Water Retention Characteristics of Superabsorbent Polymers (SAPs) Used as Soil Amendments
Abstract
1. Introduction
2. Materials and Methods
2.1. SAP and Soil
2.2. Immersing Solutions
2.3. Water Absorbency of SAP and Soil–SAP Mixtures
2.4. Water Release from SAP by Heating
2.5. Soil Water Retention Curves and Plant-Available Water
2.6. Experiments for Characterizing SAP Alone and Soil–SAP Mixtures
2.6.1. Experiment 1: Comparisons of the SAPs’ Water Absorbency in Pure Water and Salt Solutions
2.6.2. Experiment 2: Effects of Salt Types and Concentrations on the SAPs’ Water Absorbency and Recovery of Absorbency Using Tap Water
2.6.3. Experiment 3: Comparison of Water Absorbency of Soil–SAP Mixtures in Tap Water and Salt Solutions
2.6.4. Experiment 4: SAP Application Rate and Water-Holding Capacity of Soil–SAP Mixtures
2.6.5. Experiment 5: Comparison of the Rate of Water Release from Soil–SAP Mixtures under Heating
2.6.6. Experiment 6: Soil Water Retention Curves of Soil–Dry SAP Mixtures
2.6.7. Experiment 7: Soil Water Retention Curves of Soil–Gel SAP Mixtures
2.6.8. Experiment 8: SAP Application Rate and Soil Water Retention Curves
2.7. Statistical Analyses
3. Results
3.1. Water Absorption and Release by SAP Alone
3.1.1. Experiment 1
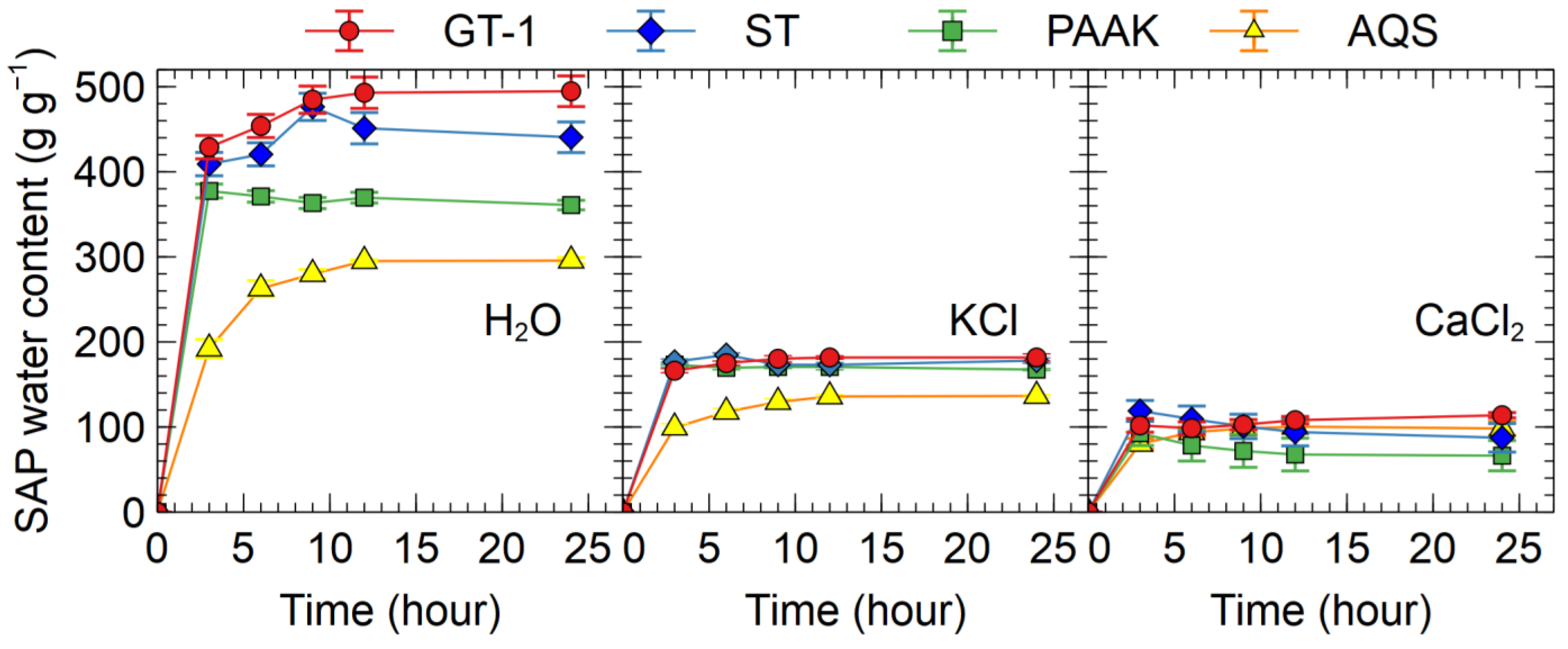
3.1.2. Experiment 2
3.2. Water Absorption and Release of Soil–SAP Mixtures
3.2.1. Experiment 3
3.2.2. Experiment 4
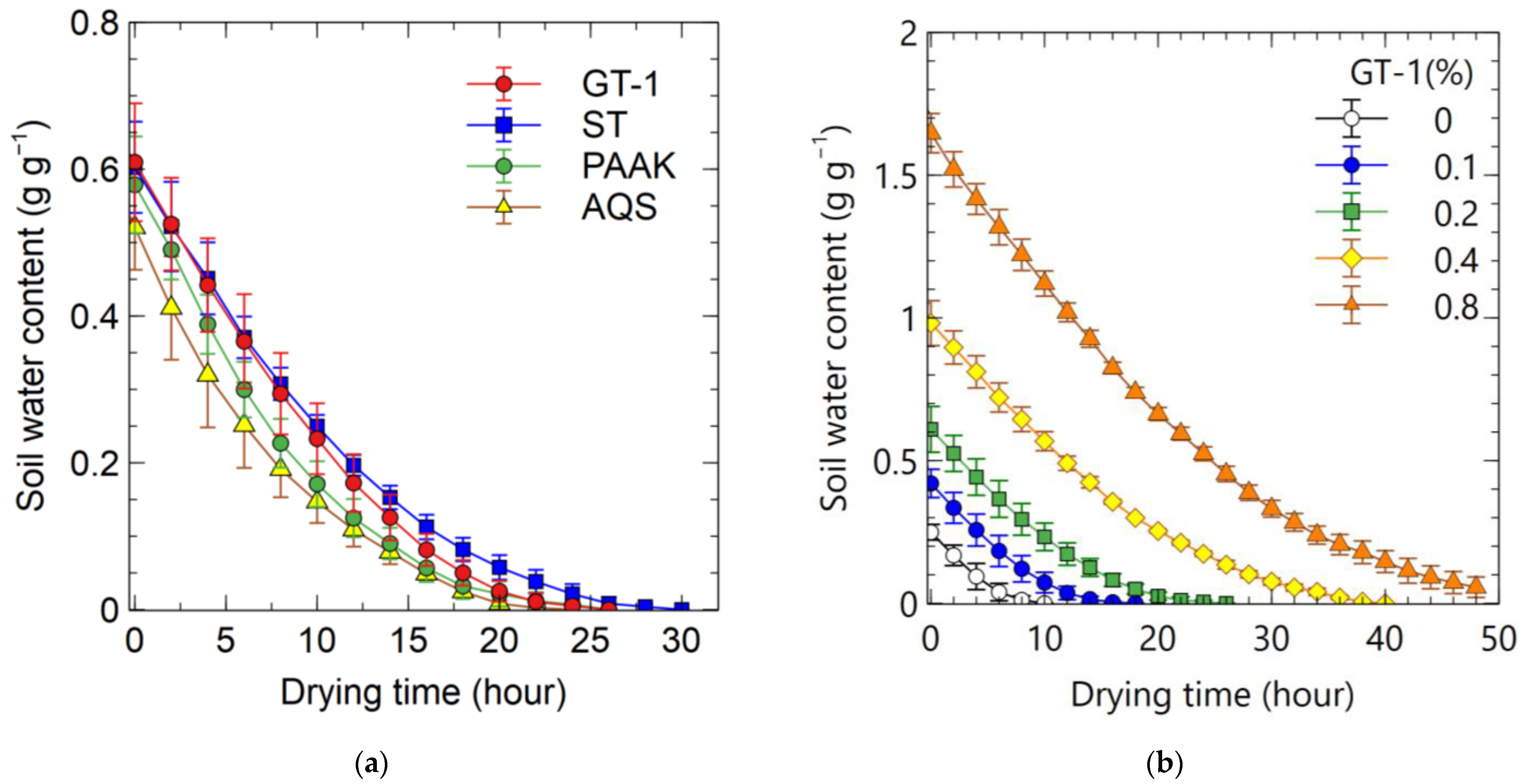
3.2.3. Experiment 5
3.3. Soil Water Retention of Soil–SAP Mixtures
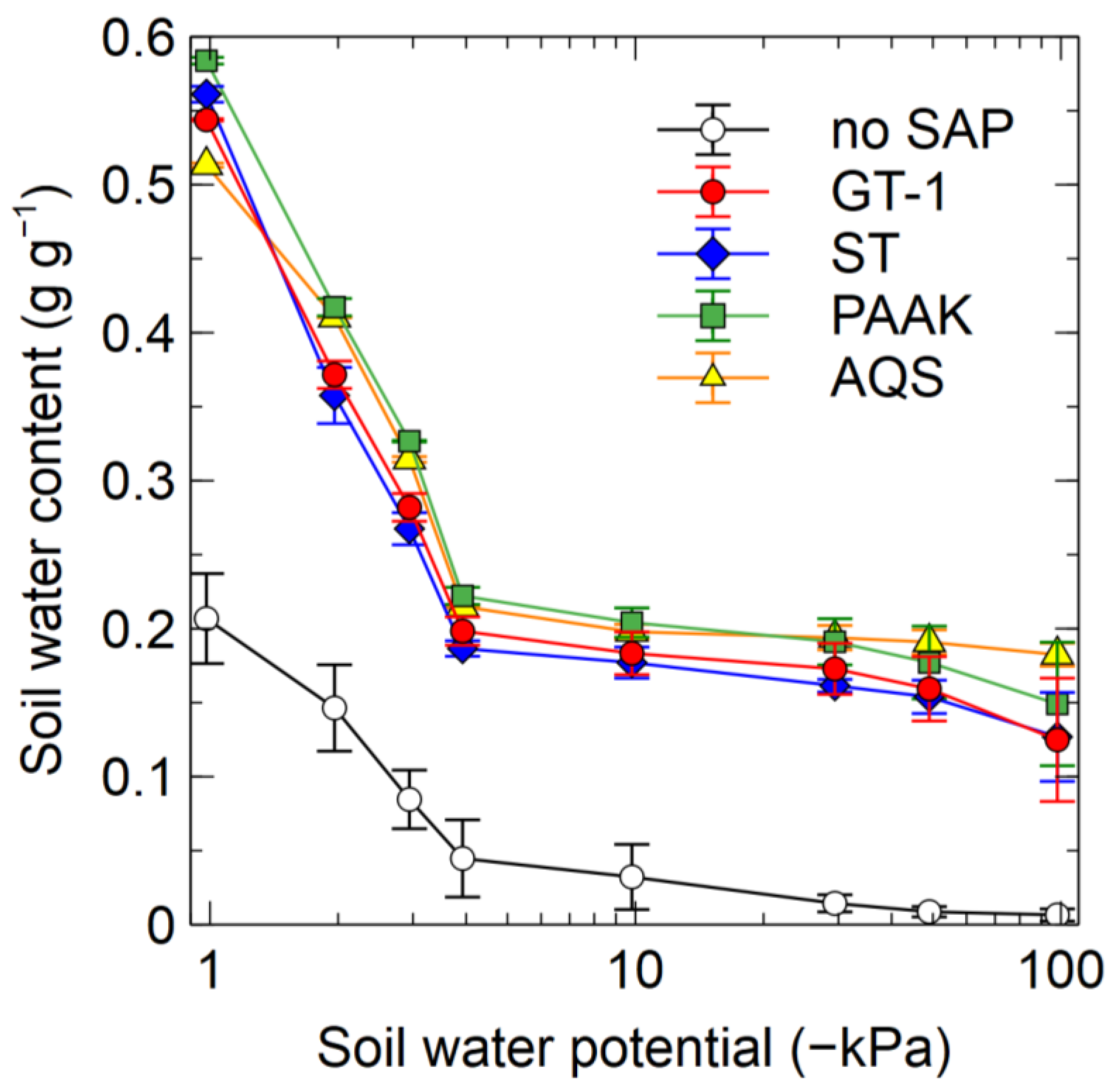
3.3.1. Experiment 6
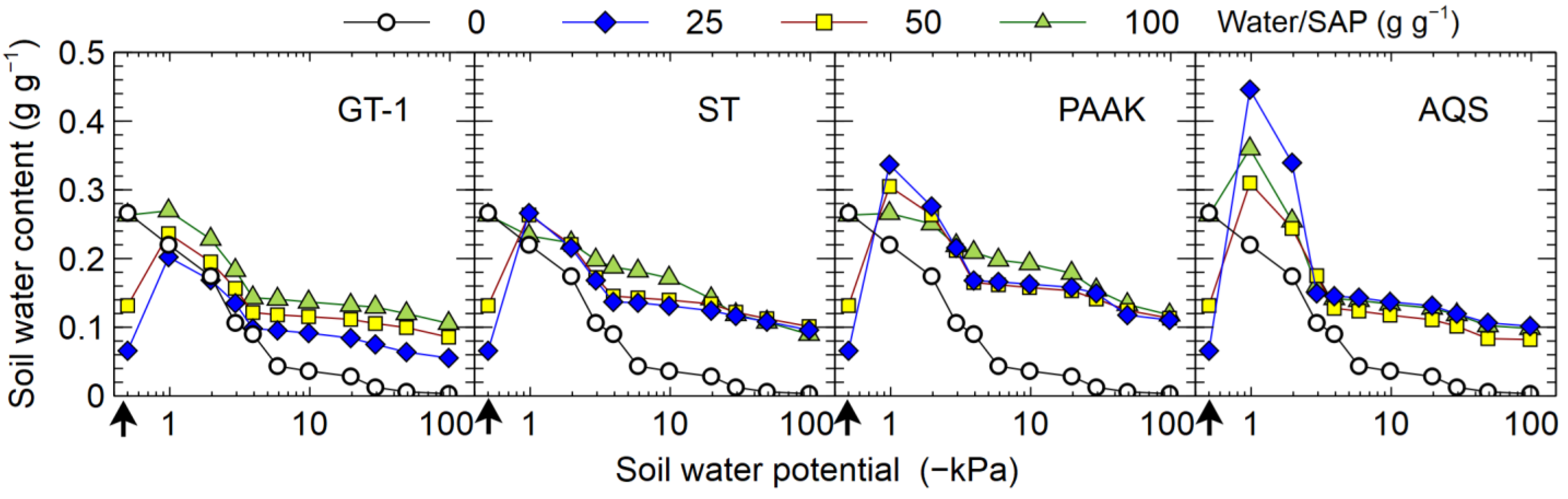
3.3.2. Experiment 7
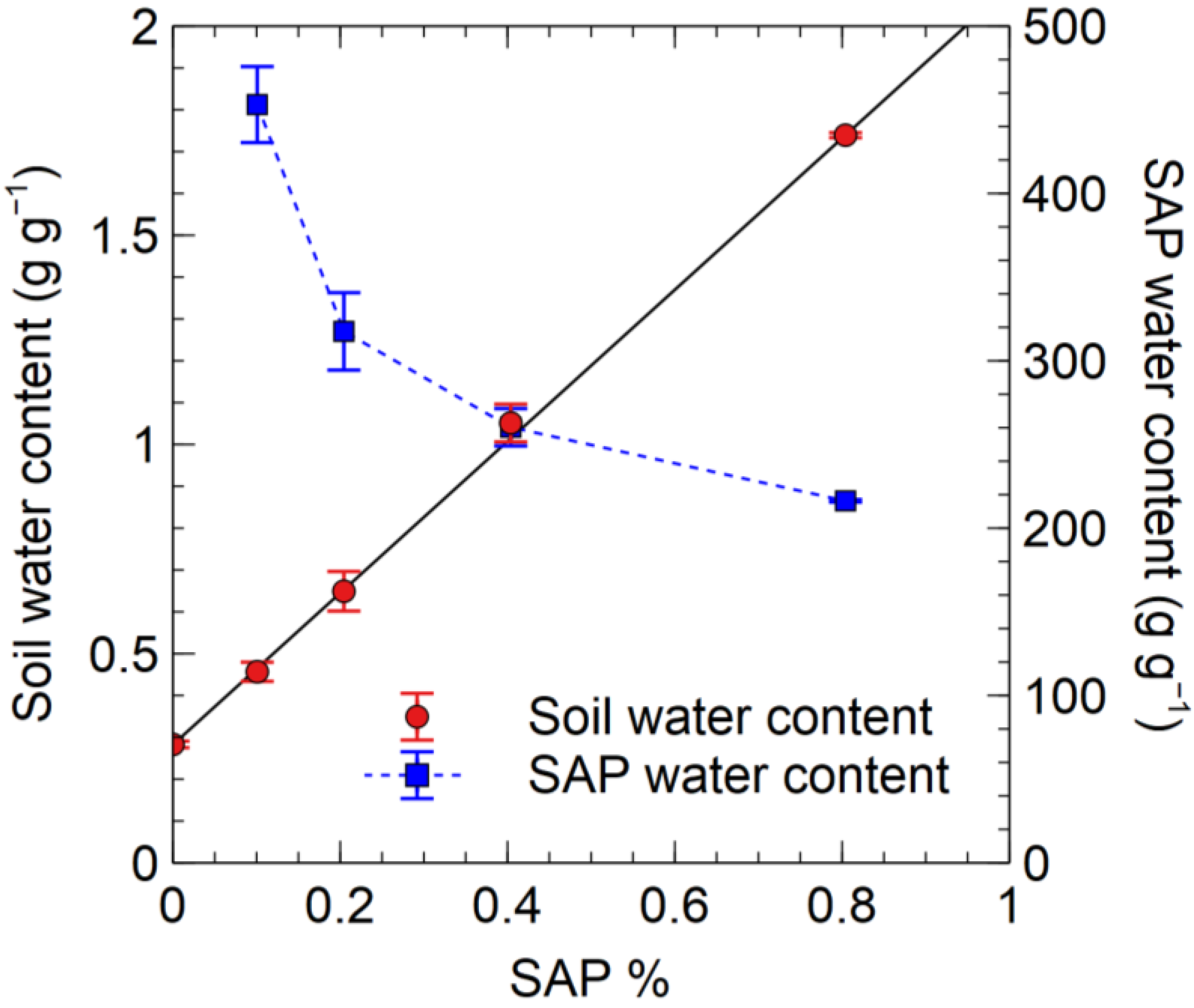
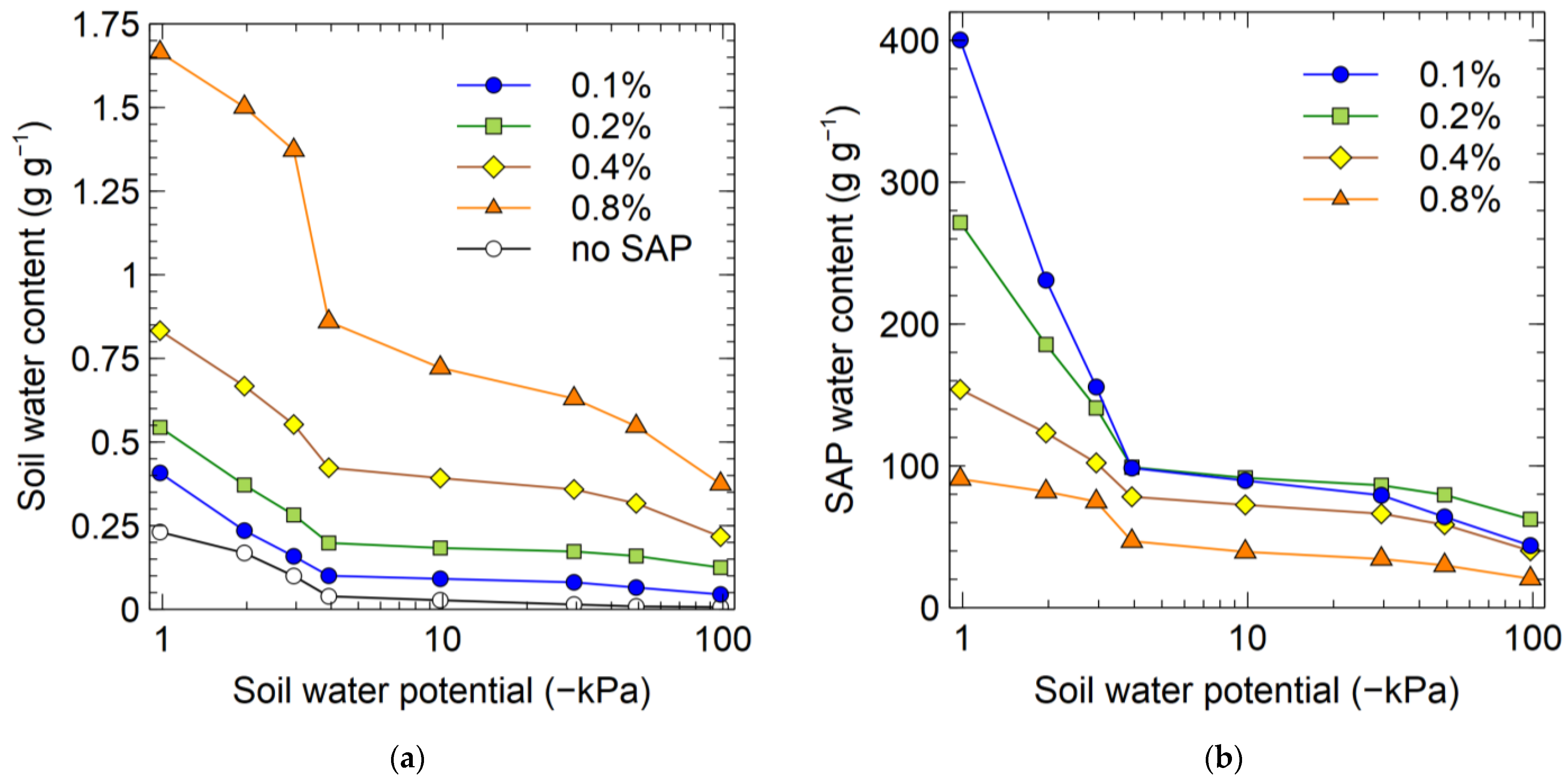
3.3.3. Experiment 8
4. Discussion
4.1. SAP Application Rate and the Water Absorbency of SAP in the Soil
4.2. Effects of Salt on the Absorbency of SAPs
4.3. Water Released from SAP by Heating
4.4. Recovery of Water Absorbency after Desiccation of SAPs
