Abstract
The agricultural soils of West Limerick, Ireland, contain very localised, extremely high natural Se concentrations that reach levels that are very toxic to grazing livestock. The Carboniferous shales that formed in anoxic deep-water marine environments are the source of the selenium, which, along with the other redox-sensitive elements of molybdenum, uranium, arsenic and vanadium, were mobilised and reprecipitated in post-glacial anoxic marshes. The result has been a history of selenosis and molybdenosis in livestock in this important dairy province. Soils collected at 10–20 cm from five different agricultural sites were analysed, and all yielded concentrations greatly in excess of the safe Se limits of 3–10 mg kg−1; the highest value recorded was 1265.8 mg kg−1 Se. The highest recorded value for Mo in these soils was 1627.5 mg kg−1, and for U, 658.8 mg kg−1. There was a positive correlation between Se, Mo U and organic matter in the soils. Analysis of non-accumulator pasture grasses (Lolium perenne (perennial ryegrass), Festuca arundinacea (tall fescue), Dactylis glomerata (cocksfoot) and Phleum pretense (timothy grass)) revealed the shoot/leaf to contain up to 78.05 mg kg−1 Se while Trifolium repens (white clover) leaves contained 296.15 mg kg−1 Se. An in situ growing experiment using the Se accumulator species Brassica oleracea revealed 971.2 mg kg−1 Se in the leaves of premier kale, which also contained 1000.4 mg kg−1 Mo. Translocation factors (TFs) were generally higher for Mo than Se across all plant species. Combined X-ray absorption near edge spectroscopy (XANES) with micro-X-ray fluorescence (μ-XRF) showed the Se was present in the soil predominantly as the reduced immobile phase, elemental Se (Se0), but also as bioavailable organoselenium species, mainly selenomethionine (SeMet). SeMet was also the main species identified within both the Se non-accumulator and Se accumulator plants. The Se soil–plant system in West Limerick is dominated by SeMet, and uptake into the cattle pasture results in selenosis in the grazing dairy herds. The hyperaccumulating Brassica oleracea species could be used to extract both the Se and Mo to reduce the toxicity of the blighted fields.
1. Introduction
Selenium uptake and accumulation in plants is important to human and animal health as plants are the main dietary source of this vital micronutrient. Whereas selenium deficiency is a widespread problem, Se toxicity is a localised but serious issue in certain areas of the world [1]. The uptake and bioavailability of selenium in plants are determined by the concentration and selenium speciation in soil and water and controlled by physico-chemical conditions [2,3,4,5]. Selenium is not essential to plants but is assimilated into a number of organoselenium compounds in a similar manner to the sulfur uptake that increases plant growth and antioxidant activity [6]. The translocation and distribution of selenium into different parts of the plant are influenced by the species of selenium in the soil [7]. When plants accumulate selenium, this can lead to human poisoning, resulting in acute selenium toxicity, characterised by loss of sight, paralysis, respiratory failure and death [8]. In livestock, chronic selenium toxicity leads to alkali disease, which is characterised by hair loss and the deformation of hooves and bones. Anthropogenic activity is a major cause of selenium toxicity, with mining-derived selenium causing a major poisoning in the Ensi District of China [4,9], whereas agricultural practices resulted in the catastrophic selenium poisoning of the Kesterson Reservoir in California [10]. Natural toxicity is often associated with selenium derived from mudstone/shale formations, such as in the Punjab area of India and western USA, although irrigation methods greatly enhanced the problem [11,12,13]. Naturally occurring selenium toxicity has been recognised in the soils of Ireland, associated with Carboniferous mudstone and shale [14,15,16,17,18]. In particular, high selenium and molybdenum have been identified in West Limerick, where there is a historical record of both selenium and molybdenum toxicity in cattle [14,15,16,17,18]; there is also a record of high uranium in the soils of the region [19]. This region is a major producer of dairy products (‘The Golden Vale’), and the local dairy farmers are well aware of the localised fields where selenium poisoning occurs; however, detailed analyses of the soils and plants of the region have not been undertaken.
Much of the extensive research on selenium in the environment has focused on high redox potential, alkaline systems where Se-oxyanions prevail [6,20,21,22], especially using controlled, experimental conditions [23]. The current study focuses on the uptake and speciation of selenium in the ‘natural’ system found in these temperate livestock grasslands of western Ireland. The extreme naturally derived concentration of selenium in these soils allows the determination of selenium bioavailability in the pasture and the potential of Se accumulator plants (Brassica sp.) in the phytoremediation of these soils [24,25]. The concentrations of molybdenum and uranium in these Irish soils are also extremely high in places; data for these elements, as well as arsenic and vanadium, are also presented here.
As with selenium, molybdenum is an essential micronutrient for plants and higher life forms. It is also extensively documented that elevated molybdenum values in soils result in high concentrations in herbage, leading to molybdenosis in livestock. Uptake of molybdenum from soil is via molybdate, MoO42−, and concentrations of 5 mg kg−1 molybdenum in herbage can prove fatal in cattle due to copper deficiency resulting from the development of non-absorbable copper thiomolybdate complexes in the rumen [26,27,28,29,30]. Background values for soil worldwide are 0.5–5 mg kg−1, but Irish soils are known to have elevated values (6.5 mg kg−1) associated with the Carboniferous mudstones, shales and limestones [27]; values of 2 to 25 mg kg−1 were recorded in herbage in Irish soils, where molybdenosis is a recorded problem [30]. The use of copper supplements is often employed to deal with molybdenosis [31], although phytoremediation strategies to deplete excess molybdenum in soils have been explored and the use of soil amendments have been successfully demonstrated [32,33].
Although it is not a focus of this work, we recorded high uranium values in the soils, and these data are also presented here. High accumulations of uranium can damage plant growth and enable uptake by livestock. Global average uranium values in soil are in the range of 1–4 mg/kg−1, but the accepted toxicity level is contentious, with values of 5 to 1000 mg/kg−1 being quoted [34]; the bioavailability of uranium is highly dependent on its speciation in soils, and this is, in turn, is dependent on a range of factors, namely, redox, pH, phosphate content, soil organic matter (SOM) and microbial populations [35,36].
Arsenic is a redox-sensitive element commanding great interest in toxicological investigations in soil and water (see [37] and references therein). We provide data on this element, but the data were relatively unremarkable and, therefore, are little discussed in this work.
