Evidence of Potential Organo-Mineral Interactions during the First Stage of Mars Terraforming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Experiment
2.2. Sampling and Measurements
2.3. Physical Fractionation of OM
2.4. Organic C, Total N, and Major and Trace Elements Determination
2.5. Fe EXAFS and XANES
2.6. Data Analysis
3. Results and Discussion
3.1. Influence of Amendment on C and n Distribution
3.2. Major and Trace Elements Concentration
3.3. Fe EXAFS and XANES LCF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Certini, G.; Scalenghe, R. Do soils exist outside Earth? Planet. Space Sci. 2010, 58, 1767–1770. [Google Scholar] [CrossRef]
- Certini, G.; Karunatillake, S.; Zhao, Y.Y.S.; Meslin, P.Y.; Cousin, A.; Hood, D.R.; Scalenghe, R. Disambiguating the soils of Mars. Planet. Space Sci. 2020, 186, 104922. [Google Scholar] [CrossRef]
- Häder, D.P.; Braun, M.; Hemmersbach, R. Bioregenerative Life Support Systems in Space Research. In Gravitational Biology I; Springer Briefs in Space Life Sciences; Springer: Cham, Switzerland, 2018; pp. 113–122. [Google Scholar]
- Peters, G.H.; Abbey, W.; Bearman, G.H.; Mungas, G.S.; Smith, J.A.; Anderson, R.C.; Douglas, S.; Beegle, L.W. Mojave Mars simulant—Characterization of a new geologic Mars analog. Icarus 2008, 197, 470–479. [Google Scholar] [CrossRef]
- Rampe, E.B.; Ming, D.W.; Blake, D.F.; Bristow, T.F.; Chipera, S.J.; Grotzinger, J.P.; Morris, R.V.; Morrison, S.M.; Vaniman, D.T.; Yen, A.S.; et al. Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 172–185. [Google Scholar] [CrossRef]
- Wamelink, G.W.W.; Frissel, J.Y.; Krijnen, W.H.J.; Verwoert, M.R.; Goedhart, P.W. Can plants grow on Mars and the Moon: A growth experiment on Mars and Moon soil simulants. PLoS ONE 2014, 9, 103138. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Vingiani, S.; Palladino, M.; El-Nakhel, C.; Duri, L.; Pannico, A.; Rouphael, Y.; De Pascale, S.; Adamo, P. Geo-mineralogical characterisation of Mars simulant MMS-1 and appraisal of substrate physico-chemical properties and crop performance obtained with variable green compost amendment rates. Sci. Total Environ. 2020, 720, 137543. [Google Scholar] [CrossRef]