4.5. Difference in the Water Absorbency of Soil–SAP Mixtures between Gel and Dry SAPs
4.6. Soil–Water Retention Curves of Soil–SAP Mixtures
4.7. Differences among SAP Types
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| SAP | MWHC in Pure Water | MWHC in KCl | MWHC in CaCl2 |
|---|---|---|---|
| GT-1 | 494 (18) a | 182 (7) a | 114 (3) |
| ST | 441 (23) b | 178 (5) ab | 87 (17) |
| PAAK | 361 (10) c | 167 (2) b | 66 (18) |
| AQS | 296 (7) d | 136 (3) c | 98 (8) |
| SAP | Soil Water Content (g g−1) | SAP Water Content (g g−1) | Time to Desiccation (hour) | Evaporation Rate (g g−1 h−1) | |
|---|---|---|---|---|---|
| 3 h | 24 h | 24 h | |||
| GT-1 | 0.751 (0.018) a | 0.665 (0.067) a | 325 (19) | 28.7 (0.7) a | 0.0418 (0.0043) |
| ST | 0.755 (0.012) a | 0.671 (0.051) a | 330 (15) | 30.0 (1.2) a | 0.0378 (0.0060) |
| PAAK | 0.740 (0.012) a | 0.650 (0.041) a | 322 (12) | 26.7 (1.8) a | 0.0475 (0.0078) |
| AQS | 0.566 (0.017) b | 0.593 (0.018) a | 293 (5) | 28.0 (1.2) a | 0.0503 (0.0044) |
| no SAP | 0.308 (0.012) c | 0.299 (0.006) b | n.a. | 11.3 (2.2) b | 0.0388 (0.0106) |
| SAP Type | SAP Application Rate (%) | Potential (kPa) | −0.98 | −1.96 | −2.94 | −3.92 | −9.81 | −29.4 | −49.0 | −98.1 |
|---|---|---|---|---|---|---|---|---|---|---|
| pF | pF1.0 | pF1.3 | pF1.5 | pF1.6 | pF2.0 | pF2.5 | pF2.7 | pF3.0 | ||
| GT-1 | 0.1 | 400.3 | 230.9 | 155.6 | 98.5 | 89.6 | 79.3 | 64.0 | 43.8 | |
| GT-1 | 0.2 | 271.6 | 185.6 | 140.8 | 99.0 | 91.5 | 86.3 | 79.5 | 62.3 | |
| GT-1 | 0.4 | 153.9 | 123.3 | 102.1 | 78.2 | 72.5 | 66.2 | 58.5 | 40.1 | |
| GT-1 | 0.8 | 90.7 | 81.8 | 74.8 | 46.9 | 39.3 | 34.3 | 29.8 | 20.4 | |
| ST | 0.2 | 278.0 | 177.0 | 132.5 | 92.4 | 87.6 | 79.9 | 76.2 | 62.6 | |
| PAAK | 0.2 | 293.3 | 209.6 | 164.1 | 111.6 | 102.5 | 96.0 | 89.0 | 74.9 | |
| AQS | 0.2 | 255.0 | 203.9 | 156.2 | 106.8 | 98.3 | 96.3 | 94.8 | 90.6 |


References
