Abstract
A methodology has been developed to assess the presence and dissipation of herbicides of a wide range of polarities in soil using in-tube solid-phase microextraction (IT-SPME) coupled online to capillary liquid chromatography (capLC). The compounds investigated were tritosulfuron (TRT), triflusulfuron-methyl (TRF), aclonifen (ACL), and bifenox (BF), with log octanol-water partition coefficients (log Kow) ranging from 0.62 to 4.48. The method provided suitable linearity at concentration levels of 0.5–4.0 µg/g for TRT and TRF, and 0.2–1.0 µg/g for ACL and BF, and intra- and interday precision (expressed as relative standard deviation) ≤4% and ≤8%, respectively. The mean recoveries ranged from 90% to 101%, and the limits of detection (LODs) and quantification (LOQs) were in the intervals of 0.05–0.1 µg/g and 0.1–0.4 µg/g, respectively. The accuracy of the method was also satisfactory. The proposed approach was successfully applied to assess the degradation of the tested herbicides in different types of soil (agricultural, urban and forest) after being exposed to different laboratory and outdoor conditions. The results obtained showed a greater persistence of the most apolar compounds ACL and BF, with percentages of degraded herbicide ≤31% regardless of the soil characteristics. In contrast, a significant degradation of highly polar herbicides TRT and TRF was observed in soils with the lowest organic matter, even after a few days of exposure. For example, the percentages of remaining TRT and TRF in this kind of soil after 20 days were ≤65%; the half-life time of TRF was only 24.8 days. These results indicate that the proposed approach can be considered as an effective tool for a better understanding of soil pollution.
1. Introduction
The use of herbicides has expanded globally over the years to meet the demands of a growing population. However, after being applied, only a portion of these substances reaches their targets while the rest is mainly deposited on the soil surface and undergoes different (bio)degradation and transport processes [1,2]. The mechanisms involved in the transformation of herbicides depend on several factors such as their physicochemical properties, soil characteristics, and climatological conditions, and in many cases, they are not completely known [3]. Therefore, monitoring the presence of herbicides in soil is considered crucial, not only to ensure environmental quality, but also to gain knowledge about their persistence, dissipation mechanisms, and final fate [4,5].
Compared to other compartments such as water and air, the analysis of herbicides in soils is a more challenging task not only because of the higher complexity of the matrix, but also because of its inhomogeneity. As a result, the distribution of organic pollutants in soils can be very nonhomogeneous, making necessary the analysis of a great number of samples to obtain the required information [6]. Thus, the development of simple and cost-effective analytical methods that can be applied to assess the presence of herbicides in soil with the proper levels of sensitivity and accuracy is of great interest.
In the past decades, several sample treatments have been proposed for the analysis of organic pollutants such as herbicides in soil samples prior to their analysis by liquid chromatography (LC), which is considered the technique of reference. An extraction with an organic solvent is typically used to isolate the targets from the sample [6,7]. However, due to the complexity of the matrix and the low concentration levels of the herbicides, a further treatment of the extracts is needed before LC separation, with a solvent evaporation followed by a redissolution and/or a sorbent-mediated re-extraction being the most commonly used options [8].
The miniaturization of extraction methods has made a substantial contribution to the development of more sustainable analytical processes, in line with current trends in analytical chemistry [9]. In this context, numerous alternatives have been proposed to process the first extract obtained from the soils, with a predominance of solid phase microextraction (SPME) using fibers [6]. Another option is in tube-SPME (IT-SPME), in which the analytes are preconcentrated in an extractive capillary coated with a (hydrophobic) polymeric sorbent; the capillary is typically installed in the injection valve of a liquid chromatograph, in replacement of the injection loop. The IT-SPME approach has been mainly used for the selective extraction and preconcentration of herbicides and other organic pollutants from water samples because the distribution of the targets between the aqueous sample and the extractive phase is favorable to the extraction. However, the extraction efficiency decreases as the polarity of the solvent of the working solution decreases. Since an organic solvent is typically used to isolate herbicides from the soil samples, the extracts obtained may be unsuitable for IT-SPME. For this reason, most IT-SPME-based procedures described thus far involve the evaporation of the extracts obtained from the soil samples, followed by the redissolution of the analytes in an aqueous solvent and subsequent injection into the extractive capillary, albeit at the expense of the analysis time [10,11].
An additional challenge commonly faced in sorbent-based extraction methods proposed for herbicides is the limited efficiency achievable for highly polar analytes. In the past decades, there has been a gradual replacement of highly apolar herbicides by more polar compounds in order to reduce bioaccumulation [12]. For these reasons, there is a clear need for sample treatment strategies that can be applied to simultaneously analyze herbicides with different polarities [13]. In this regard, a great deal of attention has been devoted to the development of new sorbents for both SPME with fibers [14] and IT-SPME devices [9,15,16,17,18]. For example, the use of capillaries coated with different types of nanoparticles has been proposed for the simultaneous analysis of herbicides of a wide range of polarities in water samples [19,20]. However, the number of studies that have focused on soil samples is still very limited [21].
Taking a different approach, we hypothesized that coupling IT-SPME online to capLC would allow us to analyze herbicides with different polarities directly in the extracts obtained from the soil samples by adjusting the solvent composition of the solutions introduced into the IT-SPME device. Therefore, in the present work, our goal was to develop an analytical tool for the analysis of herbicides in soil that met the following requirements: (i) miniaturized, in order to reduce the consumption of resources and the time of analysis, (ii) applicable to the analysis of herbicides of different hydrophilicities using a simplified sample treatment (single extraction without intermediate evaporation operations), and (iii) sensitive enough to detect changes in the concentrations of the target herbicides during their degradation. Four herbicides with log Kow ranging from 0.62 to 4.48 were selected as model compounds, namely TRT, TRF, ACL, and BF (see the Supplementary Materials, Table S1). Conditions for the extraction of the herbicides from the soil samples and subsequent analysis of the extracts by IT-SPME were optimized, and the analytical capacities of the proposed method established. The method was applied to study the persistence of the tested herbicides in soil by analyzing the spiked soil over time. Because of the low volatility of the tested herbicides (see Table S1), it was assumed that loss of the analytes along the study due to their volatilization at ambient temperature were negligible.