2. Methodology
2.1. Field Sampling
Soils in pastures grazed by livestock were sampled from five Se-enriched sites (FF, MO, TL, TJ, PK; Figure 1) that were identified by the Irish Agricultural Food Development Authority, Teagasc, combined with information from previous studies [15,17,38] and local farmers; sites FF and MO are in adjacent fields separated by a small ditch. Further information on the sites is provided in Appendix A, Figure A1, Figure A2, Figure A3 and Figure A4. The sites are all relatively low-lying grassland in gentle topography (a maximum of 20 m elevation changes), with organic-rich soils overlying Carboniferous limestones, mudstones and shales. Soil samples (500 g) were collected over the sites from 10–20 cm depth for bulk chemical analysis, and soil cores were also collected from sampling locations TL and FF to determine the changes in Se concentration with depth. A first suite of samples analysed informed further sampling and the choice of coring locations (see Table A4 for sample site locations of the analyses presented herein). The soil cores were sampled using a Van Walt corer with 4.4 cm stainless steel diameter cylinders; a complete core to bedrock (approximately 50 cm) was recovered and divided into 10 cm sections, which were used for bulk chemical analysis. An adjacent core was collected and stored under oxygen-free nitrogen at the site to maintain an anaerobic environment suitable for speciation and microbial analysis. The cores were tightly capped and stored at 4 °C to attenuate microbial activity.
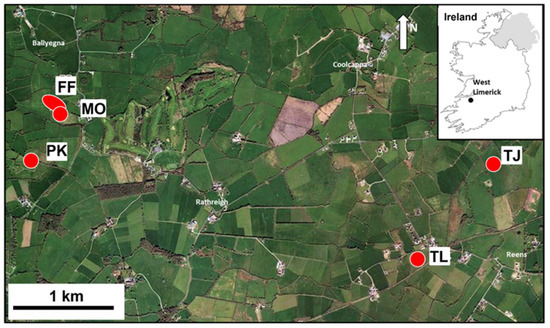
Figure 1.
Map of part of West Limerick, Ireland, showing the patchwork of dairy livestock fields in this part of Ireland’s ‘Golden Vale’. Sampling locations are shown (FF, MO, PK, TL and TJ). Satellite image, Google Earth Pro, ©Google. Image© Maxar Technologies. See Figure A1, Figure A2, Figure A3 and Figure A4.
The pasture plants grazed by the livestock were also sampled from the 5 sites (Figure A1, Figure A2, Figure A3, Figure A4 and Figure A6), where the vegetation consisted of species of perennial grasses, namely, Lolium perenne (perennial ryegrass), Festuca arundinacea (tall fescue), Dactylis glomerata (cocksfoot) and Phleum pretense (timothy grass) as well as Trifolium repens (white clover). The sampling of plant material was undertaken at the locations identified in the soil analysis as high in selenium. An in situ growing experiment using three varieties of the Se hyperaccumulator species Brassica oleracea (red flare cabbage, hispi cabbage and premier kale) [39,40] was conducted in a plot adjacent to sites FF/MO. This plot had a soil concentration of 276 mg kg−1 Se (and 91 mg kg−1 Mo, 23 mg kg−1 U; Table A1), and the Brassica sp. were grown in an area cleared of grass for 11 weeks (early May–late July) under natural conditions. To minimise stress, all the plant species analysed in this investigation were collected by digging them out, complete with soil, and placing the whole unit in 20 × 20 × 20 cm pots (see Figure A7 and Figure A8). These were transported to an experimental greenhouse and watered with local (Limerick) rainwater; sampling was undertaken within 2 days.
2.2. Soil Analysis
In preparation for chemical analysis, the 10 cm sections of core and the 500 g soil samples collected at 10–20 cm were dried at 50 °C. Rock fragments and roots were removed by sieving (0.1 mm) before the samples were powdered (<50 μm). Wax-mounted pellets were prepared using 12 g of sample and 3 g of wax pressed under a 10 t load. The pellets were analysed by X-ray fluorescence (XRF) using a PANanalytical Axios sequential X-ray fluorescence spectrometer with Omnian® and Pro-trace® standardisation. Then, 1 g of soil was separated for loss on ignition (LOI) by measuring the weight loss of the soil at 110 and 1000 °C to calculate the moisture loss (H2O wt%) and carbon dioxide (CO2 wt%) released by heating. Another 1 g was used to calculate the total organic carbon (TOC) content of the soils by measuring the weight loss of the soil at 110 and 400 °C and applying a correction factor assuming 58% of organic matter contains organic carbon [41]. The Se, Mo, U and As concentrations measured at 10–20 cm depth were plotted using Golden LLC Surfer® V15 software to provide a visual indication of the localised nature of their enrichment. The selenium concentrations derived from XRF analysis of the 10–20 cm soil samples were used to determine the plant and core sampling locations. Eh and pH values for the soils were taken in both the field and laboratory using deionised water mixed with an equal volume of soil using a Denver Instrument UB-10 pH/mV benchtop meter calibrated in a redox buffer solution of pH 7 and Eh 220 mV. X-ray diffraction analysis using a Bruker D8 Advance diffractometer was used to determine the crystalline phases in the soils.
2.3. Plant Chemical Analysis
As the grass pasture species were intermingled, they were not separated but divided into shoot (blade) and root material. White clover (from site TJ) and the Brassica oleracea varieties were separated into leaf, stem and root material. Once separated, the plant material was rinsed in deionised water to remove any soil particles. To determine the bulk elemental concentrations, the plant material was frozen at −80 °C and freeze-dried. Then, 0.1–0.2 g of powdered plant material was added to a PFA microwave vessel, and 6 mL nitric acid (HNO3) and 3 mL hydrogen peroxide were added to digest the material overnight; 1 mL of hydrofluoric acid was added to the vessels, and after 1 h, they were sealed and digested at 170 °C in a MARS microwave digestion unit for a total of 55 min at 800 W (12 samples per batch). Once the vessels depressurised, the acidified sample was poured into PTFE beakers and heated on a hot plate until the HF evaporated. The residue was dissolved in 2% ultra-pure HNO3 and reheated to incipient dryness, and the residue washed and diluted to 50 mL with 2% ultra-pure HNO3. In each batch, samples were digested in triplicate with standard reference material CRM 402 (a white clover with Se 6.7 +/− 0.25 mg kg−1), with an average recovery of 6.73 mg kg−1 and all blanks measuring <0.1 mg kg−1 Se. Then, 10 mL was filtered (>0.45 µm) and analysed for selenium (82Se), molybdenum (95Mo), arsenic (75As), uranium (238U), vanadium (51V) and iron (56Fe) using the inductively coupled plasma mass spectrometer (ICP-MS) Agilent 7500cx equipped with a pressurised octopole collision/reaction cell.
The translocation factor (TF) was calculated for the plant samples analysed as this demonstrates the plant’s ability to translocate the trace elements from roots through to the shoots or leaves of the plant (TF = Cshoot/Croot) [42] as well as revealing phytoextraction capabilities and bioavailability. Cshoot in the white clover and Brassica oleracea was subdivided into leaf and stem material and the TF calculated separately; in the grass species, the bulk shoot/leaf concentration was used. The bioaccumulation factor (BAF) was also calculated for the grass species; this is the ratio of the trace element concentration in the plant material to that in the soil (BAF = Cplant/Csoil), which is used to estimate a plant’s ability to accumulate the trace elements from soil [43]. Cplant was subdivided into shoot and root material and the BAF calculated separately.