- Duri, L.G.; El-Nakhel, C.; Caporale, A.G.; Ciriello, M.; Graziani, G.; Pannico, A.; Palladino, M.; Ritieni, A.; De Pascale, S.; Vingiani, S.; et al. Mars Regolith simulant ameliorated by compost as in situ cultivation substrate improves lettuce growth and nutritional aspects. Plants 2020, 9, 628. [Google Scholar] [CrossRef]
- Duri, L.G.; Caporale, A.G.; Rouphael, Y.; Vingiani, S.; Palladino, M.; De Pascale, S.; Adamo, P. The potential for lunar and martian regolith simulants to sustain plant growth: A multidisciplinary overview. Front. Astron. Space Sci. 2022, 8, 747821. [Google Scholar] [CrossRef]
- Mortley, D.G.; Aglan, H.A.; Bonsi, C.K.; Hill, W.A. Growth of sweetpotato in Lunar and Mars Simulants; SAE Technical Paper 2000-01-2289; SAE: Warrendale, PA, USA, 2000. [Google Scholar]
- Caporale, A.G.; Paradiso, R.; Luizzi, G.; Palladino, M.; Amitrano, C.; Arena, C.; Arouna, N.; Verrillo, M.; Cozzolino, V.; De Pascale, S. Green compost amendment improves potato plant performance on Mars regolith simulant as substrate for cultivation in space. Plant Soil 2023, 486, 217–233. [Google Scholar] [CrossRef]
- Fackrell, L.E.; Schroeder, P.A.; Thompson, A.; Stockstill-Cahill, K.; Hibbitts, C.A. Development of martian regolith and bedrock simulants: Potential and limitations of martian regolith as in-situ resource. Icarus 2021, 354, 114055. [Google Scholar] [CrossRef]
- Eichler, A.; Hadland, N.; Pickett, D.; Masaitis, D.; Handy, D.; Perez, A.; Batcheldor, D.; Wheeler, B.; Palmer, A. Challenging the agricultural viability of martian regolith simulants. Icarus 2021, 354, 114022. [Google Scholar] [CrossRef]
- Sonsri, K.; Naruse, H.; Watanabe, A. Mechanisms controlling the stabilization of soil organic matter in agricultural soils as amended with contrasting organic amendments: Insights based on physical fractionation coupled with 13C NMR spectroscopy. Sci. Total Environ. 2022, 825, 153853. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 1459–148569. [Google Scholar] [CrossRef]