In a recent study, we used IT-SPME coupled to capLC to investigate the degradation of ACL and BF in different environmental waters [22]. In the present work, we investigated the degradation of the four herbicides in different soils after being exposed to different laboratory and outdoor conditions. Therefore, the main novelty of the present study is that the IT-SPME–capLC approach has been extended to the determination of a wider variety of analytes and to more complex matrices. The results obtained along the study showed that the proposed approach can be considered as a simple and effective tool for assessing the dissipation of the tested compounds in soil samples.
2. Materials and Methods
2.1. Chemicals and Solutions
All reagents used during the study were of analytical grade. BF was purchased from Chemservice (West Chester, PA, USA); ACL, TRT, and TRF were acquired from Sigma-Aldrich (Steinheim, Germany). Methanol, ethanol, and acetonitrile, all of them HPLC grade, were purchased from VWR Chemicals (Randnor, PA, USA). Ortho-phosphoric acid was obtained from Panreac (Barcelona, Spain).
Stock solutions of the analytes were prepared by dissolving the appropriate amounts of the commercial standards in methanol and kept at −20 °C until use. Working solutions of the analytes and their mixtures were prepared by diluting the stock solutions with nanopure water. Nanopure water was obtained from an Adrona system (Riga, Latvia).
2.2. Apparatus and Chromatographic Conditions
For the chromatographic assays, a capLC instrument consisting of a capillary pump (Agilent 1100 Series, Waldbronn, Germany), a Rheodyne model 7725 six-port injection valve, and a diode array detector (DAD) equipped with an 80 nL flow cell (Agilent 1200 Series) was used. Agilent ChemStation software (3D, Rev. B.04.0316) was used for data acquisition and processing. The chromatograms were recorded between 190 and 400 nm and monitored at 225 nm for TRT and TRF, 320 nm for ACL, and 205 nm for BF. For the separation, a Zorbax SB C18 (150 mm × 0.5 mm id, 5 µm) column (Agilent) was employed. The mobile phase was a mixture of solvent A (0.1% H3PO4 and 0.2% acetonitrile in nanopure water) and solvent B (100% acetonitrile) in gradient elution mode. The percentage of solvent B was increased from 40% at 0 min to 60% at 9.0 min, to 75% at 14.0 min, and to 100% at 15.0 min; then, the composition was kept constant until the end of the run (20.0 min). The mobile phase flow rate was 9 µL/min. All the solvents were filtered through 0.22 µm nylon membranes purchased from GVS (Sandford, ME, USA) before use.
2.3. Soil Samples
Different soils sampled from the top 0–20 cm layer were used throughout the study: an agricultural soil (S1), two soils collected from two urban areas (S2 and S3), and forest soil (S4). Samples S1 and S4 were Calcaric Cambisol soils, whereas samples S2 and S3 were Fluvisol soils, according to the WRB-FAO classification [23]. All samples were collected at different points of the Comunitat Valenciana region (East Spain). After removing possible stones and roots, the soils were air-dried and sieved through 2.0 mm mesh (unless otherwise stated). Samples were stored in amber glass containers at room temperature until analysis. The soils were tested for storage moisture content, pH, and organic matter content (more detailed information about the procedures applied is given as supplementary content in Table S2).
2.4. Treatment of the Soil Samples
Portions of 0.5 g of the dried and sieved soils were processed directly or spiked with the analytes and then processed. The spiked samples were prepared by adding to the soil portions 250 µL of standard solutions containing the appropriate concentration of the analytes prepared in methanol; next, the mixtures were homogenized and left to air-dry.
The soil portions (untreated or spiked with the analytes) were mixed with the extraction solvent and then vortexed for 10 min. In studies aimed at optimizing the sample treatment, the concentrations of the analytes in the spiked soils were 2 µg/g for TRT and TRF, and 0.5 µg/g for ACL and BF. Different solvents were tested for the extraction: water, methanol, and ethanol as well as mixtures of water–methanol and water–ethanol (1:1, v/v). The soil samples were treated with 5 mL of the extraction solvent. After vortexing and decantation, the liquid phase was removed and filtered using syringe filters (15 mm diameter, 0.2 µm pore size). Aliquots of 0.5 mL of methanol were then passed through the filters to quantitatively desorb the most apolar analytes. The filtrates were combined and then mixed with water. The resulting mixtures were further processed by IT-SPME. Nylon, regenerated cellulose, polyethersulfone (PES), and polytetra-fluorethylene (PTFE) filters, all of them purchased from Agilent, were assayed.
2.5. Conditions for the IT-SPME
The capillary used for IT-SPME was a 30 cm segment of a TRB-35 (35% diphenyl-65% dimethyl polysilioxane) column of 0.32 mm i.d. and 3 µm coating thickness (Teknokroma, Barcelona, Spain). The capillary was installed in replacement of the loop of the injection valve. To connect the extractive capillaries to the valve, a 2.5 cm sleeve of 1/6 i.n. polyether ether ketone (PEEK) tubing and 1/6 i.n. PEEK nuts and ferrules (Teknokroma) were used.