2.4. Synchrotron Analysis
X-ray absorption spectroscopy (XAS) analysis of the Se K-edge (12.654 keV) was undertaken at Diamond Light Source, Didcot, UK, to determine the selenium species and distribution in soil and plant material from seleniferous sites TL-1 and FF-2. For XAS analysis, 1 cm sub-sections of fresh leaf, stem and root material were flash-frozen in isopentane that was cooled in a container of liquid nitrogen to achieve a temperature of −150 °C, which reduces/avoids ice crystal formation and sample damage. Sub-samples of soil were collected from the highly seleniferous horizons of two soil cores (at sites TL-C1 and FF-C2) and, in an anaerobic cabinet, sealed in 1 mL Eppendorfs. The soil samples were centrifuged at 6000 rpm for 15 min to remove pore fluid and make a robust sample for cryo-sectioning. These were flash-frozen in liquid nitrogen. All XAS samples were kept anaerobic and frozen, set in an optimum cutting compound (OCT) matrix to avoid element redistribution [44] and cryo-sectioned in a Leica CM3050 S Cryostat microtome at −20 °C to a thickness of 30–50 μm for plant material and 100 μm for soils; the latter was the thinnest possible, with samples sieved to 50 μm. OCT is a water-soluble mix of glycols (polyvinyl alcohol, polyethylene glycol), resins and non-reactive ingredients. The sections were placed between two pieces of Kapton® tape and transported in dry ice (−78.5 °C) to the I18 Microfocus Spectroscopy Beamline at Diamond Light Source [45].
The samples were cryo-fixed onto the sample holder in liquid nitrogen vapour and mounted on an x-y-z stage inclined at 45° to the incident beam in a liquid helium cryostat and cooled to 20 K to prevent in situ beam damage reduction or oxidation. The data were acquired using a Si (III) monochromator, and energy calibration was achieved using Au foil (11.919 keV); the XAS data were collected over the range of 12.55 to 13.30 keV. A range of powdered Se standards was measured in the transmission mode; the standards chosen provided the range of coordination environments and Se-species expected in seleniferous soils and plants. These were sodium selenide (Na2Se, Se-II), selenocystine (SeCys2-C6H12N2O4Se2, Se-II), selenomethionine (SeMet-C5H11NO2Se, Se-II), elemental selenium (Se0), sodium selenite (NaSeO3, SeIV) and sodium selenate (NaSeO4, SeVI).
Synchrotron μ-XRF maps of the samples were acquired with continuous scanning using two (one 6- and one 4-element) Si drift detectors with an incident beam energy of 13.0 keV and a fixed beam size of 5 μm, analyses every 10 μm and a 0.33 s acquisition time. Points of interest (POIs) displaying both high Se counts and representative concentrations of Se were selected for detailed X-ray absorption near edge structure (XANES) analysis for species identification using a step scanning mode and 1 s per point. Spectra from a range of points were screened to ensure the presented data were representative of the selenium species present. Two XANES scans were collected during the analysis. Even at liquid helium temperatures, the possibility of beam damage was a concern and was continually monitored. Tests showed no changes in each point in the first two scans presented here.
The μ-XRF spectra were processed into elemental maps by windowing the Se Kα peak and using PyMCA 5.2.2 software [45]. The XANES spectra were normalised using the same optimal energy range for fitting the pre- and post-edge lines for all the spectra and processed using ATHENA 0.9.26 software. Linear combination fitting (LCF) was performed over the energy range −20 to +50 eV around the Se K-edge against 4–6 standard spectra, providing a goodness of fit (R) and a percentage of the main species present [46,47,48].
3. Results
3.1. Soil Characterisation
The trace and major element compositions of the soils sampled at 10–20 cm are presented in Table A1, Table A2 and Table A3, and the Se, Mo, U, As and V concentrations are summarised in Table 1. The selenium distribution at all sites was characterised by zones of very high concentration surrounded by areas of very low values (Figure 2 and Figure 3). The highest selenium concentration of 1265.8 mg kg−1 was observed in a sample at site MO (Table 1). The remaining four sites have maximum selenium concentrations ranging from 28.4 to 682 mg kg−1 (Table 1). Sites FF and MO are in adjacent fields (Figure 1) and contain particularly high concentrations of Se, Mo, U and As (Table 1; Figure 3). In general, the molybdenum concentrations have a similar distribution to that of selenium and are highest at site FF, with one sample containing 1627.5 mg kg−1 Mo. Uranium concentrations are also high at sites FF and MO, with a maximum value of 599.5 mg kg−1 at site FF and 407 mg kg−1 at site MO. The arsenic concentrations ranged from 0.0–99.8 mg kg−1 at site FF and 0.0–115.3 mg kg−1 at site MO. The vanadium concentrations for all sites have a low range of 175.7–195.2 mg kg−1. pH values of the soils were in a narrow range, from 6.0 to 6.5 and Eh 126 to −90 mV.

Table 1.
The maximum and minimum concentrations of key elements derived from bulk samples collected at 10–20 cm depth from five agricultural sites in West Limerick, determined by XRF.
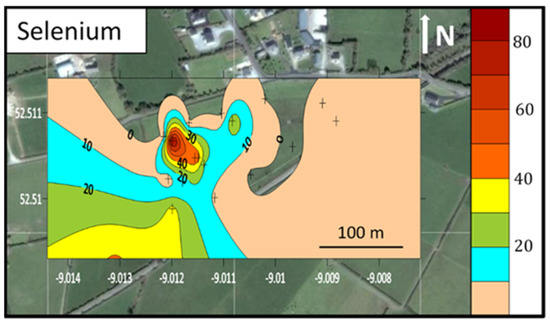
Figure 2.
Map showing the spatial distribution of selenium in soil at 10–20 cm at site TL, produced using Golden LLC Surfer® V15 software and superimposed on a satellite image of Clonaugh, West Limerick (52°30′38″ N, 9°0′43″ W) (Google Earth Pro, ©Google. Image© Maxar Technologies, using Kriging griding). Sample site marked with a+, scale in ppm, axes in longitude and latitude.
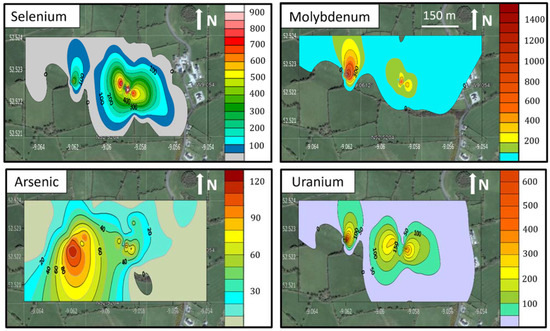
Figure 3.
