plants grown in the Sandy Entisol (Figure 4a). Shoot biomass increased with increasing carbon-humic acid concentrations added to Red Oxisol and Red-Yellow Oxisol), reaching its maximum for plants grown in the Red Oxisol samples treated with approximately 25 mg kg−1 carbon-humic acid. Compared with the control (no humic acid addition), the application of approximately 35 mg kg−1 carbon-humic acid in the Sandy Entisol increased root biomass by 89% (Figure 4b). However, the root biomass gains in response to humic acid in the Red Oxisol were lower. In the Red-Yellow Oxisol, the proliferation of the Brachiaria root system is not affected by humic acid addition. Total Brachiaria biomass increased up to 25 mg kg−1 carbon-humic acid for all three soils (Figure 4c). The root:shoot ratio increased up to concentrations of 39 and 34 mg kg−1 carbon-humic acid added to Sandy Entisol and Red Oxisol, respectively (Figure 4d). However, the root-to-shoot ratio significantly decreased for humic acid concentrations above approximately 40 mg kg−1.
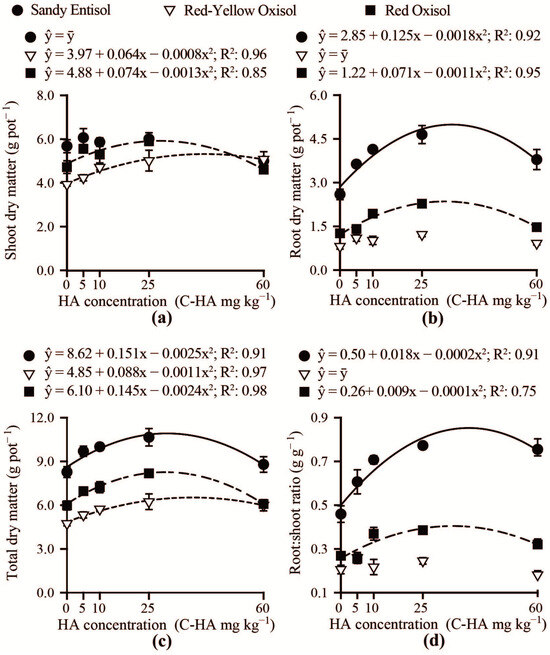
Figure 4.
Shoot (a), root (b), and total (c) dry matter production, and root:shoot biomass ratio (d) as a function of humic acid concentrations (C-HA) added to contrasting weathered soils.
The use of humic acid did not affect the accumulation of N, K, Mg and S in the Brachiaria shoot grown in the Sandy Entisol, P, S, K and Ca in Brachiaria cultivated in the Red Oxisol and N in plants grown in the Red-Yellow Oxisol (Figure 5a–f). In the Red-Yellow Oxisol, the accumulation of P, S, K, Ca, and Mg in the shoot increased, respectively, up to carbon-humic acid concentrations in the range of 19–28 mg kg−1. Concentrations of carbon-humic acid above the aforementioned range reduced the accumulation of most nutrients in Brachiaria shoot. Shoot Ca accumulation in the Sandy Entisol and Mg in the Red Oxisol increased up to optimal concentrations of 31 and 25 mg kg−1 carbon-humic acid, respectively.
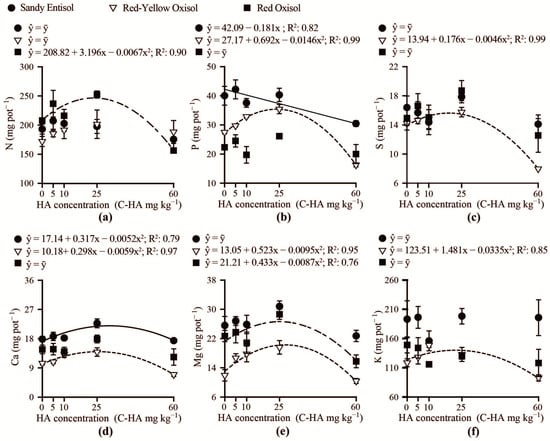
Figure 5.
Accumulation in Brachiaria shoot of nitrogen (N) (a), phosphorus (P) (b), sulfur (S) (c), calcium (Ca) (d), magnesium (Mg) (e), and potassium (K) (f) in response to humic acid concentrations (C-HA) added to contrasting weathered soils.
Shoot accumulations of Fe, Zn, Cu, Mn and B were not influenced by the addition of humic acid to Sandy Entisol (Figure 6a–e). In the Red-Yellow Oxisol, the accumulation of Fe, Zn, Cu, and Mn in the shoot increased up to concentrations of 26, 27, 19, and 26 mg kg−1 carbon-humic acid, respectively. Shoot accumulations of Fe, Zn, Cu, Mn, and B increased with humic acid addition to Red Oxisol in the range of 22–29 mg kg−1 carbon-humic acid. Concentrations of carbon-humic acid higher than the aforementioned range reduced the amounts of micronutrients in Brachiaria shoot. In the Red-Yellow Oxisol, the accumulation of B in Brachiaria shoot linearly increased as the humic acid concentration was augmented.