- Van Duivenbooden, N.; Pala, M.; Studer, C.; Bielders, C.L.; Beukes, D.J. Cropping Systems and Crop Complementarity in Dryland Agriculture to Increase Soil Water Use Efficiency: A Review. NJAS Wagening. J. Life Sci. 2000, 48, 213–236. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Lal, R. Soil Organic Matter and Water Retention. Agron. J. 2020, 112, 3265–3277. [Google Scholar] [CrossRef]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of Soil Organic Carbon on Soil Water Retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Jamei, M.; Guiras, H.; Chtourou, Y.; Kallel, A.; Romero, E.; Georgopoulos, I. Water Retention Properties of Perlite as a Material with Crushable Soft Particles. Eng. Geol. 2011, 122, 261–271. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-J.; Novak, A.; Yang, Y.; Wang, J. Role of Biochar in Improving Sandy Soil Water Retention and Resilience to Drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing Potential of Biochar for Increasing Water-Holding Capacity of Sandy Soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Yang, F.; Cen, R.; Feng, W.; Liu, J.; Qu, Z.; Miao, Q. Effects of Super-Absorbent Polymer on Soil Remediation and Crop Growth in Arid and Semi-Arid Areas. Sustainability 2020, 12, 7825. [Google Scholar] [CrossRef]
- Chehab, H.; Tekaya, M.; Mechri, B.; Jemai, A.; Guiaa, M.; Mahjoub, Z.; Boujnah, D.; Laamari, S.; Chihaoui, B.; Zakhama, H.; et al. Effect of the Super Absorbent Polymer Stockosorb® on Leaf Turgor Pressure, Tree Performance and Oil Quality of Olive Trees Cv. Chemlali Grown under Field Conditions in an Arid Region of Tunisia. Agr. Water Manag. 2017, 192, 221–231. [Google Scholar] [CrossRef]
- Takahashi, M.; Shibasaki, K.; Nakama, E.; Ishizuka, M.; Ohta, S. Application of superabsorbent polymers in forestry and revegetation fields. J. Jpn. For. Soc. 2018, 100, 229–236. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent Polymers in Agriculture and Other Applications: A Review. Polym.-Plast. Technol. Mater. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Sdiri, W.; Dabbou, S.; Nava, V.; Di Bella, G.; Ben Mansour, H. Pomological Descriptors, Phenolic Compounds, and Chemical Monitoring in Olive Fruits Irrigated with Dairy Treated Wastewater. Chemosensors 2021, 9, 130. [Google Scholar] [CrossRef]