Maps showing the spatial distribution of selenium, molybdenum, arsenic and uranium concentrations in soils at 10–20 cm at site FF, produced using Golden LLC Surfer® V15 software and superimposed on a Google Earth image of Ballyegny Beg, West Limerick (52°31′22″ N; 9°3′32″ W) (Google Earth Pro, ©Google. Image© Maxar Technologies, using Kriging griding). Values in ppm, axes longitude/latitude.
3.2. Soil Core Bulk Analysis
The variation in the trace and major elemental composition with depth was examined by analysing a complete soil core from two of the sites, FF and TL, selected from the selenium values in the 10–20 cm data. At both sites, the brown organic soil profiles were developed over a thick impermeable, grey clay encountered at a depth of 30–50 cm; the bedrock beneath was not reached. The results are shown in Table 2, and the Se values were plotted against TOC in Figure 4. The highest Se concentrations in both soil cores were at a depth horizon of 30–40 cm, with 171 mg kg−1 in core TL-C1 and 926 mg kg−1 in core FF-C2.

Table 2.
XRF data showing the concentration of selected trace and major elements for two soil cores, TL-C1 and FF-C2. R2 = the correlation with selenium. TOC = total organic carbon.
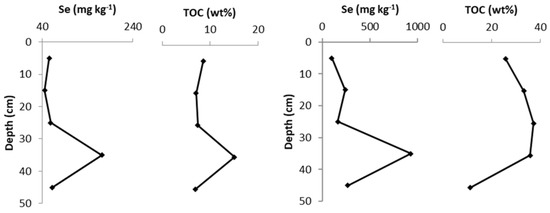
Figure 4.
Se and total organic carbon (TOC) values measured in vertical cores at site TL-C1 (left) and site FF-C2 (right).
In core FF-C2, the most seleniferous horizon also contained 191.2 mg kg−1 Mo, 94.2 mg kg−1 As and 354.9 mg kg−1 U. However, only molybdenum had a positive correlation (R2 value of 0.52) with selenium through the core profile (Figure 4). The highest uranium concentration of 658.8 mg kg−1 seen in this study was observed at a depth of 20–30 cm in core FF-C2.
In core TL-C1, the most seleniferous horizon contained the highest concentrations of arsenic (54.6 mg kg−1) seen in this profile, with a highest uranium concentration of 13 mg kg−1 but only 2.4 mg kg−1 molybdenum. These elements had high positive correlations with selenium throughout the soil profile, with an R2 value of 0.93 for uranium, 0.97 for molybdenum and 0.90 for arsenic. Sub-samples from the most seleniferous horizon of both soil cores were selected for further analysis by XAS to determine the selenium speciation (see Section 3.5).
The XRF data revealed a high SiO2 content in the top 20 cm of the profile, and this is indicative of relatively recent sediment input to the sites. CaO increases downwards towards the clay layer. A clear positive correlation of TOC with selenium is apparent at both sites (TL-C1 R2 = 0.96, and the highest TOC in FF-C2 of 37.3 wt% at 30–40 cm coincides with the maximum selenium; Figure 4). XRD analysis showed that the dominant crystalline mineral phase in both cores was quartz. The XRD analysis also showed that site TL-C1 contained minor amounts of muscovite and clinochlore, whereas FF-C2 had minor amounts of dolomite, K-feldspar, muscovite and calcite.
3.3. Se, Mo, As U and V in Grass and White Clover (Non-Accumulators)
The concentrations of Se, along with those of Mo, U and As, determined from grass shoot and root material from the five sites, are presented in Table 3. In addition, at site TL, white clover was separated into leaf, stem and root material for analysis.

Table 3.
The concentration of Se, Mo, As, U and V in selenium ‘non-accumulator’ clover and grass from pasture in West Limerick. TF = the translocation factor, a value >1 indicates the plant can translocate the element effectively from the root to shoots, stem and leaves. Analysis by ICP-MS, all experiments performed in triplicate ± the standard error. Nearest soil sample sites are in brackets.
Selenium is most concentrated in the root material of all grass samples, with the exception of one sample, FF-P2. Despite the concentration of Se in the soils at site TL being lower than those at FF, MO, and PK, the highest Se concentrations in grasses are in the two samples from this site. Sample TL-P2 contained 112.2 mg kg−1 Se in the roots and 78.1 mg kg−1 in the shoots, whereas in adjacent site TL-P1, the shoots contained 67.9 mg kg−1 in the roots and 44.9 mg kg−1 in the shoots. The lowest Se in plant material is from site PK, with sample PK-P2 containing 4.28 mg kg−1 in the roots and 1.32 mg kg−1 in the shoots; in the four 10–20 cm soil samples taken from site PK, one sample contained 682 mg kg−1 Se, but the other three had <5.4 mg kg−1 Se.
In all the grass samples, Mo was most concentrated in the shoot material compared to the root material, contrasting with Se. The highest Mo concentration was in the grass shoots of sample FF-P2 with 79.6 mg kg−1 that also contained 15.02 mg kg−1 in the roots. In general, uranium had a similar distribution to selenium and was more concentrated in the grass roots compared to the shoots, with the exception of sample FF-P2. The highest uranium concentration was in sample TJ-P1, with 6.18 mg kg−1 in the roots but it contained only 0.47 mg kg−1 in the shoots. In all grass samples, arsenic and vanadium were most concentrated in the root material compared to the shoots. The highest arsenic concentration was in sample FF-P1, with 179.67 mg kg−1 in the roots but the shoots contained only 0.30 mg kg−1 As. It was noted that the highest vanadium concentration was in sample PK-P1, with 132.91 mg kg−1 in the roots and 1.84 mg kg−1 in the shoots.
The ICP-MS data in Table 3 reveal that the highest selenium concentrations in the non-accumulator plants are in the white clover from TL-P1, with leaves containing 296.2 mg kg−1 Se, the stems 249.5 mg kg−1 Se and the roots 150.4 mg kg−1 Se. These values were significantly higher than the coexisting grasses. However, arsenic, uranium and vanadium concentrations were <1 mg kg−1 in the clover leaf and stem materials and <5 mg kg−1 in the root material.
The TFs for Se, Mo, U, As and V are presented in Table 3. The TF values for Se in grass samples were <1, except for location FF-P2, which had a value of 1.29. The highest TFs for selenium were found in the white clover sample from site TL-P1 (Table 3), where the translocation from root to leaf was 1.97, and from root to stem it was 1.66. In all five sites, the TFs for molybdenum were greater than those for selenium, with the highest value of 5.30 in site FF-P2. The TFs for uranium were <0.5, with the exception of locations FF-P2 (1.07) and MO-P1 (0.92). The TFs for arsenic and vanadium did not exceed 0.26 and 0.56, respectively. Samples of soil and plant material from TL-P1 and FF-P2 were selected for further analysis by XAS (below).