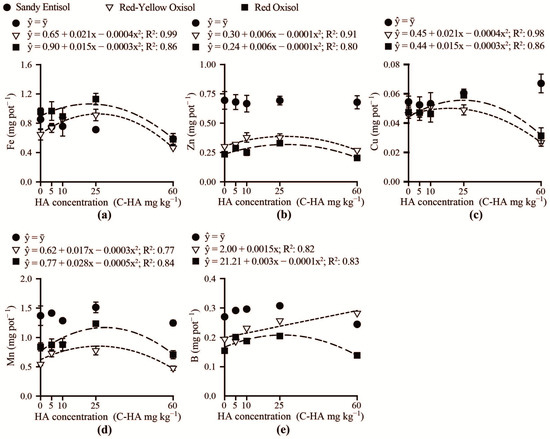
Figure 6.
Accumulation in Brachiaria shoot of iron (Fe) (a), zinc (Zn) (b), copper (Cu) (c), manganese (Mn) (d), and boron (B) (e) as related to humic acid concentrations (C-HA) added to the contrasting weathered soils.
3.3. Pearson’s Correlation Matrix
The degree of association and correlations among soil solution attributes that significantly explain most of Brachiaria shoot and root biomass production truly depend on the soil investigated (Figure 7). In the Sandy Entisol, shoot dry matter negatively correlated with pH and the contents of K and Cu in the soil solution (Figure 7a), while root dry matter positively correlated with Ca and Fe contents in the Sandy Entisol soil solution (Figure 7d). In the Red Oxisol, shoot dry matter is positively correlated with C, Fe, and P contents in its soil solution (Figure 7b), while root dry matter is correlated with pH, EC, and Mg content in its soil solution (Figure 7e). In the Red Oxisol, the root dry matter significantly increases in response to levels of Fe and Zn in the soil solution (Figure 7f), while shoot dry matter is not regulated by the availability of nutrients, EC, and pH of the solution of the soil with the highest content of clay and organic matter (Figure 7c).
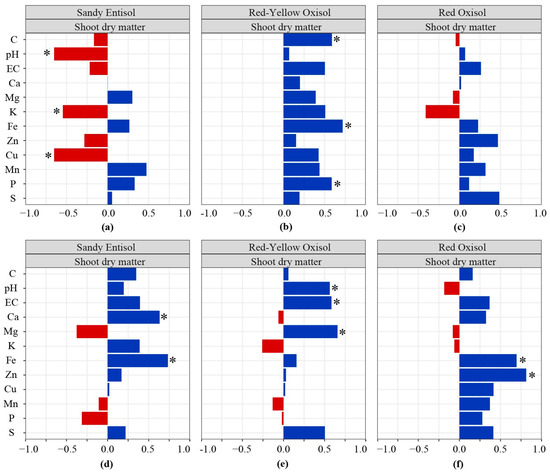
Figure 7.
Pearson’s correlation matrix was computed among dry matter (shoot (a–c)) and root (d–f) and soil solution properties and nutrients contents for each soil type. EC: electrical conductivity; Nutrients dissolved in the soil solution: carbon (C), calcium (Ca), magnesium (Mg), potassium (K), iron (Fe), zinc (Zn), copper (Cu), manganese (Mn), phosphorus (P), and sulfur (S). * Significant correlation (p < 0.05).
4. Discussion
In the Sandy Entisol, the increase in carbon in the soil solution was proportional to the concentrations of carbon added through humic acid application. However, in clayey (Red Oxisol) and especially in medium texture (Red-Yellow Oxisol) Oxisols, the levels of carbon in the soil solution exceeded those added by humic acid concentrations. Overall, disruption of soil aggregates, nutrient addition, and partial neutralization of soil acidity are key factors contributing to an increase in SOM mineralization rate and soluble carbon content in the soil [10,18,25,42,43]. Therefore, in addition to the effects of exogenous carbon added by humic acid, native carbon mineralized from SOM possibly explains and acts as a driving force regulating the high concentrations of carbon (ranging from 580 to 1605 mg L−1) determined in the soil solution. In a study conducted using humic acid application, it was found that its addition to the soil promoted a priming effect, stimulating microbial growth and increasing carbon mineralization in the soils [44]. This effect may explain the behavior observed in our study; however, further studies are needed to validate these mechanisms in different soils, mainly in field conditions.