The solutions obtained after the soil treatment (filtered and mixed with water) were processed by IT-SPME–capLC. To this aim, the solutions were manually loaded into the capillary using a 2 mL syringe (Labbox, Barcelona, Spain). Next, the position of the valve was changed, so the extractive capillary was inserted into the chromatographic flow scheme. In this way, the analytes were desorbed and transferred to the analytical column with the mobile phase for the subsequent separation and detection.
2.6. Method Validation
The linearity of the proposed analytical methodology was tested by processing soil samples (0.5 g) spiked with the herbicides at five concentration levels in the range 0.5–4.0 µg/g for TRT and TRF, and 0.2–1.0 for ACL and BF. These concentrations were selected to produce peak areas of about the same order for the four herbicides at their respective optimum wavelength (see Section 2.2). The peak areas at such wavelengths were used as analytical signals and plotted against the concentration of analytes in the samples.
The intraday precision, expressed as the relative standard deviation (RSD), was tested through the consecutive analysis of three replicates of soil (0.5 g) spiked with the analytes, whereas the interday precision (RSD) was obtained from the peak areas measured in five working sessions. In these studies, the concentrations of the analytes were 2 µg/g for TRT and TRF, and 0.5 µg/g for ACL and BF. The limits of detection (LODs) and quantification (LOQs) were established as the concentrations of analyte that resulted in signal-to-noise ratios at the corresponding detection wavelength of 3 and 10, respectively.
The recoveries were examined in the four soils tested by analyzing the soil portions spiked with the analytes. In studies aimed at testing the effect of the concentration on the recoveries, soil S4 was used; three concentration levels within the linear interval were assayed: low (0.5 µg/g for TRT and TRF and 0.2 µg/g for ACL and BF), medium (2.0 µg/g for TRT and TRF and 0.5 µg/g for ACL and BF), and high (4.0 µg/g for TRT and TRF and 1.0 µg/g for ACL and BF). The recoveries were calculated by comparing the peak areas measured for the spiked samples with those obtained for standard solutions containing an equivalent concentration of the analytes and processed directly by IT-SPME–capLC.
To study the accuracy, soil portions were spiked with the herbicides at the same three concentration levels used in the recovery studies. Then, the samples were analyzed by the proposed procedure, and the peak areas measured for the analytes were transformed into concentrations in soil using the calibration equations previously obtained. The accuracy was evaluated from the differences between the added and the measured concentrations.
2.7. Degradation Experiments
Assays on the degradation of the pesticides in the soil samples were conducted between October and December 2023. The time elapsed between sampling and the beginning of the degradation experiments was 2–4 days. Accurately weighed portions of the samples (0.5 g) were introduced in 5 mL glass vial and spiked with the analytes at concentration levels of 4 µg/g for TRT and TRF and 1 µg/g for ACL and BF, as described in Section 2.4. The vials were kept in the laboratory at ambient temperature under two conditions, in the dark or exposed to dark and natural sunlight cycles. Additionally, the soil portions were kept outdoors, exposed to dark and sunlight. During the study, the temperature of the laboratory ranged from 20 °C to 25 °C, while the outdoor temperature ranged from 8 °C to 30 °C. A set of vials with 0.5 g portions of soil spiked with the herbicides was prepared and kept together under each of the tested conditions. The dissipation of the herbicides was assessed by analyzing the soil portions at different times within the 0–40 day interval. During this study, continuous quality control tests were conducted to verify that the responses obtained for the soil portions freshly spiked with known amounts of the analytes were comparable to those measured during the method validation (differences < 20%). Control experiments were also performed with unspiked soil samples to confirm the absence of memory effects.
3. Results
3.1. Extraction of the Herbicides from Soil Samples
The preliminary solvent extraction of the herbicides from the soil samples was optimized. The sample of the mass and volume of the extraction solvent were selected according to the goal of our study (development of a miniaturized method) and sensitivity provided by the IT-SPME–capLC system [22]. Under the selected chromatographic conditions, the retention times were 13.4 min, 13.9 min, 16.2, and 17.7 min for TRT, TRF, ACL, and BF, respectively. The peak areas used for the calculations were those measured at the optimum wavelength for each analyte (see Section 2.2). At the selected wavelengths, the chromatographic profiles were suitable, and no difficulties were found in establishing the baseline for the measurement of the peak areas.
At the sensitivity levels provided by the IT-SPME–capLC approach, background peaks produced by the materials and reagents used along the analysis are often detected in the chromatograms. In particular, peaks arising from the filters used for processing the extracts represent a difficulty in the quantification of some of the analytes [22]. For this reason, a preliminary study was carried out to select the proper filters. The materials tested were nylon, regenerated cellulose, PES, and PTFE. Aliquots of 1 mL of an aqueous solution containing 200 ng/mL of TRT and TRF and 50 ng/mL mL of ACL and BF were filtered; then, the filtered solution was loaded into the IT-SPME capillary and processed. The results were compared with those obtained by directly processing 1 mL of the same solution into the IT-SPME device. As expected, the chromatograms obtained for the filtered solutions showed different additional peaks related to the materials of the filters, which were also detected in blanks. To eliminate such unwanted peaks, the filters were prewashed with 3 mL of methanol.
An additional problem was the significant decrease in the responses of the analytes in the filtered solutions. In fact, no peaks were detected with the nylon filters, while for the other filters, a drastic reduction in the peak areas of the most apolar analytes ACL and BF was observed (see Figure S1a). The best results were obtained with the cellulose filters, which were then selected for further work. To completely desorb the analytes retained on the cellulose filters, methanol was passed through the filters after passing the sample solution, and the two filtrates were collected together. It was observed that passing 0.5 mL of methanol through the filters was sufficient to completely desorb the analytes. Under such conditions, the recoveries obtained for all analytes were satisfactory (97–102%) (see Figure S1b).