3.4. Se, Mo, U, As and V in Brassica (Se-Accumulators)
The concentrations of Se, Mo, U, As and V determined by ICP-MS in three Brassica oleracea varieties (premier kale, hispi cabbage and red flare cabbage), grown in a 4 × 3 m experimental plot adjacent to site FF, are shown in Table 4. In all three Brassica varieties, selenium was most concentrated in the leaves, followed by the roots, then stems. The selenium concentration in the leaves ranged from 971.7 mg kg1 in the premier kale to 787.8 mg kg−1 in the red flare cabbage. The concentration in the roots ranged from 643 mg kg−1 in the hispi cabbage to 390 mg kg−1 in the red flare cabbage, and for the stems, from 539.9 mg kg−1 in the hispi cabbage to 336.2 mg kg−1 in the red flare cabbage. In all three Brassica varieties, molybdenum was most concentrated in the leaves, followed by the root material and then stem material, similar to selenium; the Mo concentration in the leaves ranged from 1000.4 mg kg−1 in the premier kale to 271.6 mg kg−1 in the red flare cabbage. As with the non-accumulator plants, the uptake of arsenic, uranium and vanadium was low with the highest concentrations of arsenic and uranium in the roots of the hispi cabbage of 0.76 mg kg−1 and 9.38 mg kg−1 respectively; for vanadium, it was 1.11 mg kg−1 in the roots of premier kale. Premier kale proved the best accumulator of both selenium and molybdenum, demonstrating that the choice of plant variety is important in phytoextraction.

Table 4.
The mean concentration of Se, Mo, As, U and V in selenium accumulator Brassica oleracea varieties from the experimental plot in a pasture in West Limerick. TF = the translocation factor; a value >1 indicates the plant can translocate the element effectively from the root to shoots, stem and leaves. Analysis by ICP-MS, all experiments performed in triplicate ± the standard error.
The TFs from the root to leaf ranged from 1.29–2.02 for selenium, and as with the non-accumulator species, the translocation of molybdenum was more efficient than selenium, with a TF from root to leaf of 1.45–3.24. The TFs for arsenic, uranium and vanadium were all below 1. These positive TFs for selenium and molybdenum are a further indication that Brassica oleracea will be effective in phytoextraction. Samples were selected from the premier kale and hispi cabbage for further analysis by XAS to determine the distribution of selenium and the selenium species present in the plants.
3.5. μ-XRF and XAS
Cryo-sectioned samples of selenium non-accumulator plants (tall fescue grass and white clover), Se accumulator plants (premier kale and hispi cabbage) and seleniferous soil from site TL-C1 and site FF-C2 at 30–40 cm depth were selected for XRF and speciation analysis using µ-XAS. Spectra of the Se standards (see above) were produced (Figure 5). A series of µ-XRF maps were then collected (Figure 6, Figure 7, Figure 8, Figure 9 and Figure A6), revealing the selenium distribution, and from these XANES spectra were collected from representative points of interest (POIs) and compared to the Se standards. The Se K-edge energies for reference standards, based on the maximum of the first derivative of XANES spectra (e0), were 12665.0 eV for sodium selenate (NaSeO4, SeVI), 12662 eV for sodium selenite (NaSeO3, SeIV), 12658 eV for selenomethionine (C5H11NO2Se, Se-II), 12657 eV for red elemental selenium (Se0), 12657 eV for sodium selenide (Na2Se, Se-II) and 12656 eV for selenocystine (C6H12N2O4Se2, Se-II). Table 5, Table 6, Table 7 and Table 8 show the results of the LCF of selenium species in plant and soil samples, undertaken on merged duplicates of the normalised XANES spectra using ATHENA 0.9.26 software. These fits are illustrated in Figure A5.
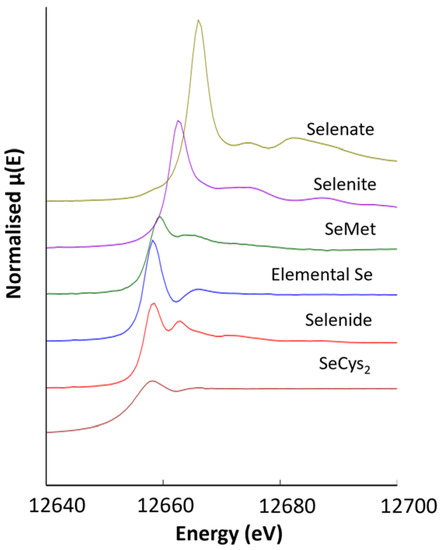
Figure 5.
Normalised XANES spectra for inorganic and organic selenium standards in the oxidation states-II, 0, IV and VI.
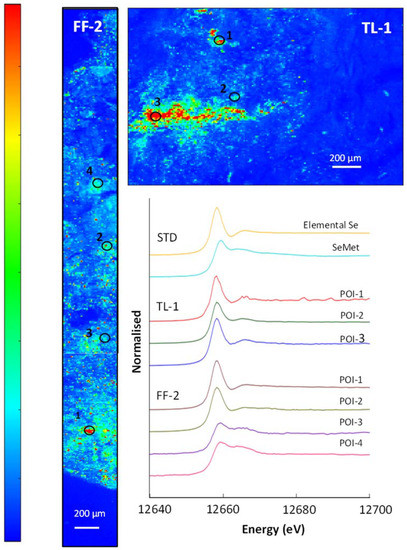
Figure 6.
(Top) and (left): µ-XRF Se K-edge maps showing a section through a soil pellet. The colours are a ‘pseudo-temperature’ scale, with high selenium counts in red and areas with no counts in dark blue. (Right): Normalised XANES spectra showing selenium species in the selected points of interest (POIs) compared to elemental selenium and the SeMet standard.
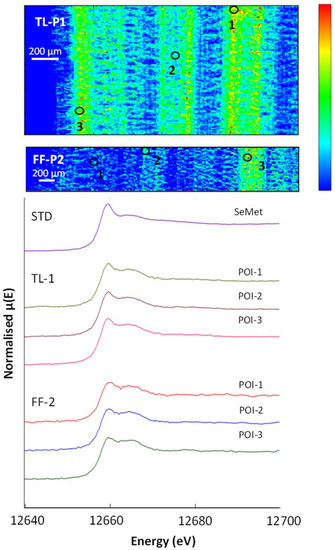
Figure 7.
(Top): µ-XRF Se K-edge maps showing a grass leaf (shoot) from sites TL-P1 and FF-P2. The µ-XRF is on a temperature scale, with high selenium counts in red and areas with no counts in dark blue. (Bottom): normalised XANES spectra showing selenium species in selected POIs and the SeMet standard.