Furthermore, humic acid added to soils interacts with soil constituents and SOM pools, regulating the rates of carbon adsorption-desorption processes and carbon concentrations in soil solution [10,20]. In two contrasting texture soils, the concentrations of carbon in solution exceeded the amounts of carbon added as humic acid, ranging from 50 to 290 mg L−1, and were sharply influenced by SOM content and sources of P added to the soil [18]. In experiments with contrasting soils incubated in closed pot systems, Carmo et al. [25] observed that carbon concentrations in solution varied over time, reaching concentrations ranging from 90 mg L−1 (sandy soil) to 680 mg L−1 (clayey soil).
Across different concentrations of humic acid, the pH of the Sandy Entisol underwent significant changes, ranging from 5.5 (no humic acid) to 6.7 (60 mg kg−1 carbon-humic acid). In the less-buffered sandy growth medium, the carboxylic, phenolic, and other polar groups of humic acid, when dissociated, release H+ ions, generating negative charges that can complex metals, forming organic metallic complexes (OMCs) with varying stability and solubility [8,29]. Additionally, the alkaline nature of humic acid, particularly in the less-buffered soil (Sandy Entisol), is expected to increase the soil solution pH [31]. The low buffering capacity of Sandy Entisol and Red-Yellow Oxisol soils contributed to the increase in EC in response to the concentrations of carbon-humic acid added to the soils (Figure 2). Electrical conductivity, in a soil type-dependent manner, serves as an indirect index allowing for the inference of the amount of ions and salts dissolved in the soil solution [18,25]. In line with Maluf et al. [27], the increased ion content in the soil solution is likely due to the capacity of humic acid to compete with metals and their accompanying anions for soil colloid binding and adsorption sites, as well as the composition of humic acid itself, including the high loadings of K added by the humic acid sample used in this study [31].
The effects of humic acid application on soil solution properties are more pronounced in the Sandy Entisol samples compared to buffered Oxisols (Red-Yellow Oxisol and Red Oxisol). Sandy soil is characterized by a low cation exchange capacity (CEC), allowing humic acid to displace cations and anions adsorbed in the soil solid phase, consequently increasing the concentrations of ions in the soil solution. This may explain the increased availability of Ca, K, Fe, Zn, and Cu in the soil solution. Nutrient availability in the soil solution is regulated by factors such as soil water content, pH, soil type, fertilizer rate, soil organic matter content, and humic acid concentrations [18,25]. The differences in the magnitude of humic acid concentration effects on soil solution properties are also potentially explained by the higher nutrient and acidity buffering capacity of Red-Yellow Oxisol and Red Oxisol compared with Sandy Entisol.
The increased levels of K in the solution of soils treated with humic acid can be attributed to the loading charges of K added to the soils by the humic acid itself (Figure 3). Generally, K is the most concentrated nutrient in commercial humic acid and humates products, as potassium hydroxide (KOH) is the main strong base used to extract humic substances from lignite and leonardite [31]. Potassium competes with other nutrients for soil colloid adsorption sites; therefore, at high concentrations, it can displace cationic micronutrients that were previously retained on the soil sorption complex [45].
In soil solution, P concentrations in the range of 0.2–0.3 mg L−1 are considered ideal for abundant plant growth [46]. However, in this study, the levels of P in the soil solution exceeded the threshold levels already reported as ideal for plant growth in nutritive solutions. For instance, in a typical Brazilian Oxisol treated with different carbon-humic acid concentrations and single superphosphate, the concentration of soil solution P was approximately 0.5 mg L−1 [18].
The reduction of P in the soil solution of Sandy Entisol in response to humic acid concentration was possibly related to the low clay content in the soil. This may result in high competition between the polar groups of humic acid and phosphate for soil adsorption sites. Colloidal surface binding sites have a greater affinity for P than humic acid; therefore, the soil solution of humic acid-treated soils is expected to have lower P levels as the amount of humic acid added to the soil increases [18]. The main mechanisms involving the application of humic acid and its effect on P availability in the soil are complex, involving processes such as interaction with ions and metal oxides in the soil through competitive adsorption, complexation of phosphorus with other metals with higher availability, electrostatic interaction, precipitation reactions, and changes in the physical and chemical properties (pH, CEC, aggregate stability, and water-holding capacity) of the soil. Additionally, humic acid influence the microbial community and enzyme activity to affect the migration and transformation of soil phosphorus, and they can also stimulate specific P transporters in the roots [47,48,49].
In the Sandy Entisol, the increase in Ca, K, Zn and Cu concentrations in the solution as a function of carbon-humic acid concentrations was not sufficient to increase Brachiaria shoot biomass The negative correlation between pH and shoot dry matter (Figure 7) indicates that the highest pH reached in the Sandy Entisol (pH 6.7) was one of the reasons hindering Brachiaria shoot growth. As the soil pH approaches values close to 7.0, the availability of most cationic micronutrients sharply decreases, which contributes to the prevention of nutrient uptake by Brachiaria, thereby reducing its growth. In contrast to the shoot, in the Sandy Entisol, the root dry matter and total dry matter, as well as the root:shoot ratio, increased in response to humic acid concentrations. Calcium levels in the soil solution of Sandy Entisol were a major driving force in improving the proliferation of the Brachiaria root system, as evidenced by a positive correlation between Ca concentration in the solution and root dry matter produced in the Sandy Entisol (Figure 7). Calcium plays a role in cell wall stabilization [50,51] and is capable of alleviating potential imbalances of nutrients in the soil solution, such as high levels of Mg, N predominantly as ammonium, and excessive K levels supplied to crops [52].