- Sarvaš, M.; Pavlenda, P.; Takáčová, E. Effect of Hydrogel Application on Survival and Growth of Pine Seedlings in Reclamations. J. For. Sci. 2008, 53, 203–209. [Google Scholar] [CrossRef]
- Dabhi, R.; Bhatt, N.; Pandit, B. Effect on the Absorption Rate of Agricultural Super Absorbent Polymers under the Mixer of Soil and Different Quality of Irrigation Water. Int. J. Eng. Res. 2014, 3, 1402–1406. [Google Scholar]
- Nazarli, H.; Zardashti, M.R.; Darvishzadeh, R.; Najafi, S. The Effect of Water Stress and Polymer on Water Use Efficiency, Yield and Several Morphological Traits of Sunflower under Greenhouse Condition. Not. Sci. Biol. 2010, 2, 53–58. [Google Scholar] [CrossRef]
- Crous, J.W. Use of Hydrogels in the Planting of Industrial Wood Plantations. South. For. A J. For. Sci. 2017, 79, 197–213. [Google Scholar] [CrossRef]
- Pandey, P.P.; Sharma, R.; Neelkanthe, S.S. Climate Change: Cmbating Drought with Antitranspirants and Super Absorbent. Plant Arch. 2017, 17, 1146–1156. [Google Scholar]
- Skrzypczak, D.; Mikula, K.; Kossińska, N.; Widera, B.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. Biodegradable Hydrogel Materials for Water Storage in Agriculture—Review of Recent Research. Desalination Water Treat. 2020, 194, 324–332. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable Superabsorbent Hydrogel IncreasesWater Retention Properties of Growing Media and Plant Growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Kaliappa, S. Superabsorbent Polymers: A State-of-Art Review on Their Classification, Synthesis, Physicochemical Properties, and Applications. Rev. Chem. Eng. 2023, 39, 127–171. [Google Scholar] [CrossRef]