Next, different solvents were tested for the extraction of the herbicides from the spiked soil samples, as described in Section 2.4. Sample S1 was used in this part of the study. The liquid phase was separated, and a portion of 1.0 mL was passed through a prewashed filter, followed by 0.5 mL of methanol. Finally, 1 mL of the collected mixture was loaded into the IT-SPME device and processed. The percentages of analytes recovered were calculated by comparing the peak areas with those obtained for solutions containing equivalent concentrations of the analytes in the same solvent and processed directly into the IT-SPME–capLC system.
As observed in Figure 1, when using water as the solvent for extraction, high recoveries were obtained for the polar analytes TRT and TRF, but for the most hydrophobic analytes, the recoveries were unsuitable. Conversely, the highest recoveries were found for the most apolar compounds with the alcohols. Nearly quantitative recoveries were obtained for all analytes with 1:1 water–alcohol mixtures (v/v). However, the peak profile observed for TRT was significantly worse when using ethanol. Consequently, 1:1 water–methanol (v/v) was selected as the solvent for the treatment of the soil samples. It has to be noted that even when using 1:1 water–methanol for the soil treatment, an additional portion of methanol was found to be necessary for the complete desorption of the most apolar analytes from the filters. The option finally selected was successive passage through the filters of 1 mL of the 1:1 water–methanol extract, followed by 0.5 mL of methanol.
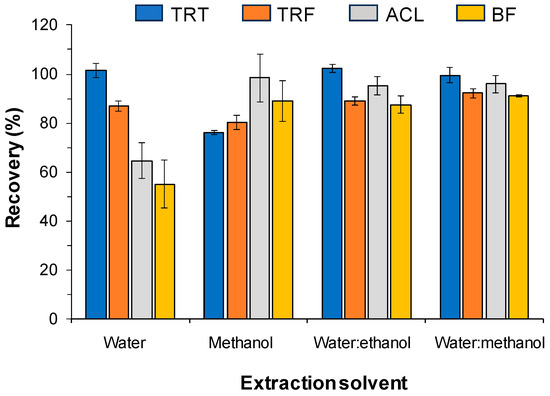
Figure 1.
Mean recoveries obtained for the spiked soil (sample S1) with different extraction solvent. Concentrations in soil: TRT and TRF, 2 µ/g; ACL and BF, 0.5 µg/g. For other experimental conditions, see the main text.
3.2. IT-SPME Conditions
As stated earlier, the efficiency of the IT-SPME approach critically depends not only on the hydrophobicity of the analytes, but also on the solvent of the working solution. As the extracts obtained from soil contained methanol, poor extraction efficiencies were found when they were directly loaded onto the extractive capillary of the IT-SPME device. To overcome this problem, the percentage of organic solvent was reduced by adding water to the extracts. The volume of water was optimized to achieve a favorable extraction of all the analytes, despite their wide range of hydrophobicities, with the minimum dilution of the analytes.
First, solutions of the same concentration of the analytes prepared in different water–methanol mixtures were tested, and the responses (peak areas) were compared. Constant responses were observed for methanol contents in the 10–33.3% range; a higher percentage of methanol resulted in a decrement in the responses (see Figure S2a). Thus, for a minimum dilution of the extracts, a final methanol percentage of 33.3% was chosen. This percentage was achieved by simply adding 1.5 mL of water to the extract collected after the filtration stage.
The volume of extract (containing 33.3% of methanol) processed into the IT-SPME was also optimized (Figure S2b). Increasing the volume of working solutions resulted in an increase in the peak areas of the most apolar herbicides ACL and BF. However, a slight decrement in the responses was observed for TRT and TRF when increasing the volume of the sample. As a compromise, a volume of 1 mL was chosen.
According to the above results, the optimized procedure can be summarized in three steps, as depicted in Figure 2: extraction of the herbicides from soil, filtration of the extract, and solvent adjustment for IT-SPME–capLC. The specific conditions used in each step are also shown in this figure. It should be noted that the main novelty of the proposed methodology is related to the third step of the process.
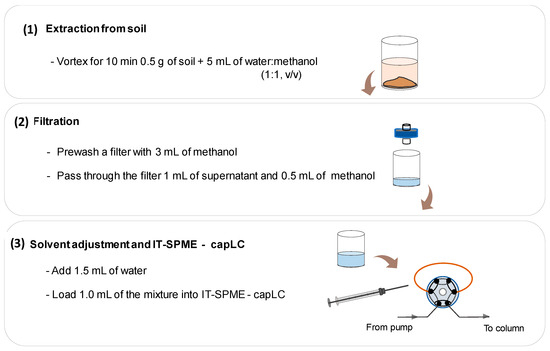
Figure 2.
Schematic diagram and conditions of the analysis process. For other experimental conditions, see the main text.
3.3. Analytical Performance
The analytical performance of the proposed approach was evaluated. To this aim, the recoveries, linearity, LODs and LOQs, precision, and accuracy were tested (see Section 2.6). Portions of 0.5 g of soil were spiked with the analytes as described in Section 2.4, and then subjected to the optimized extraction/IT-SPME–capLC procedure. Four different soils were tested: agricultural soil (S1), urban soil (S2 and S3), and forest soil (S4). The soils were previously tested for the storage moisture content, pH, and organic matter content (see Table 1). None of the target analytes were found in the samples. Therefore, further assays were carried out with the spiked soil.

Table 1.
Properties of the tested soils (mean values, n = 3).