Increases in K, P, S, Fe, Zn, Cu and contents in the soil solution contributed to the augmentation of shoot dry matter of Brachiaria plants grown in the Red Oxisol and Red-Yellow Oxisol (Figure 3 and Figure 4). In the humic acid-treated Red-Yellow Oxisol samples, the increase in Brachiaria shoot dry matter was positively correlated with P levels in the soil solution (Figure 7). Humic acid use enhances P availability, its efficiency of use, and uptake by plants, thereby improving plant growth in an humic acid concentration-dependent manner [15,18,19]. One mechanism involved in the increase in P availability in soils treated with humic acid is the blocking of phosphate adsorption sites on the surface of colloids in a soil type-dependent manner [27].
In the Red Oxisol, the increase in Zn and Fe in the solution contributed to an increase in Brachiaria root dry matter, though shoot dry matter was not affected by these nutrients (Figure 7). Increased root proliferation in the humic acid-treated Sandy Entisol and Red Oxisol samples, without compromising Brachiaria shoot growth, may be beneficial over time. The root is a plant organ capable of storing photoassimilates and energy, favoring the regrowth of grass plants in subsequent growing seasons [53]. Pinheiro et al. [4] demonstrated a positive relationship between root dry matter and the regrowth of Brachiaria in response to the foliar application of 30 mg L−1 humic acid after 60 days of grass seed emergence. Accordingly, in the aforementioned study, humic acid did not influence Brachiaria shoot biomass at the first cut, while it increased root proliferation and shoot biomass at the second grass cutting, indicating increased bioactivity of humic acid over time and its high capacity in promoting plant regrowth [4]. Daur [6] reported an increase in the shoot dry matter, N, P, K, Mg, Fe, Cu, and Zn in Panicum antidolate retz treated with 90 kg ha−1 humic acid incorporated at 0–15 cm soil layer, which accounted for 60 mg kg−1 humic acid. The optimal concentration of carbon-humic acid that positively affected plant growth and soil properties in this study varied between 60 and 95 mg kg−1 humic acid. This range of humic acid added to soil with positive effects on plants and soil properties is similar to that reported by Daur [6].
Based on the results reported in this study, the effects of humic acid concentration on plant and soil properties fitted quadratic functions, whereby an optimum humic acid concentration is defined after which the response stabilizes and, subsequently, reduces, reflecting a sharp reduction in plant biomass [15,18]. The decrease in shoot and root biomass of Brachiaria observed after the highest concentration of humic acid is possibly due to the formation of encrusted layers of humic materials in cell walls, consequently reducing hydraulic conductivity and impairing root growth [32], as well as increasing the number of border cells [33]. Additionally, high humic acid concentrations may increase lipid peroxidation of the root cell plasmatic membrane, with negative impacts on plants [32].
Humic acid concentrations close to 25 mg kg−1 carbon-humic acid added to Red Oxisol improved the accumulation of N, Mg, Fe, Zn, Cu, Mn, and B in Brachiaria shoot, which were 18–52% higher than the control (humic acid-not treated plants) (Figure 5 and Figure 6). The increase in Fe, Zn, and Cu in the shoot was related to higher nutrient availability in the soil solution, while P accumulated in Brachiaria shoot was not favored by the higher levels of P contents in the solution of humic acid-treated soils (Figure 3 and Figure 5). In the Red-Yellow Oxisol, the increase in Fe, Zn, and Mn in the soil solution contributed to a greater nutrient accumulation in Brachiaria shoot, improving Brachiaria shoot growth, resembling the same results reported for Brachiaria plants grown in the Red Oxisol.
Humic acid improves the availability of nutrients in solution by triggering soil and plant processes, including those linked to the formation of organic metallic complexes with Fe, Zn, Mn, and Cu, thus boosting micronutrient acquisition by crops [9,11,28]. The complexation process involves reactions between the metal and carboxylic, phenolic, and other polar compounds enriched in N and S radicals found in the humic substances structure [8,29]. The mechanism involved in the increase in B uptake by plants under the influence of humic acid is not well known, while the formation of B-humate complexes available for plants is claimed as a possible explanation for the enhanced B acquisition by plants [54]. Overall, the highest concentration of carbon-humic acid (60 mg kg−1) inhibited nutrient accumulation in Brachiaria shoot.