- Starkey, T.E.; Enebak, S.A.; South, D.B.; Cross, R.E. Particle Size and Composition of Polymer Root Gels Affect Loblolly Pine Seedling Survival. Nativ. Plants J. 2012, 13, 19–26. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Shainberg, I.; Goldstein, D.; Warrington, D.N.; Levy, G.J. Water Retention and Hydraulic Conductivity of Cross-Linked Polyacrylamides in Sandy Soils. Soil Sci. Soc. Am. J. 2007, 71, 406–412. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of Hydrogel Application on Soil Water Retention Characteristics. J. Plant Nutri. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Fallahi, H.-R.; Taherpour Kalantari, R.; Aghhavani-Shajari, M.; Soltanzadeh, M.-G. Effect of Super Absorbent Polymer and Irrigation Deficit on Water Use Efficiency, Growth and Yield of Cotton. Not. Sci. Biol. 2015, 7, 338–344. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Takahashi, M.; Shibasaki, K.; Nakama, E.; Ishizuka, M. Physical, chemical, and biological characteristics of soils amended with superabsorbent polymers. Jpn. J. For. Environ. 2020, 62, 51–59. [Google Scholar] [CrossRef]
- Sanz Gómez, J. Characterization and Effects of Cross-Linked Potassium Polyacrylate as Soil Amendment. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Sapin, 2015. [Google Scholar]
- Koupai, J.A.; Eslamian, S.S.; Kazemi, J.A. Enhancing the Available Water Content in Unsaturated Soil Zone Using Hydrogel, to Improve Plant Growth Indices. Ecohydrol. Hydrobiol. 2008, 8, 67–75. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-96189-7. [Google Scholar]
- Qadir, M.; Ghafoor, A.; Murtaza, G. Amelioration Strategies for Saline Soils: A Review. Land Degrad. Dev. 2000, 11, 501–521. [Google Scholar] [CrossRef]
- Banedjschafie, S.; Durner, W. Water Retention Properties of a Sandy Soil with Superabsorbent Polymers as Affected by Aging and Water Quality. J. Plant Nutr. Soil Sci. 2015, 178, 798–806. [Google Scholar] [CrossRef]
- Śpitalniak, M.; Lejcuś, K.; Dąbrowska, J.; Garlikowski, D.; Bogacz, A. The Influence of a Water Absorbing Geocomposite on Soil Water Retention and Soil Matric Potential. Water 2019, 11, 1731. [Google Scholar] [CrossRef]