- Perko, H.A.; Nelson, J.D.; Green, J.R. Mars Soil Mechanical Properties and Suitability of Mars Soil Simulants. J. Aerosp. Eng. 2006, 19, 169–176. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the martian surface. Annu. Rev. Earth Planet. Sci. 2014, 42, 291–315. [Google Scholar] [CrossRef]
- Delage, P.; Karakostas, F.; Dhemaied, A.; Belmokhtar, M.; Lognonné, P.; Golombek, M.; De Laure, E.; Hurst, K.; Dupla, J.C.; Kedar, S.; et al. An Investigation of the Mechanical Properties of Some Martian Regolith Simulants with Respect to the Surface Properties at the InSight Mission Landing Site. Space Sci. Rev. 2017, 211, 191–213. [Google Scholar] [CrossRef]
- Kasyap, S.S.; Senetakis, K. A Grain-Scale Study of Mojave Mars Simulant (MMS-1). Sensors 2021, 21, 4730. [Google Scholar] [CrossRef]
- Ramkissoon, N.K.; Pearson, V.K.; Schwenzer, S.P.; Schröder, C.; Kirnbauer, T.; Wood, D.; Seidel, R.G.W.; Miller, M.A.; Olsson-Francis, K. New simulants for martian regolith: Controlling iron variability. Planet. Space Sci. 2019, 179, 10472. [Google Scholar] [CrossRef]
- Lalonde, L.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Giannetta, B.; Plaza, C.; Siebecker, M.G.; Aquilanti, G.; Vischetti, C.; Plaisier, J.; Juanco, M.; Sparks, D.L.; Zaccone, C. Iron speciation in organic matter fractions isolated from soils amended with biochar and organic fertilizers. Environ. Sci. Technol. 2020, 54, 5093–5101. [Google Scholar] [CrossRef]
- Giannetta, B.; Siebecker, M.G.; Zaccone, C.; Plaza, C.; Rovira, P.; Vischetti, C.; Sparks, D.L. Iron(III) fate after complexation with soil organic matter in fine silt and clay fractions: An EXAFS spectroscopic approach. Soil Till. Res. 2020, 200, 104617. [Google Scholar] [CrossRef]
- Giannetta, B.; Plaza, C.; Thompson, A.; Plante, A.F.; Zaccone, C. Iron speciation in soil size fractions under different land uses. Geoderma 2022, 418, 115842. [Google Scholar] [CrossRef]
- Paradiso, R.; Arena, C.; Rouphael, Y.; d’Aquino, L.; Makris, K.; Vitaglione, P.; De Pascale, S. Growth, photosynthetic activity and tuber quality of two potato cultivars in controlled environment as affected by light source. Plant Biosyst. 2019, 153, 725–735. [Google Scholar] [CrossRef]