Indirect effects of humic substances on plants include alterations in growth media properties, thereby affecting soil nutrient availability [11,15,16,27], as observed for several solution properties of the three humic acid-treated soils (Figure 2 and Figure 3). The direct effects of humic acid on plants are explained by the interaction between humic acid molecules with the cell surface, nutrient carriers, and the activity of H+-ATPase in the cell plasma membrane, auxin-like hormones, in addition to enhancing the proliferation of the root system, the emission of lateral roots, and overall plant growth [8,11,12,13,14]. The intensity of both direct and indirect effects of humic acid on plants and soil properties depends on the concentration and composition of humic acid [8,14,31].
However, only the shoot biomass of Brachiaria grown in the medium-texture soil (Red-Yellow Oxisol) was positively controlled (p < 0.05) by the solution carbon concentration. Therefore, the carbon content in the soil solution was not a key factor in controlling Brachiaria growth in response to humic acid concentrations. In soil solution, 5–93% of carbon compounds are potentially degradable [55], which is why the effect of humic substances on plants is not exclusively dependent on the amount of carbon in the soil solution but rather relies on the bioactivity of carbon dissolved pools found in soil.
Regardless of the soil type investigated, the effects of humic acid on plant and solution attributes were clustered into four groups based on their degree of similarity (Figure 8). The following groups emerged from the use of the PCA approach: group I—no humic acid added to Sandy Entisol, Red-Yellow Oxisol, and Red Oxisol, and application of 5 and 10 mg kg−1 carbon-humic acid in the Red Oxisol, and 5 mg kg−1 carbon-humic acid in the Red-Yellow Oxisol; Group II—application of 5, 10, 25, and 60 mg kg−1 carbon-humic acid in the Sandy Entisol; Group III—application of 10 and 25 mg kg−1 carbon-humic acid in the Red-Yellow Oxisol and 25 mg kg−1 carbon-humic acid in the Red Oxisol; and Group IV—application of 60 mg kg−1 carbon-humic acid in the Red-Yellow Oxisol and Red Oxisol. The response of Brachiaria to humic acid was evaluated by univariate and cluster analyses. A low or null effect of humic acid addition was observed for the group comprising the lowest concentrations of carbon-humic acid added to Oxisols (Figure 8). Thus, for the red circle-group, humic acid use slightly or did not affect the soil solution properties, Brachiaria nutritional status, and biomass production. In the Sandy Entisol, carbon-humic acid concentrations sharply altered the main soil solution properties and promoted a sharp increase in Brachiaria root proliferation as well (dark green triangle group). The positive effect of humic acid on nutritional status and shoot growth of Brachiaria was observed for the intermediary concentrations of carbon-humic acid added to Oxisols (pale green square group). In the group clustered around the highest carbon-humic acid concentration (60 mg kg−1 carbon-humic acid) added to Oxisols (orange hexagon group), humic acid reduced nutrients accumulated in shoot, hampering Brachiaria growth.
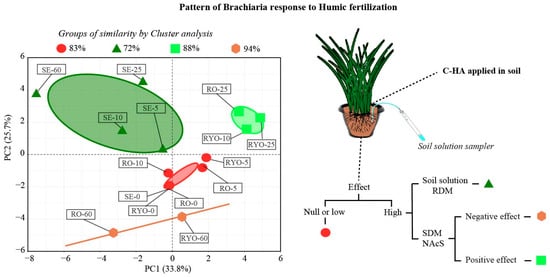
Figure 8.
Cluster analysis and hypothetical model and diagram for the humic acid effects on Brachiaria root and shoot growth. SE: Sandy Entisol; RYO: Red-Yellow Oxisol; RO: Red Oxisol; −0, −5, −10, −25 and −60 represent the concentrations of carbon as humic acid as follows: 0, 5, 10, 25 and 60 mg kg−1 carbon-humic acid. Soil solution: effect of humic acid on soil solution properties; RDM: root dry matter production; SDM: shoot dry matter production; NAcS: nutrient accumulation in shoot.
Despite the promising results elucidating the main mechanisms involved in the application of humic acid to the soil, complementary studies should be developed to evaluate these effects under field conditions, with different crops and different management practices. Under field conditions, due to environmental stresses compared to greenhouse conditions, the effects of humic acid application may be greater because of the role of humic acid in stress mitigation. Furthermore, in field conditions, the optimal concentrations may differ from those found in this study due to differences in the volume of soil explored and the soil–plant–microorganism interaction.