- Adomako, J.T.; Nishide, T.; Senge, M. The Influence of the Polymers on Moisture Storage in Soils—The Effective Utilization and Persistence of High Water Absorbing Syntheic Polymers (I). Trans. Jpn. Soc. Irri. Drain. Rec. Eng. 1994, 1994, 79–87. [Google Scholar] [CrossRef]
- Ouchi, S.; Nishikawa, A.; Fujita, F. Soil-improving effect of a Super-absorbent polymer (Part 1) Total volume, three phase distribution, and available water of super-water-absorbent polymer mixed soils. Jpn. J. Soil Sci. Plant Nutr. 1989, 60, 15–21. [Google Scholar]
- Yabashi, S.; Amamiya, Y. Study on the changes of soil physical properties by adding water holding substances VIII In the cases of Kanto loam soils. Tec. Bull. Fac. Hort. Chiba Univ. 1993, 47, 195–202. [Google Scholar]
- Kargas, G.; Londra, P.A. Effect of Tillage Practices on the Hydraulic Properties of a Loamy Soil. Desalination Water Treat. 2015, 54, 2138–2146. [Google Scholar] [CrossRef]
- Misiewicz, J.; Lejcuś, K.; Dąbrowska, J.; Marczak, D. The Characteristics of Absorbency Under Load (AUL) for Superabsorbent and Soil Mixtures. Sci. Rep. 2019, 9, 18098. [Google Scholar] [CrossRef]
- Lejcuś, K.; Śpitalniak, M.; Dąbrowska, J. Swelling Behaviour of Superabsorbent Polymers for Soil Amendment under Different Loads. Polymers 2018, 10, 271. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Snoeck, D.; Schröfl, C.; De Belie, N.; Klemm, A.J.; Ichimiya, K.; Moon, J.; Wyrzykowski, M.; Lura, P.; Toropovs, N.; et al. Testing Superabsorbent Polymer (SAP) Sorption Properties Prior to Implementation in Concrete: Results of a RILEM Round-Robin Test. Mater. Struct. 2018, 51, 28. [Google Scholar] [CrossRef]
- Japanese Industrial Standard K 7223-1996; Testing Method for Water Absorption Capacity of Super Absorbent Polymers. Japan Standrds Association: Tokyo, Japan, 1996.
- Japanese Industrial Standard K 7224-1996; Testing Method for Absorption Rate of Super Absorbent Polymers. Japan Standrds Association: Tokyo, Japan, 1996.