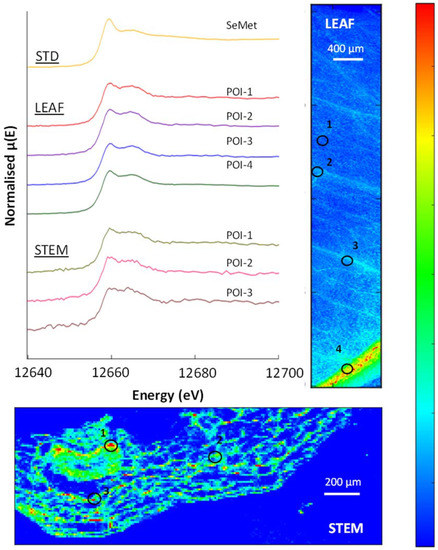
Figure 8.
µ-XRF Se K-edge maps showing a white clover leaf and a cross-section through a stem and a leaf from the vein out towards the margin. The µ-XRF is on a pseudo-temperature scale, with high selenium counts in red and areas with no counts in dark blue. (Top left): normalised XANES spectra showing selenium species in selected POIs and the SeMet standard.
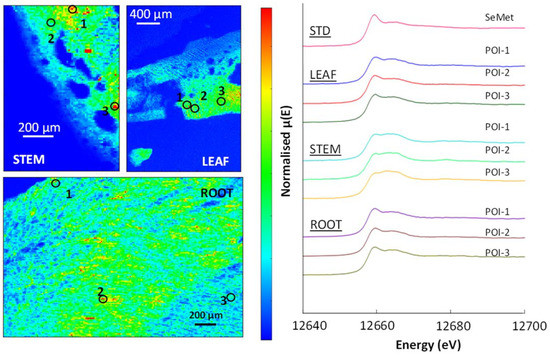
Figure 9.
(Left): µ-XRF Se K-edge maps showing cross-sections through a premier kale leaf, stem and root. The µ-XRF is on a temperature scale, with high selenium counts in red and areas with no counts in dark blue. (Right): normalised XANES spectra showing selenium species in selected POIs and the SeMet standard.

Table 5.
Linear combination fits for points of interest (POIs) selected in the seleniferous horizon (30–40 cm) of two soils from West Limerick. The goodness of fit is determined by the R-factor (approximate error ±3%). (Note: there was no detectable selenate).

Table 6.
Linear combination fits for selected selenium non-accumulator species measured in ATHENA. The goodness of fit is determined by the R-factor. The improvement in the fit for values <4% is marginal.

Table 7.
Linear combination fits for selected Brassica oleracea (Se accumulator) species from site FF measured in ATHENA. The goodness of fit is determined by the R-factor. The improvement in the fit for the selenate values <4% was marginal.

Table 8.
Bioaccumulation factors (BAF) calculated for sites TL-P1 and FF-P2 (0–10 cm).
3.6. Soil Selenium Species
The µ-XRF map (Figure 6) from site TL-C1 shows an inhomogeneous distribution of selenium in the soil, with several points and a distinct layer of higher selenium counts. The linear combination fits in Table 5 show that elemental selenium (Se0) was the most abundant selenium species, with 65–93% identified in the three selected POIs; the ‘layer’ represented by POI-3 contained 81% elemental selenium and 19% selenide. The µ-XRF map (Figure 6) also shows several areas of lower selenium concentration dispersed through the soil, represented by POI-2 and POI-4. The best fits for these areas are a combination of elemental selenium and organic selenium species, SeCys2 in POI-4 and both SeCys2 and SeMet in POI-2 (Table 5). In site FF-C2, elemental selenium was also the most abundant selenium species in POI-1 and POI-2 (93% and 77%), with 7% and 16% selenide, respectively, also present. However, SeMet was the most abundant selenium species in two areas (POI-3 and POI-4), with 70% and 76%, respectively. POI-3 also contains 24% SeCys2, while POI-4 has 19% Se0 with 11% selenite; the latter is only seen as a small component of the soil selenium. In both soil samples, there was no positive correlation, at this scale, of selenium with other elements identified. For instance, the soil samples contained abundant iron, but the µ-XRF maps show that the areas of high iron did not correlate with selenium.
3.7. Selenium in Non-Accumulator Species
The µ-XRF maps in Figure 7 pick out the morphology of shoots (blades) of grass from TL-P1 and FF-P2 which are defined by their selenium content, with the highest selenium concentration associated with the leaf veins. The LCF data (Table 6) for the selenium non-accumulator plants showed SeMet was consistently the dominant selenium species in the shoot, stem and root material analysed. In the FF-P2 shoot and TL-P1 root, SeCys2 was present, while in the grass blade (TL-P1), none was detected. Se-organic species comprise 86–92% of the selenium present in the grass. In all grass samples, the LCF revealed a small but significant component of selenite, typically 9%, but convincing evidence of the presence of selenate was lacking. The µ-XRF map in (Figure 8) shows the outer part of a white clover stem, with the cells of the cortex and phloem exposed by their selenium content. SeMet is the most abundant Se-species (86–88%), again with a component (~9%) of selenite. In the clover leaf, the mid-rib (POI-4; 82%), veins and blade, SeMet was again the dominant Se-species, with 8% SeCys2 and 11% selenite.
3.8. Selenium in Brassica oleracea
The µ-XRF maps of the accumulator species indicate significant Se concentrations throughout the plants, with a particularly high concentration at the epidermis (Figure 8, Figure 9, Figure 10 and Figure A6). Similar to the selenium non-accumulator plants, SeMet was the most abundant species in the leaf, stem and root material in both Brassica plants analysed; POIs revealed SeMet in the range 67–97%, and LCF revealed significant SeCys2 to be present, with variable amounts (2–18%) of selenite. The two organic species accounted for 78% to 96% of Se-species present, although the stems of the premier kale contained significant (~16%) selenite (Table 7).
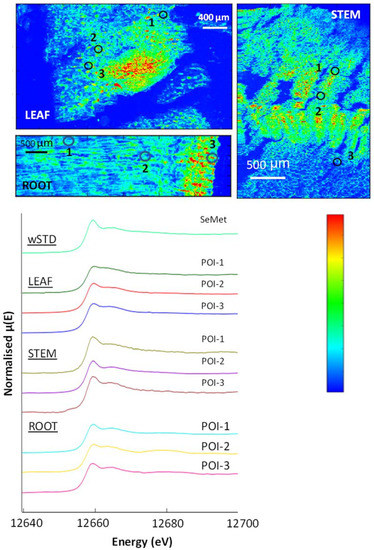
Figure 10.
µ-XRF Se K-edge maps showing a hispi cabbage leaf cross-section through a stem and root (top). The µ-XRF is on a temperature scale, with high selenium counts in red and areas with no counts in dark blue. (Bottom): normalised XANES spectra showing selenium species in selected POIs and the SeMet standard.