5. Conclusions
Chemical properties of soil solution and Brachiaria traits in response to humic acid concentrations were influenced differently depending on the soil type. The availability of most nutrients and the pH of solutions in the sandy soil (Sandy Entisol) and medium-texture Oxisol (Red-Yellow Oxisol) were notably affected by humic acid concentrations. At approximately 35 mg kg−1 carbon-humic acid, humic acid proved to be an effective input for enhancing Fe availability in the solution of the clayey Oxisol. The impact of humic acid addition on Brachiaria was more pronounced for root growth than shoot growth. Brachiaria exhibited greater growth in sandy soil compared with medium-texture and clayey Oxisols, regardless of humic acid concentration. In less-buffered soils such as Sandy Entisol and Red-Yellow Oxisol, the addition of 30–40 mg kg−1 carbon-humic acid resulted in a substantial increase in root growth by 76–89%, while the overall biomass production of Brachiaria increased by approximately 30% across all soil types. Carbon concentrations in the soil solution increased in response to humic acid concentrations, although this increase was also influenced by the enhanced decomposition of native soil organic matter. However, the positive effects of humic acid concentrations on Brachiaria growth were not solely driven by carbon concentrations in the soil solution. Therefore, establishing guidelines for the effective use of humic acid to enhance Brachiaria growth in highly fertilized Brazilian weathered soils will require determining not only the optimal humic acid concentration but also considering the stratification of soil types concerning their responses to native organic matter decomposition and humic acid concentration used to treat plants.
Author Contributions
Conceptualization, M.N.V. and C.A.S.; methodology, M.N.V., S.D.R. and C.A.S.; software, M.N.V. and E.G.d.M.; validation, M.N.V., E.G.d.M., S.D.R. and C.A.S.; formal analysis, E.G.d.M., S.D.R. and C.A.S.; investigation, M.N.V., E.G.d.M., S.D.R. and C.A.S.; resources, C.A.S.; data curation, M.N.V., E.G.d.M. and S.D.R.; writing—original draft preparation, M.N.V., E.G.d.M., S.D.R. and C.A.S.; writing—review and editing, M.N.V., E.G.d.M., S.D.R. and C.A.S.; visualization, M.N.V. and E.G.d.M.; supervision, E.G.d.M., S.D.R. and C.A.S.; project administration, C.A.S.; funding acquisition, C.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) (Grant Code-001) and the National Council for Scientific and Technological Development (CNPq), grants # 151485/2022-4, 7, 311212/2023-9, and 307447/2019-7.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council for Scientific and Technological Development (CNPq), and the Minas Gerais State Research Foundation (FAPEMIG) for financial support and scholarships provided to the authors. Special thanks to CNPq for the fellowship for the first author (E.G.d.M.) (grants #151485/2022-4 and #153474/2024-6).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study’s design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- ABIEC Beef Report: Perfil da Pecuária No Brasil. Available online: http://abiec.com.br/publicacoes/beef-report-2021/ (accessed on 10 April 2024).
- Jank, L.; Barrios, S.C.; do Valle, C.B.; Simeão, R.M.; Alves, G.F. The Value of Improved Pastures to Brazilian Beef Production. Crop Pasture Sci. 2014, 65, 1132. [Google Scholar] [CrossRef]
- Pereira, L.E.T.; Herling, V.R.; Tech, A.R.B. Current Scenario and Perspectives for Nitrogen Fertilization Strategies on Tropical Perennial Grass Pastures: A Review. Agronomy 2022, 12, 2079. [Google Scholar] [CrossRef]
- Pinheiro, P.L.; Passos, R.R.; Peçanha, A.L.; Canellas, L.P.; Olivares, F.L.; de Sá Mendonça, E. Promoting the Growth of Brachiaria Decumbens by Humic Acids (HAs). Aust. J. Crop Sci. 2018, 12, 1114–1121. [Google Scholar] [CrossRef]
- Verlinden, G.; Coussens, T.; De Vliegher, A.; Baert, G.; Haesaert, G. Effect of Humic Substances on Nutrient Uptake by Herbage and on Production and Nutritive Value of Herbage from Sown Grass Pastures. Grass Forage Sci. 2010, 65, 133–144. [Google Scholar] [CrossRef]
- Daur, I. Feed value of blue panic (Panicum antidotale Retz.) grass at different growth stages and under varying levels of humic acid in saline conditions. Turk. J. Field Crops 2016, 21, 210. [Google Scholar] [CrossRef]