The recoveries of the analytes in the spiked samples were calculated by comparing the peak areas obtained by analyzing the extracts collected after applying the analytical process (Figure 2) with those obtained for standard solutions containing the same concentration of the herbicides, which were also prepared in water containing 33.3% of methanol (v/v). The results obtained are shown in Figure 3a. As shown in this figure, in most instances, nearly quantitative recoveries were found regardless of the hydrophobicity of the herbicide and the soil. For sample S2, the recoveries obtained for ACL and BF were slightly lower, which in light of the values in Table 1 can be attributed to the higher adsorption of these herbicides to the soil organic matter [8]. Reducing the particle size to 0.4 mm did not significantly affect the recoveries, as depicted in Figure 3b.
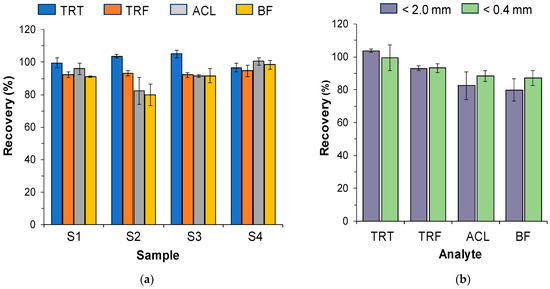
Figure 3.
Mean recoveries obtained for the spiked samples: (a) the four soils tested; (b) soils of <2.0 mm and <0.4 mm particle sizes for sample S1. Concentrations in soils: TRT and TRF, 2 µ/g; ACL and BF, 0.5 µg/g. For other experimental conditions, see the main text.
To evaluate the effect of the concentration on the recovery, portions of one of the soil samples (S4) were spiked at three concentration levels and then processed. The results obtained are shown in Table 2. As observed, for the four herbicides, the recoveries were comparable at the three concentration levels assayed. The mean recoveries of the analytes for the four tested soils are also summarized in Table 2. According to these results, it can be deduced that the recoveries were similar for all the soils tested.

Table 2.
Recovery percentages (%) obtained for the soil (S4) spiked at different concentration levels with the proposed method.
The results obtained in the linearity study as well as the LODs and LOQs are listed in Table 3.

Table 3.
Analytical performance of the proposed method.
The above table shows that the responses measured for the analytes (peak areas) were linear for concentrations ranging from 1.0 to 4.0 µg/g for TRT and TRF and from 0.2 to 1.0 µg/g for ACL and BF.
The precision was also satisfactory, with intraday and interday RSD coefficients ≤4% and ≤8%, respectively. It should be emphasized that, despite the low mass of soil used in the proposed method, the RSD values obtained for different replicates of a soil sample (different extracts obtained for different portions of the same soil) were comparable to the RSDs measured for three consecutive injections of the same extract (see Table S3). Hence, the mass of soil used can be considered adequate, provided that the raw sample is conveniently homogenized and sieved to ensure the representativeness.
The accuracy was investigated by analyzing soil spiked with different amounts of the analytes. As observed in Table 3, good concordance was found between the concentrations added and those found by applying the proposed method. The accuracy expressed in terms of recoveries ranged from 86% to 106%.
3.4. Study of the Dissipation of Herbicides in Soil
To study the degradation of the tested herbicides over time, portions of the soil samples were spiked with the analytes at concentration levels of 4 µg/g for TRT and TRF and 1 µg/g for ACL and BF, and kept under different conditions until analysis (see Section 2.4). Individual portions were taken at the selected times and analyzed.
The effect of the light was studied under laboratory conditions using portions of a spiked soil (S1) that had been kept in the dark or exposed to natural sunlight. The percentages of the herbicides that remained in the soil over time are shown in Figure 4. These values were calculated as the ratio between the peak area at a given time and the peak area at the beginning of the study.
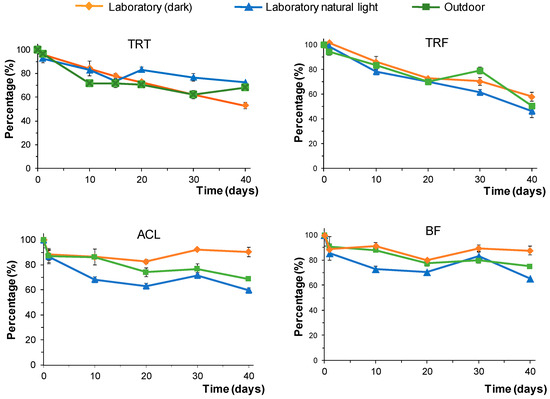
Figure 4.
Variation in the percentage of undegraded herbicides over time in soil (S1) under different conditions: samples kept in the laboratory in the dark, samples kept in the laboratory exposed to natural sunlight, and samples exposed to outdoor conditions. Concentrations in soils: TRT and TRF, 2 µ/g; ACL and BF, 0.5 µg/g.
For samples kept in the dark, the degradation of TRT and TRF was faster than the degradation of the other two compounds tested. The percentages of TRT and TRF that remained in the soil decreased over time almost linearly. The percentages of undegraded TRT after 40 days was 53%, whereas 58% of TRF remained in the soil after the same period. In contrast, small variations in the amounts of undegraded ACL and BF were found, with the percentages of undegraded herbicides higher than 80%. Exposing the samples to the sunlight had a limited impact on the percentages of undegraded TRT and TRF. However, for ACL and BF, the degradation was slightly faster in samples exposed to natural sunlight. For these compounds, small differences were also found when comparing the percentages of undegraded herbicides in samples exposed to sunlight under both laboratory and outdoor conditions.
The effect of the type of soil on the degradation was studied for samples exposed to outdoor conditions. Examples of the chromatograms obtained for one of the tested soils (S4) along the study are depicted in Figure 5. In all instances, the peaks of the analytes could be satisfactorily monitored, and only minor background peaks were detected. These minor peaks were also observed in the control (non-spiked) soils.