- Kang, S.-H.; Hong, S.-G.; Moon, J. Absorption Kinetics of Superabsorbent Polymers (SAP) in Various Cement-Based Solutions. Cem. Concr. Res. 2017, 97, 73–83. [Google Scholar] [CrossRef]
- Yaseen, R.; Hegab, R.; Kenawey, M.; Eissa, D. Effect of Super Absorbent Polymer and Bio Fertilization on Maize Productivity and Soil Fertility under Drought Stress Conditions. Egypt. J. Soil Sci. 2020, 60, 377–395. [Google Scholar] [CrossRef]
- Pettygrove, S.G.; Asano, T. Irrigation with Reclaimed Municipal Wastewater—A Guidance Manual; Department of Land, Air & Water Resources, University of California, Davis: Sacramento, CA, USA, 1985. [Google Scholar]
- Australian and New Zealand Environment and Consevation Council. Australian Water Quality Guidelines for Fresh and Marine Waters 1992; Environment and Conservation Council: Canberra, New Zealand, 1992. [Google Scholar]
- Yu, J.; Shainberg, I.; Yan, Y.L.; Shi, J.G.; Levy, G.J.; Mamedov, A.I. Superabsorbents and Semiarid Soil Properties Affecting Water Absorption. Soil Sci. Soc. Am. J. 2011, 75, 2305–2313. [Google Scholar] [CrossRef]
- Chirino, E.; Vilagrosa, A.; Vallejo, V.R. Using Hydrogel and Clay to Improve the Water Status of Seedlings for Dryland Restoration. Plant Soil 2011, 344, 99–110. [Google Scholar] [CrossRef]
- Feng, W.; Gao, J.; Cen, R.; Yang, F.; He, Z.; Wu, J.; Miao, Q.; Liao, H. Effects of Polyacrylamide-Based Super Absorbent Polymer and Corn Straw Biochar on the Arid and Semi-Arid Salinized Soil. Agriculture 2020, 10, 519. [Google Scholar] [CrossRef]
- Orikiriza, L.J.B.; Agaba, H.; Eilu, G.; Kabasa, J.D.; Worbes, M.; Hüttermann, A. Effects of Hydrogels on Tree Seedling Performance in Temperate Soils before and after Water Stress. J. Environ. Prot. 2013, 04, 713–721. [Google Scholar] [CrossRef]
- Khodadadi Dehkordi, D. Effect of Superabsorbent Polymer on Soil and Plants on Steep Surfaces: Effect of Superabsorbent Polymer. Water Environ. J. 2018, 32, 158–163. [Google Scholar] [CrossRef]
- Hüttermann, A.; Zommorodi, M.; Reise, K. Addition of Hydrogels to Soil for Prolonging the Survival of Pinus Halepensis Seedlings Subjected to Drought. Soil Tillage. Res. 1999, 50, 295–304. [Google Scholar] [CrossRef]
- Bo, Z.; Renkuan, L.; Yunkai, L.; Tao, G.; Peiling, Y.; Ji, F.; Weimin, X.; Zhichao, Z. Water-Absorption Characteristics of Organic-Inorganic Composite Superabsorbent Polymers and Its Effect on Summer Maize Root Growth. J. Appl. Polym. Sci. 2012, 126, 423–435. [Google Scholar] [CrossRef]
- Bakass, M.; Bellat, J.P.; Mokhlisse, A.; Bertrand, G. The Adsorption of Water Vapor on Super Absorbent Product at Low Temperatures and Low Mass. J. Appl. Polym. Sci. 2006, 100, 1450–1456. [Google Scholar] [CrossRef]
- Savi, T.; Marin, M.; Boldrin, D.; Incerti, G.; Andri, S.; Nardini, A. Green Roofs for a Drier World: Effects of Hydrogel Amendment on Substrate and Plant Water Status. Sci. Total Environ. 2014, 490, 467–476. [Google Scholar] [CrossRef]
- Bai, W.; Song, J.; Zhang, H. Repeated Water Absorbency of Super-Absorbent Polymers in Agricultural Field Applications: A Simulation Study. Acta. Agr. Scand. Sect. B Soil Plant Sci. 2013, 63, 433–441. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hong, S.J.; Shin, W.S.; Kwon, Y.R.; Lim, S.H.; Kim, H.C.; Kim, J.S.; Kim, J.W.; Kim, D.H. Preparation of a Biodegradable Superabsorbent Polymer and Measurements of Changes in Absorption Properties Depending on the Type of Surface-crosslinker. Polym. Adv. Technol. 2020, 31, 273–283. [Google Scholar] [CrossRef]
- Okumura, T.; Duan, K.; Tanaka, K.; Nakayama, A. Application of water-absorbent polymer for seedling plantation in sandy region. J. Jpn. Soc. Reveg. Tech. 1990, 16, 16–18. [Google Scholar] [CrossRef]
| SAP | Polymer | Countercation | Bulk Density (Mg m−3) | Grain Diameter |
|---|---|---|---|---|
| SANFRESH GT-1 (GT-1) | Acrylic acid | nondisclosure | 0.36 | 150–500 µm |
| SANFRESH ST-500D (ST) | Acrylic acid | Na | 0.62 | 300–500 µm |
| A prototype product (PAAK) | Acrylic acid | K | 0.69 | 300–500 µm |
| AQUASORB™ 3005 K4 (AQS) | Acrylic acid + acrylamide | K | 0.75 | 1.7–2.8 mm |
| Experiment | Dependent Variable | Independent Variable | Material | Solution | Method |
|---|---|---|---|---|---|
| 1 | SAP water content | SAP types, solution types, time | SAP | Pure water, 10 mM KCl, 5 mM CaCl2 | Teabag |
| 2 | SAP water content | SAP type, types and concentrations of salt solution | SAP | 10 mM KCl, 20 mM KCl, 5 mM CaCl2, 10 mM CaCl2, tap water | Teabag |