4. Discussion
4.1. Selenium and Molybdenum in the West Limerick Soils
In localised zones, the selenium concentrations in the soils collected from the five dairy cattle pasture sites in West Limerick are extreme and far above the global soil average concentration of 0.44 mg kg−1 [1] and the trigger action value (TAV) for agricultural land of 3–10 mg kg−1 (Table A1 and Table 9).

Table 9.
Trigger action values (TAVs) and upper safe limits for elemental concentrations in agricultural soils vary [1,49,50] and bioavailability is highly dependent on elemental species. Most publications quote the lower limits for Se, and Mo presented here [51,52].
The West Limerick soils in these zones are within the defined ‘seleniferous’ range of 1–1200 mg kg−1 [2,53,54], and the value of 1265.8 mg kg−1 at site MO is one of the highest naturally formed (unmineralised) soil selenium values recorded worldwide. These Se-hotspots occur in lower-lying areas surrounded by soils with low selenium values, many of the latter with concentrations below <0.1 mg kg−1 Se that would be considered ‘selenium deficient’ [12,55]. The boundary between the high and low selenium zones is sharp and defined by small (as small as 1 m) changes in elevation. The Se-rich zones in the soils are also high in molybdenum, and there is, generally, a positive correlation between selenium and molybdenum. The average world soil average of molybdenum is 1.1 mg kg−1, and the TAV range is 5–10 mg kg−1. Sites FF and MO have many values massively above the TAV, and these soils are defined as molybdeniferous to extremely molybdeniferous [17,49]. The soil sample collected from site FF-1, with a concentration of 1627.5 mg kg−1, represents one of the highest naturally formed (unmineralised) concentrations recorded worldwide. The average world natural uranium soil concentration is 3 mg kg−1, and many of the values in these Limerick soils are considerably above global averages, with some very high values for natural soils, up to 658.8 mg kg−1 in core FF-C2. In all sites studied, the arsenic concentrations (Table A1, Table A2 and Table A3) also exceed the world soil average of 6.83 mg kg−1, but most values were within the TAV range of 10–65 mg kg−1 [49] (Table 9).
In the bulk samples analysed by XRF, there is a close correlation between high selenium, molybdenum and uranium, but these positive correlations are not seen on the scale of the μXRF. Although the arsenic concentrations in the bulk samples do not have the anomalously high values of selenium, molybdenum and uranium, the arsenic concentrations correlate with those of selenium, with the highest arsenic concentrations coincident with the highest selenium and molybdenum values.
Given that Se, Mo, U and As are all redox-sensitive elements that are mobilised in oxic weathering conditions and immobilised in reducing conditions, their co-incidence in these West Limerick soils is to be expected. The local source of these elements is the Carboniferous deep-water shales (the Clare Shales for sites FF, MO, PK, TJ; the Rathkeale Shales for site TL [56]), and local hydrology will have transported the elements as the oxyanions (, , and () into the marshy areas that now form the high Se zones. High organic matter characterises these soils, and the thick clay layer observed in cores and trenches would have provided an excellent under-seal, leading to waterlogging and providing a perfect low Eh sink for the reduction, immobilisation and concentration of the Se, Mo, U and As. The West Limerick region was at the margins of the ice advance in the last glacial maximum, and periglacial conditions will have existed until ~11 ka BP [57]. From that time until the anthropogenic draining of the marshy areas, this redox system will have operated. At present, there is no addition of either selenium or other ‘redox’ elements to these soil systems.
The analysis of the soil at sites FF and TL reveals the presence of Se0 and selenide, the organic species SeMet and SeCys2 and very limited selenite. The reduction of selenium in anoxic soils by microbial activity by metal/metalloid reducers is well recorded [58], and several strains of bacteria are able to reduce the selenate via selenite to Se0, producing selenium nanoparticles [59,60,61]. This process will have been operating in the organic-rich West Limerick soils, where the natural microbial consortium will have managed the potentially toxic levels of selenium by converting it to the highly immobile Se0, and selenide, greatly reducing its bioavailability. Selenide can be absorbed into organic matter or occur as FeSe, and as there is no evidence of a positive Se-Fe correlation in the soils, it is likely to be associated with the extensive organic matter present in these soils [62,63].
Selenate is the most common selenium uptake route into plants [5,64], mimicking the sulfate pathway, but selenate does not play a significant role in these neutral to mildly acidic soils in West Limerick. However, the SeMet and SeCys2 present in these soils are highly mobile and easily taken up by plants after being released from decaying Se-bearing organic matter [65]. It has been noted that these organo-selenium compounds can be taken up by wheat and Brassicas at rates over 20-fold higher than selenate or selenite [3,66], and plants have been shown to take up the SeCys and SeMet from soils via root amino acids [66]. The oxyanion selenite is a minor bioavailable component of the soils, and its uptake to roots has been associated with inorganic phosphate (Pi) transporters [67], although there is also evidence that the aquaporin NIP2;1 is able to take up selenite into rice [66,68,69,70].
The system that operated in West Limerick has similarities to that operating at Kesterson in California, where selenium derived from Cretaceous shales was concentrated by (in this case, human) irrigation processes and transported as selenate to the Kesterson wetlands. There, it was immobilised in the anoxic conditions as Se0 and remobilised by microbial activity, taken up by plants and recycled via selenium in decaying organic matter [71,72], with disastrous effects on local wildlife.
4.2. Selenium in Pasture Plants
The pasture grasses take up both the Se and Mo in significant amounts, making it bioavailable for livestock (Table 3 and Table 4). The concentration of Se in the roots and shoots of the grasses is variable, but values greatly exceed the recommended limit in pastures of 2–5 mg kg−1 Se [7,73,74]. The adjacent sites FF and MO have very high soil selenium, and this is reflected in the grass, with the shoot samples containing 34 and 48 mg kg−1, respectively, and the roots 26 and 84 mg kg−1. However, the highest concentrations (as high as 78.05 mg kg−1) in the shoots were in grass samples from site TL-P1. Site TL, with ‘relatively’ low soil selenium (maximum 88 mg kg−1), is not greatly different in character from the other sites; the site is well-grassed, with a grey clay layer at >60 cm depth, but it does have a lower TOC than other sites (Table A1, Table A2 and Table A3). As a consequence, site TL-P1 has a higher BAF than FF-P2 (Table 8), demonstrating the selenium is relatively immobilised in the latter. As in many cases elsewhere [61,75], selenium in these West Limerick soils is strongly associated with the organic matter, and a study by [75] showed a decrease in selenium uptake when soils contained >11% organic matter, while soils with 5–11% organic matter showed optimum uptake (see Figure 4). Thus, the difference in uptake can be related to the amounts of organic matter present at these two sites, and the very high levels of organic matter at site FF-P2 will have enhanced stimulation of the microbial reduction of selenium to less bioavailable species such as Se0 due to the increase in available electron donors [75,76,77]. The organic nature of the FF and MO soils may explain why the selenium content of the plants, although high, is not as high as might be expected given the soil selenium concentration. Root-to-shoot translocation in the grass is, with one exception, <1, consistent with experimental studies of wheat that showed restricted translocation in systems where the selenium was present as an organic selenium species in the roots; in selenate-dominated soil systems, translocation is much more efficient [78].