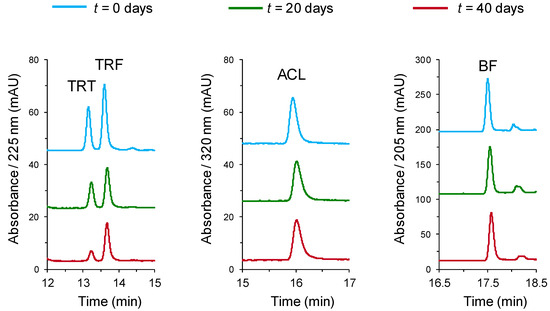
Figure 5.
Chromatograms obtained for soil S4 spiked with the analytes at their respective optimum detection wavelengths and at different exposure times. Initial concentrations in soil: TRT and TRF, 4 µ/g; ACL and BF, 1 µg/g. For other experimental conditions, see the main text.
The percentages of herbicide remaining in the soil samples over time were calculated as described above. The results obtained are depicted in Figure 6. As observed in this figure, the herbicides ACL and BF were relatively stable, regardless of the type of soil. The percentages of ACL were above 85% throughout the study, except in sample S1, where this compound dissipated faster, reaching an undegraded herbicide percentage of 69% at day 40. Similar results were found for BF, with a remaining percentage after 40 days of 75% in soil S1, whereas more than 90% of this compound was found in the rest of the soils.
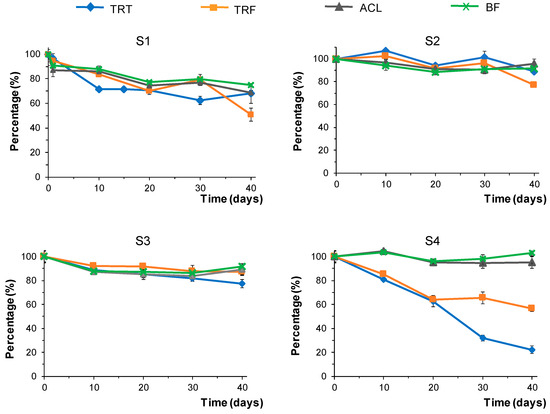
Figure 6.
Variation in the percentage of undegraded herbicides over time in the different soils tested in samples exposed to outdoor conditions.
In contrast, the percentages of TRT and TRF that remained in the sample were highly dependent on the type of soil. After 40 days, the percentage of undegraded TRT ranged from 90% to 22%, and values ranging from 89% to 51% were found for TRF. The results obtained for S2 and S3, which corresponded to the same type of soil (urban), were quite similar, whereas the highest variability was observed for S4. In this soil, ACL and BF remained approximately stable, whereas for TRT and TRF, a significant reduction in the percentage of undegraded herbicide was observed, even by day 10. Both compounds suffered similar degradation up to day 20. For longer periods, the dissipation of TRF was more moderate. However, a constant degradation rate was observed for TRT within the tested time interval, with y = 100.62 − 2.04x, y being the percentage of undegraded TRT and x being the time expressed in days (R2 = 0.98); the half-life time calculated from the above equation was 24.8 days.
4. Discussion
The widespread use of herbicides has prompted an increasing demand on analytical methods applicable to evaluate their persistence and long-term effects on ecosystems. In the present study, a method was specifically developed for the study of the dissipation of four herbicides of different polarities in soil samples. So far, only a few analytical methods have been reported for the study of the degradation of the tested herbicides in soil, and most of them involved two or more consecutive extractions and re-extractions, followed by solvent evaporation steps. As a result, up to several hours are required for the treatment of the sample before LC analysis. This can be seen in Table S4, which summarizes the most relevant features of methods previously proposed for the determination of the tested herbicides in soil. The proposed method only involves the extraction of the target compounds from the soil, followed by the direct processing of the extracts by IT-SPME coupled to capLC; the time required to prepare the sample is only ≈15 min. Thus, the main advantages of the proposed approach over the previously described methods are that the sample treatment is considerably simplified, and the consumption of solvents and other resources is substantially reduced due to its miniaturization. In addition, it can be applied to the simultaneous analysis of herbicides of different polarities. Moreover, the selectivity is excellent due to the combined effect of the solvent extraction and the IT-SPME. The proposed method is adequate to monitor the presence of the tested herbicides at low to sub µg/g levels, which are concentrations typically used in degradation studies. Therefore, the proposed method can be considered as a rapid and cost-effective tool for degradation studies.
The information available regarding the degradation in soils of the herbicides included in this study is still very limited [4,8,24,25,26,27]. ACL has been the most studied compound, although the reported results show a wide variability in terms of its persistence, with reported half-life times ranging from 13 to 990 days [4,8,24]. A half-life for TRF in soil of 32 days was also reported in [26]. According to a previous study, the stability of TRF in water increases in the presence of dissolved organic matter [27], but, to the best of our knowledge, no data have been reported for soil samples. No data have been found for the degradation in soil of the other herbicides tested. The application of the proposed method has produced relevant results in this regard. The results obtained demonstrate that the organic matter content has a stronger influence on the persistence of the tested herbicides than parameters such as natural sunlight exposure or the environment in which the samples are kept (laboratory, outdoor).
According to the literature, the stability of herbicides in soil is positively correlated to their ability to bind to soil organic matter [8]. In turn, the fraction of herbicide adsorbed to the organic matter is a function of both the affinity of the herbicide for the organic matter and the content of organic matter in the soil. It can be assumed that the low stability found for TRT and TFS, in comparison to the other two herbicides tested, is due their low tendency to bind to the organic matter, as indicated by their much lower Kow coefficients (see Table S1). On the other hand, the low organic matter content in soil S4, and to a lesser extent in soil S1, can explain the faster degradation of TRT and TRF compared to the dissipation observed in soils S2 and S3. The fact that the percentages of undegraded herbicides were rather similar in soils S2 and S3 for the four compounds tested suggests that the herbicide hydrophobicity has a lower impact on their degradation when the organic content is high enough.