- Paradiso, R.; Ceriello, A.; Pannico, A.; Sorrentino, S.; Palladino, M.; Giordano, M.; Fortezza, R.; De Pascale, S. Design of a module for cultivation of tuberous plants in microgravity: The ESA Project “Precursor of Food Production Unit” (PFPU). Front. Plant Sci. 2020, 11, 417. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Harris, D.; Horwath, W.R.; van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.-E.; Brown, G.E.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- O’Day, P.A.; Rivera, N.; Root, R.; Carroll, S.A. X-ray absorption spectroscopic study of Fe reference compounds for the analysis of natural sediments. Am. Mineral. 2004, 89, 572–585. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Poulet, F.; Bibring, J.-P.; Mustard, J.F.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.E.; Gondet, B.; Gomez, C.; the Omega Team. Phyllosilicates on Mars and implications for early martian climate. Nature 2005, 438, 623–627. [Google Scholar] [CrossRef]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.P.; Poulet, F.; Bishop, J.; Dobrea, E.N.; et al. Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Torrent, J.; Barrón, V. Key Role of Phosphorus in the Formation of the Iron Oxides in Mars Soils? Icarus 2000, 145, 645–647. [Google Scholar] [CrossRef]
- Barrón, V.; Torrent, J. Evidence for a simple pathway to maghemite in Earth and Mars soils. Geochim. Cosmochim. Acta 2002, 66, 2801–2806. [Google Scholar] [CrossRef]
- Giannetta, B.; Oliveira de Souza, D.; Aquilanti, G.; Celi, L.; Said-Pullicino, D. Redox-driven changes in organic C stabilization and Fe mineral transformations in temperate hydromorphic soils. Geoderma 2022, 406, 115532. [Google Scholar] [CrossRef]
- Giannetta, B.; Cassetta, M.; Oliveira de Souza, D.; Mariotto, G.; Aquilanti, G.; Zaccone, C. Coupling X-ray absorption and Raman spectroscopies to characterize iron species in a karst pedosedimentary record. Soil Syst. 2022, 6, 24. [Google Scholar] [CrossRef]
- Giannetta, B.; Zaccone, C.; Plaza, C.; Siebecker, M.G.; Rovira, P.; Vischetti, C.; Sparks, D.L. The role of Fe(III) in soil organic matter stabilization in two size fractions having opposite features. Sci. Total Environ. 2019, 653, 667–674. [Google Scholar] [CrossRef]