- Neves, R.G.; Freitas, G.S.; Deminicis, B.B.; de Sá Mendonça, E.; Peçanha, A.L.; Dobbss, L.B.; Chambela Neto, A.; da Sá Deminicis, R.G. Dry Matter Yield, Growth Index, Chemical Composition and Digestibility of Marandu Grass under Nitrogen and Organic Fertilization. Semin. Ciências Agrárias 2019, 40, 1901. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical Framework Integrating the Main Mechanisms Involved in the Promoting Action of Rhizospheric Humic Substances on Plant Root- and Shoot- Growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S.; et al. Humic Substances: Determining Potential Molecular Regulatory Processes in Plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- de Morais, E.G.; Silva, C.A.; Maluf, H.J.G.M. Soaking of Seedlings Roots in Humic Acid as an Effective Practice to Improve Eucalyptus Nutrition and Growth. Commun. Soil Sci. Plant Anal. 2021, 52, 1399–1415. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic Substances Biological Activity at the Plant-Soil Interface. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant Biostimulants: Physiological Responses Induced by Protein Hydrolyzed-Based Products and Humic Substances in Plant Metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Tavares, O.C.H.; Santos, L.A.; Ferreira, L.M.; Sperandio, M.V.L.; da Rocha, J.G.; García, A.C.; Dobbss, L.B.; Berbara, R.L.L.; de Souza, S.R.; Fernandes, M.S. Humic Acid Differentially Improves Nitrate Kinetics under Low- and High-Affinity Systems and Alters the Expression of Plasma Membrane H+-ATPases and Nitrate Transporters in Rice. Ann. Appl. Biol. 2017, 170, 89–103. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A Meta-Analysis and Review of Plant-Growth Response to Humic Substances. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 37–89. [Google Scholar]
- Lyons, G.; Genc, Y. Commercial Humates in Agriculture: Real Substance or Smoke and Mirrors? Agronomy 2016, 6, 50. [Google Scholar] [CrossRef]
- Maluf, H.J.G.M.; Silva, C.A.; de Morais, E.G.; Paula, L.H.D. de Is Composting a Route to Solubilize Low-Grade Phosphate Rocks and Improve MAP-Based Composts? Rev. Bras. Ciência Solo 2018, 42, e0170079. [Google Scholar] [CrossRef]
- Rosa, S.D.; Silva, C.A.; Maluf, H.J.G.M. Phosphorus Availability and Soybean Growth in Contrasting Oxisols in Response to Humic Acid Concentrations Combined with Phosphate Sources. Arch. Agron. Soil Sci. 2020, 66, 220–235. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic Substances: Structure, Function and Benefits for Agroecosystems—A Review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the Role of Humic Acids on Crop Performance and Soil Health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Nascimento, C.A.C.; Bertolino, K.M.; Picoli, L.B. Humic Acid Enhances Phosphorus Transport in Soil and Uptake by Maize. J. Plant Nutr. Soil Sci. 2024, 187, 401–414. [Google Scholar] [CrossRef]
- Zavaschi, E.; de Abreu Faria, L.; Ferraz-Almeida, R.; do Nascimento, C.A.C.; Pavinato, P.S.; Otto, R.; Vitti, A.C.; Vitti, G.C. Dynamic of P Flux in Tropical Acid Soils Fertilized with Humic Acid–Complexed Phosphate. J. Soil Sci. Plant Nutr. 2020, 20, 1937–1948. [Google Scholar] [CrossRef]
- Gerke, J. Review Article: The Effect of Humic Substances on Phosphate and Iron Acquisition by Higher Plants: Qualitative and Quantitative Aspects. J. Plant Nutr. Soil Sci. 2021, 184, 329–338. [Google Scholar] [CrossRef]
- Macdonald, L.M.; Farrell, M.; Van Zwieten, L.; Krull, E.S. Plant Growth Responses to Biochar Addition: An Australian Soils Perspective. Biol. Fertil. Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Do Carmo, D.L.; Silva, C.A.; de Lima, J.M.; Pinheiro, G.L. Electrical Conductivity and Chemical Composition of Soil Solution: Comparison of Solution Samplers in Tropical Soils. Rev. Bras. Ciência Solo 2016, 40, e0140795. [Google Scholar] [CrossRef]
- De Morais, E.G.; Silva, C.A. Novel Slow-Release NPK Biochar-Based Fertilizers with Acidulated Apatite: Evaluation of the Fertilization Value in a Short-Term Experiment. J. Soil Sci. Plant Nutr. 2023, 23, 4937–4954. [Google Scholar] [CrossRef]
- Maluf, H.J.G.M.; Silva, C.A.; Curi, N.; Norton, L.D.; Rosa, S.D. Adsorption and Availability of Phosphorus in Response to Humic Acid Rates in Soils Limed with CaCO3 or MgCO3. Ciência Agrotecnologia 2018, 42, 7–20. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–174. [Google Scholar]
- Chotzen, R.A.; Polubesova, T.; Chefetz, B.; Mishael, Y.G. Adsorption of Soil-Derived Humic Acid by Seven Clay Minerals: A Systematic Study. Clays Clay Miner. 2016, 64, 628–638. [Google Scholar] [CrossRef]