The highest pH was measured for S4, which could also be related to the faster dissipation of TRT and TRF in this soil. However, the difference between the pH values measured for samples S2 and S4 was almost the same as the difference between the pH values of samples S2 and S3, and no significant differences in the degradation of any of the herbicides were observed between the latter two soils. This indicates that soil pH was not the main cause of the degradation observed for TRT and TRF in sample S4. No correlation was found between the soil moisture content and the dissipation of any of the pesticides in the tested soils, although the four soils were sampled in areas with low moisture contents [23].
In a previous study, the dissipation of ACL and BF in different environmental waters was studied. The half-life times found for ACL ranged from 5.43 to 53.75 days, whereas the values obtained for BF ranged from 2.18 to 26.43 days [22]. According to the results obtained in the present study, ACL and BF can persist in soils for periods much longer than in environmental waters. Moreover, in all the soils tested, the most apolar compounds, ACL and BF, exhibited higher stability. Therefore, these herbicides pose greater risks to non-targeted organisms, not only due to their higher potential for bioaccumulation, but also because of their increased persistence in soils.
It should be remarked that the above results were obtained under controlled conditions. In the future, real degradation studies aimed at establishing the flows and final fate of the target compounds would be necessary to evaluate the impact of factors affecting their (bio)transformation including the analysis of a wider variety of soils and soils exposed to field conditions.
5. Conclusions
In the present study, a miniaturized method was specifically developed to evaluate the persistence of herbicides in soil. The proposed method involves the extraction of the herbicides by using an appropriate solvent, followed by direct processing of the extracts by IT-SPME–capLC. The main advantages of the proposed approach over existing procedures are its rapid and straightforward sample treatment process, and its applicability to the simultaneous analysis of herbicides with a wide range of polarities. Consequently, this method can be considered as a simple and cost-effective tool for assessing the dissipation and final fate of the tested compounds in soil.
The described methodology has been optimized to measure the target herbicides at low to sub µg/g levels, which are concentrations typically used in degradation studies. If lower concentrations need to be measured, the procedure would have to be modified to enhance the sensitivity, for example, by using a longer extractive capillary or a capillary with a modified extractive phase for IT-SPME [16,20]. In addition, the proposed method was only tested on four herbicides in a laboratory study. Thus, future studies are necessary to evaluate the proposed approach with a broader variety of herbicides and soil types. The application of this methodology to study the distribution and dissipation of herbicides in soil/water systems is also a promising area for further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/soilsystems8030071/s1, Figure S1: Effect on the percentage of analyte recovered in the filtration of: (a) the type of filter; and (b) passing 0.5 mL of methanol after the sample with the cellulose filters; Figure S2: Effect on the peak areas of: (a) the proportion of MeOH to water (v/v) in the extract; (b) the volume of extract containing 33.3% (v/v) of methanol loaded into the IT-SPME; Table S1: Chemical structures and some properties of the studied compounds compiled from different databases; Table S2: References of the procedures applied to the determination of the moisture content, pH, and organic matter content in the soil samples; Table S3: Variation in the responses obtained for three injections of a sample extract and for three sample extracts obtained for three replicates of a soil (each injected by triplicate); Table S4: Relevant features of methods proposed for the determination of the tested herbicides in soil.
Author Contributions
Conceptualization, C.E.R.-P., R.H.-H. and P.C.-F.; Methodology, C.E.R.-P., R.H.-H. and P.C.-F.; Validation, C.E.R.-P., R.H.-H. and P.C.-F.; Formal analysis, C.E.R.-P., R.H.-H. and P.C.-F.; Investigation, C.E.R.-P., R.H.-H. and P.C.-F.; Resources, C.E.R.-P., R.H.-H. and P.C.-F.; Data curation, C.E.R.-P., R.H.-H. and P.C.-F.; Writing—original draft preparation, C.E.R.-P., R.H.-H. and P.C.-F.; Visualization, C.E.R.-P., R.H.-H. and P.C.-F.; Supervision, R.H.-H. and P.C.-F.; Project administration, P.C.-F.; Funding acquisition, C.E.R.-P., R.H.-H. and P.C.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This article is part of project ref. PID2021-124554NB-I00 funded by the Spanish MCIN/AEI/10.13039/501100011033 and ERDF A way of making Europe, project ref. PDC2021-121604-I00 funded by the Spanish MCIN/AEI/10.13039/501100011033 and by European Union NextGenerationEU/PRTR, and project ref. PROMETEO 2020/078 funded by the Generalitat Valenciana-Conselleria de Educación, Universidades y Empleo. C.E.R.-P. was a recipient of a predoctoral contract from MCIN/AEI/10.13039/501100011033 and the European Union NextGenerationEU/PRTR (project PDC2021-121604-I00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study will be available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wołejko, E.; Wydro, U.; Odziejewicz, J.I.; Koronkiewicz, A.; Jabłońska-Trypuć, A. Biomonitoring of soil contaminated with herbicides. Water 2022, 14, 1534. [Google Scholar] [CrossRef]
- Göldner, V.; Speitling, M.; Karst, U. Elucidation of the environmental reductive metabolism of the herbicide tritosulfuron assisted by electrochemistry and mass spectrometry. Chemosphere 2023, 330, 138687. [Google Scholar] [CrossRef] [PubMed]