- Scheidegger, A.; Borkovec, M.; Sticher, H. Coating of silica sand with goethite: Preparation and analytical identification. Geoderma 1993, 58, 43–65. [Google Scholar] [CrossRef]
- Penn, R.L.; Zhu, C.; Xu, H.; Veblen, D.R. Iron oxide coatings on sand grains from the Atlantic coastal plain: High-resolution transmission electron microscopy characterization. Geology 2001, 29, 843–846. [Google Scholar] [CrossRef]
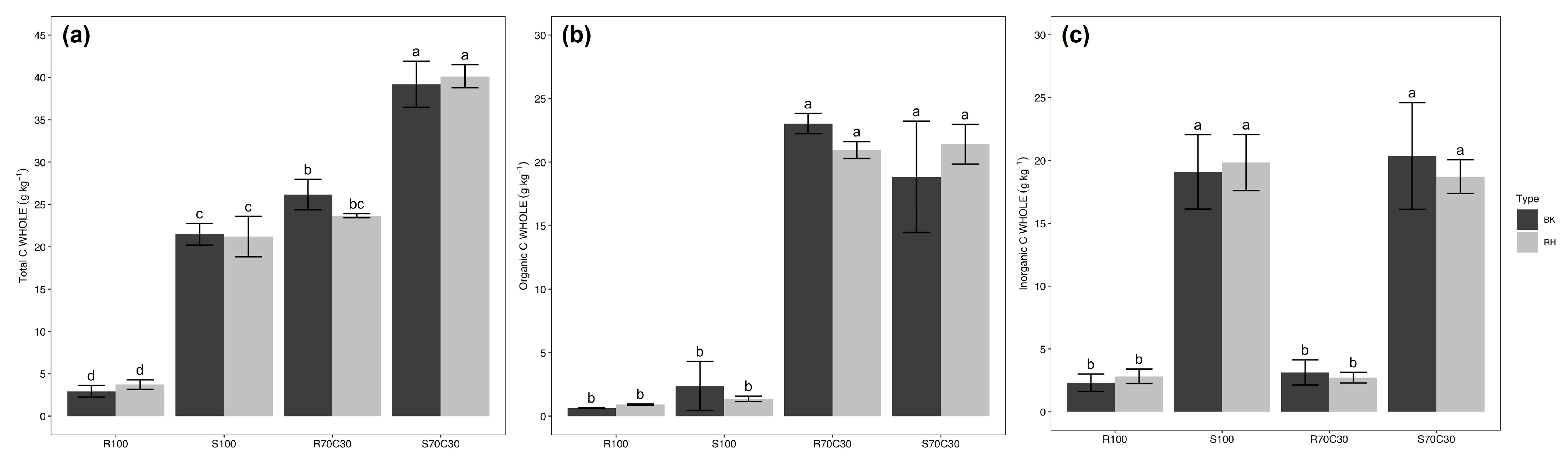
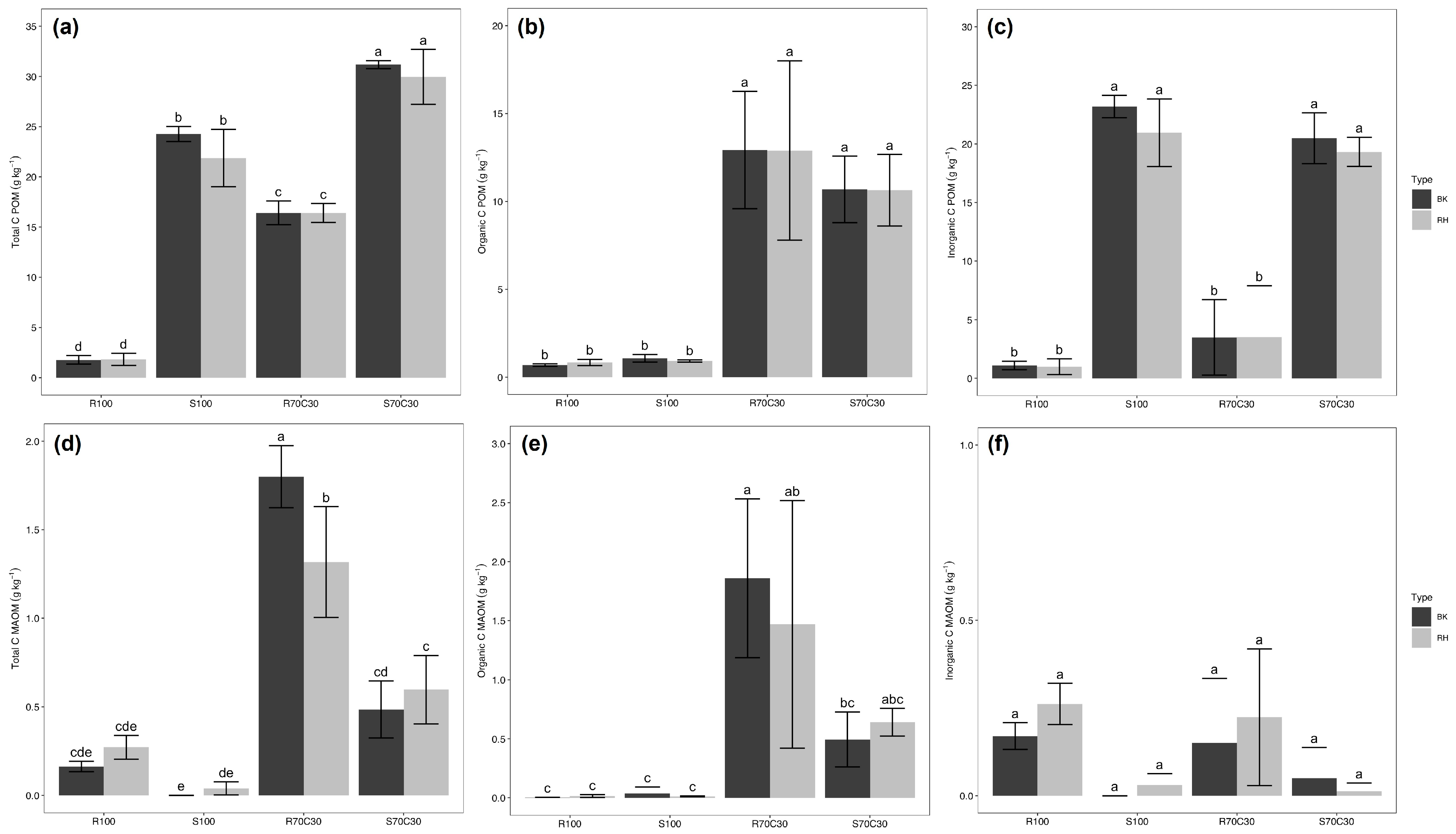
| Experimental Factors | Total C | Organic C | Inorganic C | Total N | C/N |
|---|---|---|---|---|---|
| g kg−1 | |||||
| R100 | 1.78 c | 0.51 c | 1.27 b | <0.03 c | |
| S100 | 14.81 b | 0.96 c | 13.85 a | <0.03 c | |
| R70C30 | 14.30 b | 12.13 b | 2.20 b | 0.83 a | 13.76 |
| S70C30 | 23.59 a | 10.45 a | 13.16 a | 0.60 b | 16.71 |
| Substrate (S) | *** | *** | *** | *** | ** |
| WHOLE | 22.31 a | 11.19 a | 11.12 a | 0.76 a | 14.87 b |
| POM | 17.96 b | 6.33 b | 11.63 a | 0.34 b | 18.60 a |
| MAOM | 0.58 c | 0.52 c | 0.11 b | 0.09 c | 10.89 c |
| OM fraction (OMF) | *** | *** | *** | *** | *** |
| RH | 13.42 | 5.98 | 7.45 | 0.39 | 14.53 |
| BK | 13.82 | 6.05 | 7.79 | 0.38 | 15.49 |
| RHvs. BK (RB) | ns | ns | ns | ns | ns |
| S × OMF | *** | ns | *** | * | ** |
| S × RB | ns | ns | ns | ns | ns |
| OMF × RB | ns | ns | ns | ns | ns |
| S × OMF × RB | ns | ns | ns | ns | ns |
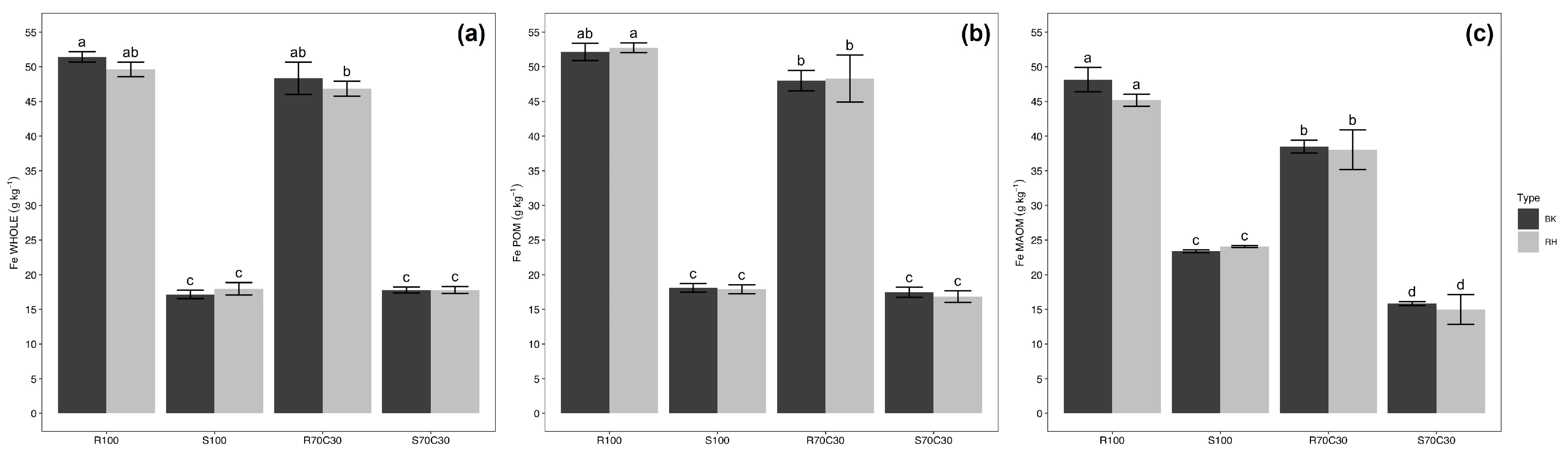
| Experimental Factors | Si | Ca | Fe | Al | K | Na | Mg | P | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||||
| R100 | 234 a | 29.0 c | 49.9 a | 14.6 a | 10.3 b | 11.4 a | 5.0 d | 7.5 b | 0.96 a | 111 a | 41.6 b |
| S100 | 215 b | 64.3 a | 18.7 c | 7.6 b | 10.4 ab | 11.6 a | 14.4 a | 19.7 a | 0.73 c | 73 c | 30.4 c |
| R70C30 | 214 b | 30.5 c | 44.7 b | 14.5 a | 9.8 b | 10.9 ab | 5.8 c | 7.4 b | 0.87 b | 113 a | 47.7 a |
| S70C30 | 186 c | 58.9 b | 16.8 d | 6.5 b | 11.4 a | 10.2 b | 12.5 b | 19.0 a | 0.65 d | 99 b | 49.1 a |