| 3 | Soil water content | SAP types, salt types, time | Soil–SAP mixture | Tap water, 10 mM KCl, 5 mM CaCl2 | Teabag |
| 4 | Soil water content | SAP application rates | Soil–SAP (GT-1) mixture | Tap water | Teabag |
| 5 | Water release rate, time to desiccation | Soil water content | Soil–SAP mixture | Teabag | |
| 6 | Soil water content | SAP types, water potential | Soil–SAP mixture | Tap water | Sand column, pressure chamber |
| 7 | Soil water content | SAP types, water contents of SAP gels, water potential | Soil–gel SAP mixture | Tap water | Sand column, pressure chamber |
| 8 | Soil water content | SAP application rates, water potential | Soil–SAP (GT-1) mixture | Tap water | Sand column, pressure chamber |
| SAP | Salt Solution | MWHC in Salt Solution (g g−1) | MWHC in Tap Water (g g−1) |
|---|---|---|---|
| GT-1 | 10 mM KCl | 105.0 (1.8) ab | 187.2 (4.5) ab |
| ST | 105.6 (1.3) a | 177.3 (1.4) ab | |
| PAAK | 99.7 (1.2) b | 174.6 (3.1) b | |
| AQS | 75.6 (0.8) c | 137.1 (3.3) c | |
| GT-1 | 20 mM KCl | 82.7 (0.8) a | 170.8 (4.4) ab |
| ST | 85.6 (1.2) a | 163.3 (1.7) b | |
| PAAK | 78.1 (1.2) b | 157.2 (1.8) b | |
| AQS | 56.2 (0.8) c | 121.2 (1.3) c | |
| GT-1 | 5 mM CaCl2 | 32.3 (1.6) b | 129.0 (5.0) a |
| ST | 26.6 (3.9) b | 73.3 (0.4) b | |
| PAAK | 24.6 (4.0) b | 62.9 (0.3) b | |
| AQS | 45.7 (0.8) a | 127.2 (0.7) a | |
| GT-1 | 10 mM CaCl2 | 7.2 (1.0) b | 8.4 (1.0) b |
| ST | 6.5 (1.1) b | 4.5 (0.3) c | |
| PAAK | 5.6 (1.0) b | 3.8 (0.1) c | |
| AQS | 25.0 (2.7) a | 81.6 (1.3) a |
| SAP | Soil Water Content (g g−1) | SAP Water Content (g g−1) | Time to Desiccation (h) | Evaporation Rate (g g−1 h−1) | |
|---|---|---|---|---|---|
| 3 h | 24 h | 24 h | |||
| GT-1 | 0.751 (0.018) a | 0.665 (0.067) a | 325 (19) | 28.7 (0.7) a | 0.0418 (0.0043) |
| ST | 0.755 (0.012) a | 0.671 (0.051) a | 330 (15) | 30.0 (1.2) a | 0.0378 (0.0060) |
| PAAK | 0.740 (0.012) a | 0.650 (0.041) a | 322 (12) | 26.7 (1.8) a | 0.0475 (0.0078) |
| AQS | 0.566 (0.017) b | 0.593 (0.018) a | 293 (5) | 28.0 (1.2) a | 0.0503 (0.0044) |
| no SAP | 0.308 (0.012) c | 0.299 (0.006) b | No data | 11.3 (2.2) b | 0.0388 (0.0106) |
| Salt Solution | SAP | Soil Water Content (g g−1) | SAP Water Content (g g−1) |
|---|---|---|---|
| 10 mM-KCl | GT-1 | 0.549 (0.013) a | 274 (8) a |
| ST | 0.540 (0.013) a | 276 (7) a | |
| PAAK | 0.562 (0.016) a | 282 (8) a | |
| AQS | 0.467 (0.016) b | 238 (8) b | |
| 5 mM-CaCl2 | GT-1 | 0.307 (0.012) | 151 (6) |
| ST | 0.320 (0.017) | 157 (9) | |
| PAAK | 0.322 (0.021) | 158 (10) | |
| AQS | 0.345 (0.015) | 172 (8) |
| SAP | −0.98 to −3.9 kPa | −3.92 to −98.1 kPa | <−98.1 kPa | Total |
|---|---|---|---|---|
| GT-1 | 0.345 (0.051) ab [63.5%] | 0.073 (0.039) [13.4%] | 0.125 (0.004) a [23.0%] | 0.543 (0.001) bc [100%] |
| ST | 0.375 (0.000) a [66.7%] | 0.060 (0.021) [10.7%] | 0.127 (0.026) a [22.6%] | 0.562 (0.004) ab [100%] |
| PAAK | 0.362 (0.003) a [62.0%] | 0.073 (0.031) [12.5%] | 0.149 (0.036) a [25.5%] | 0.584 (0.002) a [100%] |
| AQS | 0.298 (0.000) b [58.2%] | 0.032 (0.006) [6.3%] | 0.182 (0.007) a [35.5%] | 0.512 (0.001) c [100%] |
| No SAP | 0.192 (0.036) c [85.4%] | 0.032 (0.019) [14.2%] | 0.001 (0.000) b [0.4%] | 0.225 (0.015) d [100%] |
| SAP Rate % | −0.98 to −3.92 kPa | −3.92 to −98.1 kPa | <−98.1 kPa– | Total |
|---|---|---|---|---|
| 0 | 0.221 [89.7%] | 0.017 [7.1%] | 0.007 [3.0%] | 0.246 |
| 0.1 | 0.307 [75.4%] | 0.056 [13.7%] | 0.045 [10.9%] | 0.408 |
| 0.2 | 0.345 [63.5%] | 0.073 [13.5%] | 0.125 [23.0%] | 0.544 |
| 0.4 | 0.410 [49.2%] | 0.206 [24.8%] | 0.217 [26.1%] | 0.833 |
| 0.8 | 0.805 [48.3%] | 0.485 [29.1%] | 0.375 [22.5%] | 1.664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, M.; Kosaka, I.; Ohta, S. Water Retention Characteristics of Superabsorbent Polymers (SAPs) Used as Soil Amendments. Soil Syst. 2023, 7, 58. https://doi.org/10.3390/soilsystems7020058
Takahashi M, Kosaka I, Ohta S. Water Retention Characteristics of Superabsorbent Polymers (SAPs) Used as Soil Amendments. Soil Systems. 2023; 7(2):58. https://doi.org/10.3390/soilsystems7020058
Chicago/Turabian StyleTakahashi, Masamichi, Izumi Kosaka, and Seiichi Ohta. 2023. "Water Retention Characteristics of Superabsorbent Polymers (SAPs) Used as Soil Amendments" Soil Systems 7, no. 2: 58. https://doi.org/10.3390/soilsystems7020058
APA StyleTakahashi, M., Kosaka, I., & Ohta, S. (2023). Water Retention Characteristics of Superabsorbent Polymers (SAPs) Used as Soil Amendments. Soil Systems, 7(2), 58. https://doi.org/10.3390/soilsystems7020058