- Erguven, G.O.; Bayhan, H.; Demir, G.; Ikizoglu, B.; Kanat, G. Monitoring aclonifen remediation in soil with a laboratory-scale research. J. Chem. 2016, 2016, 5059049. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Gambín, M.; Navarro, S. Leaching behavior appraisal of eight persistent herbicides on a loam soil amended with different composted organic wastes using screening indices. J. Environ. Manage. 2020, 273, 111179. [Google Scholar] [CrossRef] [PubMed]
- Caraba, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the effects of the herbicide aclonifen and its soil metabolites on soil and aquatic environments. Agriculture 2023, 13, 1226. [Google Scholar] [CrossRef]
- Kenessov, B.; Koziel, J.A.; Bakaikina, N.V.; Orazbayeva, D. Perspectives and challenges of on-site quantification of organic pollutants in soils using solid-phase microextraction. Trends Anal. Chem. 2016, 85, 111. [Google Scholar] [CrossRef]
- Bakanov, N.; Honert, C.; Eichler, L.; Lehmann, G.U.C.; Schulz, R.; Brühl, C.A. A new sample preparation approach for the analysis of 98 current-use pesticides in soil and herbaceous vegetation using HPLC-MS/MS in combination with an acetonitrile-based extraction. Chemosphere 2023, 331, 138840. [Google Scholar] [CrossRef] [PubMed]
- Sharipov, U.; Kočárek, M.; Jursík, M.; Nikodem ABorůvka, L. Adsorption and degradation behavior of six herbicides in different agricultural soils. Environ. Earth Sci. 2021, 80, 702. [Google Scholar] [CrossRef]
- Kanu, A.B. Recent developments in sample preparation techniques combined with high-performance liquid chromatography: A critical review. J. Chromatogr. A 2021, 1654, 462444. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Bao, T.; Chen, Z. Polydopamine-based immobilization of zeolitic imidazolate framework-8 for in-tube solid-phase microextraction. J. Chromatogr. A 2015, 1388, 9. [Google Scholar] [CrossRef]
- Pang, J.; Song, X.; Huang, X.; Yuan, D. Porous monolith-based magnetism-reinforced in-tube solid phase microextraction of sulfonylurea herbicides in water and soil samples. J. Chromatogr. A 2020, 1613, 460672. [Google Scholar] [CrossRef] [PubMed]
- Kuster, M.; López de Alda, M.; Barceló, D. Liquid chromatography-tandem mass spectrometric analysis and regulatory issues of polar pesticides in natural and treated waters. J. Chromatogr. A 2009, 1216, 520. [Google Scholar] [CrossRef] [PubMed]
- Hensen, B.; Olsson, O.; Kümmerer, K. A strategy for an initial assessment of the ecotoxicological effects of transformation products of pesticides in aquatic systems following a tiered approach. Environ. Int. 2020, 137, 105533. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Zhu, X.; Huang, X. Mixed functional monomers-based monolithic adsorbent for the effective extraction of sulfonylurea herbicides in water and soil samples. J. Chromatogr. A 2018, 1531, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, W.K.; Zhang, H.X.; Shi, Y.P. Simultaneous determination of bifenox, dichlobenil and diclofop methyl by hollow carbon nanospheres enhanced magnetic carboxylic multi-walled carbon nanotubes. Anal. Chim. Acta 2018, 1011, 40. [Google Scholar] [CrossRef] [PubMed]
- Serra-Mora, P.; Rodríguez-Palma, C.E.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Improving the on-line extraction of polar compounds by IT-SPME with silica nanoparticles modified phases. Separations 2018, 5, 10. [Google Scholar] [CrossRef]
- Yao, W.; Fan, Z.; Zhang, S. Preparation of metal-organic framework UiO-66-incorporated polymer monolith for the extraction of trace levels of fungicides in environmental water and soil samples. J. Sep. Sci. 2019, 42, 2679. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Innovations in extractive phases for in-tube solid-phase microextraction coupled to miniaturized liquid chromatography: A critical review. Molecules 2020, 25, 2460. [Google Scholar] [CrossRef] [PubMed]
- Serra-Mora, P.; Herráez-Hernández, R.; Campíns-Falcó, P. Minimizing the impact of sample preparation on analytical results: In-tube solid-phase microextraction coupled on-line to nano-liquid chromatography for the monitoring of tribenuron methyl in environmental waters. Sci. Total Environ. 2020, 721, 137732. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Herráez-Hernández, R.; Campíns-Falcó, P. Bimodal copper oxide nanoparticles doped phase for the extraction of highly polar compounds by in-tube solid-phase microextraction coupled on-line to nano-liquid chromatography. J. Chromatogr. A 2020, 1617, 460819. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Cardoso, A.T.; Maciel, E.V.S.; Lanças, F.M. Current materials for miniaturized sample preparation: Recent advances and future trends. Trends Anal. Chem. 2023, 165, 117120. [Google Scholar] [CrossRef]
- Rodríguez-Palma, C.E.; Herráez-Hernández, R.; Campíns-Falcó, P. Study of the degradation of diphenyl-ether herbicides aclonifen and bifenox in different environmental waters. Chemosphere 2023, 336, 139238. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, J.F. (Ed.) The Soils of Spain; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–14. ISBN 978-3-319-20541-0. [Google Scholar]
- Vega, D.; Cambon, J.-P.; Bastide, J. Triflusulfuron-methyl dissipation in water and soil. J. Agric. Food Chem. 2000, 48, 3733. [Google Scholar] [CrossRef] [PubMed]
- Abbate, C.; Tuttobene, R.; Avola, G.; Gennari, M. The effect of citrus pulp amendment on sunflower production and the dissipation of the herbicide aclonifen. Ital. J. Agron. 2007, 3, 341. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef]
- Gigliotti, G.; Onofri, A.; Pannacci, E.; Businelli, D.; Trevisan, M. Influence of dissolved organic matter from waste material on the phytotoxicity and environmental fate of triflusulfuron Methyl. Environ. Sci. Technol. 2005, 39, 7446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).