| Substrate (S) | *** | *** | *** | *** | * | *** | *** | *** | *** | * | ** |
| WHOLE | 248 a | 42.6 b | 33.4 a | 9.2 c | 10.2 b | 7.5 c | 9.4 | 0.99 b | 0.78 b | 78 b | 28.6 b |
| POM | 206 b | 48.6 a | 33.9 a | 11.1 b | 9.8 b | 8.7 b | 9.7 | 0.96 b | 0.83 a | 72 b | 27.7 b |
| MAOM | 176 c | 42.2 b | 32.5 b | 13.1 a | 11.6 a | 18.0 a | 8.2 | 41.9 a | 0.81 ab | 162 a | 78.3 a |
| OM fraction (OMF) | *** | ** | *** | *** | * | *** | ns | *** | * | *** | *** |
| RH | 210 | 43.7 | 33 | 10.9 | 10.5 | 10.9 | 9.0 | 13.0 | 0.80 | 97 | 41.4 |
| BK | 214 | 45.5 | 33.6 | 11.2 | 10.5 | 11.2 | 9.3 | 13.0 | 0.81 | 104 | 44.4 |
| RH vs. BK (RB) | ns | ns | ns | ns | ns | ns | * | ns | ns | *** | ns |
| S × OMF | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** |
| S × RB | ns | ns | ns | ns | ns | ns | ns | ns | ns | ** | ns |
| OMF × RB | ns | ns | ns | ns | ns | ns | ns | ns | ns | *** | ns |
| S × OMF × RB | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns |
| Component 1 | (%) | Component 2 | (%) | Component 3 | (%) | Component 4 | (%) | |
|---|---|---|---|---|---|---|---|---|
| R100 BK POM | Smectite | 38 | Maghemite | 20 | Hematite | 19 | Ferrihydrite | 23 |
| R100 RH POM | Smectite | 44 | Maghemite | 15 | Hematite | 17 | Ferrihydrite | 24 |
| R100 BK MAOM | Smectite | 45 | Maghemite | 22 | Nontronite | 3 | Ferrihydrite | 30 |
| R100 RH MAOM | Smectite | 35 | Maghemite | 20 | Nontronite | 9 | Ferrihydrite | 36 |
| R70C30 BK POM | Smectite | 31 | Maghemite | 17 | Hematite | 20 | Ferrihydrite | 32 |
| R70C30 RH POM | Smectite | 33 | Maghemite | 19 | Hematite | 21 | Ferrihydrite | 27 |
| R70C30 BK MAOM | Smectite | 51 | Maghemite | 21 | - | Ferrihydrite | 28 | |
| R70C30 RH MAOM | Smectite | 34 | Maghemite | 26 | Nontronite | 10 | Ferrihydrite | 30 |
| S100 BK POM | Chlorite | 58 | Smectite | 23 | Goethite | 6 | Siderite | 13 |
| S100 RH POM | Chlorite | 81 | - | Goethite | 16 | Siderite | 3 | |
| S100 BK MAOM | Chlorite | 57 | Smectite | 7 | Goethite | 36 | - | |
| S100 RH MAOM | Chlorite | 55 | Smectite | 7 | Goethite | 38 | - | |
| S70C30 BK POM | Chlorite | 74 | Siderite | 4 | Goethite | 13 | Fe(III)-OM | 9 |