- De Morais, E.G.; Silva, C.A.; Rosa, S.D. Nutrient Acquisition and Eucalyptus Growth Affected by Humic Acid Sources and Concentrations. Semin. Ciências Agrárias 2018, 39, 1417. [Google Scholar] [CrossRef]
- Berbara, R.L.L.; García, A.C. Humic Substances and Plant Defense Metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; pp. 297–319. [Google Scholar]
- Canellas, L.P.; Olivares, F.L. Production of Border Cells and Colonization of Maize Root Tips by Herbaspirillum Seropedicae Are Modulated by Humic Acid. Plant Soil 2017, 417, 403–413. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Körpern. Fresenius Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Silva, S.R.; de Barros, N.F.; de Novais, R.F.; Comerford, N.B. Eucalyptus Growth and Phosphorus Nutritional Efficiency as Affected by Soil Compaction and Phosphorus Fertilization. Commun. Soil Sci. Plant Anal. 2018, 49, 2700–2714. [Google Scholar] [CrossRef]
- Kalra, Y. Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient Use Efficiency in Plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes.Pt: Pacote Experimental Designs (Portugues); R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Suzuki, R.; Terada, Y.; Shimodaira, H. Pvclust: Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of Labile Carbon Fractions to Tillage and Organic Matter Management and Their Potential as Comprehensive Soil Quality Indicators across Pedoclimatic Conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes That Influence Dissolved Organic Matter in the Soil: A Review. Sci. Agric. 2020, 77, e20180164. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Tahhan, R.; Khresat, S. Quality of Fresh Plant Residue Affects Sequestration of Residue Derived Organic Material by Humic Acid. Eurasian J. Soil Sci. 2020, 9, 222–230. [Google Scholar] [CrossRef]
- Buragohain, P.; Sreedeep, S.; Lin, P.; Ni, J.; Garg, A. Influence of Soil Variability on Single and Competitive Interaction of Ammonium and Potassium: Experimental Study on Seven Different Soils. J. Soils Sediments 2019, 19, 186–197. [Google Scholar] [CrossRef]
- Basso, C.J.; Ceretta, C.A.; Durigon, R.; Poletto, N.; Girotto, E. Dejeto Líquido de Suínos: II—Perdas de Nitrogênio e Fósforo Por Percolação No Solo Sob Plantio Direto. Ciência Rural. 2005, 35, 1305–1312. [Google Scholar] [CrossRef]
- Urrutia, O.; Erro, J.; Guardado, I.; San Francisco, S.; Mandado, M.; Baigorri, R.; Claude Yvin, J.; Ma Garcia-Mina, J. Physico-chemical Characterization of Humic-metal-phosphate Complexes and Their Potential Application to the Manufacture of New Types of Phosphate-based Fertilizers. J. Plant Nutr. Soil Sci. 2014, 177, 128–136. [Google Scholar] [CrossRef]
- Jindo, K.; Soares, T.S.; Peres, L.E.P.; Azevedo, I.G.; Aguiar, N.O.; Mazzei, P.; Spaccini, R.; Piccolo, A.; Olivares, F.L.; Canellas, L.P. Phosphorus Speciation and High-affinity Transporters Are Influenced by Humic Substances. J. Plant Nutr. Soil Sci. 2016, 179, 206–214. [Google Scholar] [CrossRef]
- Yang, F.; Sui, L.; Tang, C.; Li, J.; Cheng, K.; Xue, Q. Sustainable Advances on Phosphorus Utilization in Soil via Addition of Biochar and Humic Substances. Sci. Total Environ. 2021, 768, 145106. [Google Scholar] [CrossRef] [PubMed]
- López-Velázquez, J.G.; López-López, M.E.; Rubio-Trías, A.; Ayón-Reyna, L.E.; Díaz-Corona, D.A.; Olivas Orozco, G.I.; Molina-Corral, J.; Vega-García, M.O. Cell Wall Stabilization and Calcium Absorption on Mango Fruit Treated with a Quarantine Hot Water Treatment Combined with Calcium Salts and Stored at Chilling Temperature. J. Food Biochem. 2022, 46, e14266. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–281. [Google Scholar]
- Rengel, Z.; Cakmak, I.; White, P.J. Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]
- Soares Filho, C.V.; Cecato, U.; Ribeiro, O.L.; Roma, C.F.D.C.; Jobim, C.C.; Beloni, T.; Perri, S.H.V. Root System and Root and Stem Base Organic Reserves of Pasture Tanzania Grass Fertilizer with Nitrogen under Grazing. Semin. Ciências Agrárias 2013, 34, 2415. [Google Scholar] [CrossRef]
- Ekinci, M.; Esringü, A.; Dursun, A.; Yildirim, E.; Turan, M.; Karaman, M.R.; Arjumend, T. Growth, Yield, and Calcium and Boron Uptake of Tomato (Lycopersicon esculentum L.) and Cucumber (Cucumis sativus L.) Asaffected by Calcium and Boron Humate Application in Greenhouse Conditions. Turk. J. Agric. For. 2015, 39, 613–632. [Google Scholar] [CrossRef]
- Kalbitz, K.; Kaiser, K. Contribution of Dissolved Organic Matter to Carbon Storage in Forest Mineral Soils. J. Plant Nutr. Soil Sci. 2008, 171, 52–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).