| S70C30 RH POM | Chlorite | 74 | Siderite | 4 | Goethite | 14 | Fe(III)-OM | 8 |
| S70C30 BK MAOM | Chlorite | 33 | Smectite | 41 | Goethite | 24 | Fe(III)-OM | 2 |
| S70C30 RH MAOM | Chlorite | 32 | Smectite | 41 | Goethite | 15 | Fe(III)-OM | 12 |
| Component 1 | (%) | Component 2 | (%) | Component 3 | (%) | Component 4 | (%) | |
|---|---|---|---|---|---|---|---|---|
| R100 BK POM | Illite | 9 | Hematite | 40 | Maghemite | 7 | Ferrihydrite | 44 |
| R100 RH POM | Illite | 11 | Hematite | 40 | Maghemite | 8 | Ferrihydrite | 41 |
| R100 BK MAOM | Nontronite | 29 | Hematite | 30 | Siderite | 7 | Ferrihydrite | 34 |
| R100 RH MAOM | Illite | 29 | Hematite | 25 | Siderite | 6 | Ferrihydrite | 40 |
| R70C30 BK POM | Illite | 7 | Hematite | 42 | - | Ferrihydrite | 51 | |
| R70C30 RH POM | Illite | 10 | Hematite | 42 | - | Ferrihydrite | 48 | |
| R70C30 BK MAOM | Nontronite | 22 | Hematite | 30 | Siderite | 6 | Ferrihydrite | 42 |
| R70C30 RH MAOM | Nontronite | 23 | Hematite | 29 | Siderite | 6 | Ferrihydrite | 42 |
| S100 BK POM | Chlorite | 55 | Hematite | 9 | Ferrihydrite | 22 | Fe(III)-OM | 14 |
| S100 RH POM | Chlorite | 56 | Hematite | 10 | Ferrihydrite | 24 | Fe(III)-OM | 10 |
| S100 BK MAOM | Chlorite | 52 | Goethite | 20 | Siderite | 4 | Fe(III)-OM | 24 |
| S100 RH MAOM | Chlorite | 50 | Goethite | 22 | Siderite | 3 | Fe(III)-OM | 25 |
| S70C30 BK POM | Chlorite | 54 | Hematite | 7 | Ferrihydrite | 23 | Fe(III)-OM | 16 |
| S70C30 RH POM | Chlorite | 53 | Hematite | 6 | Ferrihydrite | 20 | Fe(III)-OM | 21 |
| S70C30 BK MAOM | Chlorite | 36 | Goethite | 11 | Ferrihydrite | 11 | Fe(III)-OM | 42 |
| S70C30 RH MAOM | Chlorite | 40 | Goethite | 10 | Ferrihydrite | 16 | Fe(III)-OM | 34 |
| Component 1 | (%) | Component 2 | (%) | Component 3 | (%) | Component 4 | (%) | |
|---|---|---|---|---|---|---|---|---|
| R100 BK POM | Illite | 2 | Hematite | 41 | Purpurite | 10 | Ferrihydrite | 47 |
| R100 RH POM | Nontronite | 9 | Hematite | 44 | - | Ferrihydrite | 47 | |
| R100 BK MAOM | Nontronite | 29 | Hematite | 30 | Siderite | 7 | Ferrihydrite | 34 |
| R100 RH MAOM | Nontronite | 25 | Hematite | 32 | Siderite | 5 | Ferrihydrite | 38 |
| R70C30 BK POM | Illite | 6 | Hematite | 41 | Purpurite | 4 | Ferrihydrite | 45 |
| R70C30 RH POM | Illite | 10 | Hematite | 38 | Maghemite | 13 | Ferrihydrite | 40 |
| R70C30 BK MAOM | Nontronite | 25 | Hematite | 31 | Siderite | 5 | Ferrihydrite | 39 |
| R70C30 RH MAOM | Nontronite | 24 | Hematite | 31 | Siderite | 5 | Ferrihydrite | 40 |
| S100 BK POM | Chlorite | 53 | Hematite | 8 | Ferrihydrite | 22 | Fe(III)-OM | 17 |
| S100 RH POM | Chlorite | 54 | Hematite | 10 | Ferrihydrite | 24 | Fe(III)-OM | 12 |
| S100 BK MAOM | Chlorite | 50 | Goethite | 16 | Siderite | 13 | Fe(III)-OM | 21 |
| S100 RH MAOM | Chlorite | 52 | Goethite | 20 | Siderite | 4 | Fe(III)-OM | 24 |
| S70C30 BK POM | Chlorite | 52 | Hematite | 8 | Ferrihydrite | 25 | Fe(III)-OM | 15 |
| S70C30 RH POM | Chlorite | 50 | Maghemite | 21 | Ferrihydrite | 5 | Fe(III)-OM | 24 |
| S70C30 BK MAOM | Chlorite | 40 | Goethite | 13 | Ferrihydrite | 16 | Fe(III)-OM | 31 |
| S70C30 RH MAOM | Chlorite | 40 | Goethite | 13 | Ferrihydrite | 16 | Fe(III)-OM | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetta, B.; Caporale, A.G.; Olivera de Souza, D.; Adamo, P.; Zaccone, C. Evidence of Potential Organo-Mineral Interactions during the First Stage of Mars Terraforming. Soil Syst. 2023, 7, 92. https://doi.org/10.3390/soilsystems7040092
Giannetta B, Caporale AG, Olivera de Souza D, Adamo P, Zaccone C. Evidence of Potential Organo-Mineral Interactions during the First Stage of Mars Terraforming. Soil Systems. 2023; 7(4):92. https://doi.org/10.3390/soilsystems7040092
Chicago/Turabian StyleGiannetta, Beatrice, Antonio G. Caporale, Danilo Olivera de Souza, Paola Adamo, and Claudio Zaccone. 2023. "Evidence of Potential Organo-Mineral Interactions during the First Stage of Mars Terraforming" Soil Systems 7, no. 4: 92. https://doi.org/10.3390/soilsystems7040092
APA StyleGiannetta, B., Caporale, A. G., Olivera de Souza, D., Adamo, P., & Zaccone, C. (2023). Evidence of Potential Organo-Mineral Interactions during the First Stage of Mars Terraforming. Soil Systems, 7(4), 92. https://doi.org/10.3390/soilsystems7040092