The white clover (Trifolium repens.) collected from site TL-P1 was high in selenium in all parts of the plant (296.15 mg kg−1 in leaf), representing a BAF factor of ~5 compared to ~1 for grass shoots from the same location (Table 8). This is in direct contrast to a previous comparative study [79] that, using selenate, showed a much higher amount of selenium in ryegrass than white clover, although another study [80] noted 79.39 mg kg−1 in white clover leaves grown in the seleniferous soils of the Enshi District, China. In West Limerick, SeMet and SeCys2 are the main soil species, and selenate is largely absent, revealing the importance of the bioavailable species in defining relative Se uptake [81]. In West Limerick, the white clover behaves as a secondary selenium accumulator (defined as containing >100 mg kg−1 Se [7]), and, therefore, white clover should not be used to reseed pasture on selenium-bearing organic-rich soils.
There is very limited evidence for the presence of selenite in the West Limerick soils (Table 5), but there is a persistent amount of selenite seen in the plants (Table 6 and Table 7). This will not have been produced in situ in the plants but must have been taken up from the soil. Selenite is the likely oxyanion to be present in anoxic soils and is taken up directly into plants and converted (both enzymatically or non-enzymatically) to selenide for incorporation into the amino acids SeMet and SeCys2 [66]. Selenite uptake into plants has been linked to phosphate transporters [67,82], but competition with the poorly mobile phosphate would be reduced by the high organic content of these West Limerick soils. Thus, the uptake of selenite would be fast, leading to the fast depletion of selenite in the soil and incorporation in the plants. Grass shoots sampled from sites FF-P1/2, MO-P1, TJ-P1 and PK-P1 all contained molybdenum concentrations that exceed the maximum tolerable level for livestock of 5–10 mg kg−1 [26,27,28], and sample FF-P2 contained 79.63 mg kg−1 Mo. Compared to selenium, the translocation factor from root to shoot in grass is >1 and some values exceed 5; the low concentrations in samples from site TL-P1 reflected the low concentrations in the soil (Table 1), and this site was associated with the Rathkeale Formation, a different Carboniferous shale/mudstone unit to the likely source unit for the other areas. In molybdeniferous soils, molybdate is the mobile bioavailable Mo-species, and this will be stable at lower Eh values than selenate in mildly acidic soils. Molybdenum can be held in anoxic soils as sulfide or sorbed to organic matter but is not immobilised by dissimilatory metal-reducing bacteria in a similar way to selenium [77] and will thus be available as molybdate for uptake by plants. The translocation of the molybdate from roots to shoots is undertaken by sulfate transporters and will have been facilitated in these soils by the lack of competition from selenate [83]. Uranium, arsenic and vanadium pose less of a risk to livestock health as these elements have a poor translocation from roots to shoots. There are extensive studies on uranium uptake in plants, especially in phytoremediation studies [84,85]. Site FF had exceptionally high soil uranium in the top 30 cm, but uptake by plants at that site (and other sites) was limited to ~4.0 mg kg−1. There is a high organic component in the soils, and in these humic-rich environments, bioavailable organic uranium complexes can form and persist as mobile U (VI). However, in anoxic environments, the uranium will tend to remain bound to the organic and mineral matter [86].
Selenium concentrations in plants largely reflect the concentrations in the soil environment [1], and the data presented here demonstrate that the pasture in these areas of former marshy ground has toxic levels of selenium and molybdenum. The main fodder for the cattle will be grass (15 kg/per day), and the recommended daily intake for dairy cows is 300 μg/kg DM Se [87]. Even though translocation from root to shoot in the grass is <1, the blades of grass provide a significant health hazard to the cattle, which would be taking in between 1 and 2 orders of magnitude of excess selenium. The predominant mobile Se-species identified in the root and shoot material of the grass and white clover was SeMet, which can also cause phytotoxicity in plant tissues in high concentrations due to the misincorporation of SeMet in place of methionine in protein synthesis [7,88,89]. However, it is selenocysteine that is thought to be the major source of problematic misincorporation into proteins [90], and the only evidence of phytotoxicity observed was the slight chlorosis (yellowing) of the tall fescue grass blades at site FF and in the hispi cabbage (see below); these samples had the highest concentration of plant SeCyst2 (Table 3 and Table 4).
4.3. Selenium and Molybdenum Accumulator Brassica oleracea
All three Brassica oleracea varieties demonstrate the hyperaccumulation of selenium, with high selenium throughout the plants. Although selenate transporters were unlikely to be active (see above), these samples show typical features of hyperaccumulators with a high SeMet content, effective translocation from roots to leaves (average 861 mg kg−1 in the leaves) and a concentration of selenium in the xylem; the latter is the main transport pathway [6,90,91]. The Brassica sp. also accumulated significant concentrations of molybdenum in the same pattern as selenium, with the highest concentrations in the leaves (Table 4; see also). Molybdenum toxicity and phytoremediation have been much less studied than selenium but has been successfully addressed by using soil amendments and Brassica sp. [92,93] and the ground cover species Axonopus compressus, grown on mine tailings [33]. The concentration of ~800 mg kg−1 Mo in the Brassica leaves observed in this study would pose a huge risk to livestock and human health; the intake limit to avoid toxicity for humans is 400 µg−1 per day [1,94]. However, the ability of the Brassica species to accumulate both selenium and molybdenum in concentrations throughout the plant demonstrates how effective they could be for the phytoremediation of seleniferous soils in West Limerick. Each 500 g plant would remove 400 µg of selenium if harvested. A Brassica oleracea root system can typically reach 50 cm, which means it would tap the whole seleniferous and molybdeniferous soil profile. In addition, the Brassica plants could be used for ‘biofortification’ to increase dietary intake in selenium- and molybdenum-deficient areas; most soils in the British Isles are selenium- and molybdenum-deficient [1,55,58,95].
5. Conclusions
In the highly restricted zones of the livestock pasture fields of West Limerick, there are extreme concentrations of Se, Mo and U in soils with the highest recorded concentrations of 1265.8 mg kg−1 Se, 1627.5 mg kg−1 Mo and 658.8 mg kg−1 U. The general concentrations in these elements are orders of magnitude above their TAVs. The soils enriched in these elements are in low-lying areas that were, historically, mashes or shallow lakes. The boundaries of the enriched zones are often sharp and marked by very small changes in elevation, representing the margins of the original marshy areas.
For soil analysis at a depth of 10–20 cm, 500 g samples within the enriched zones reveal that the concentrations of Se, Mo and U have a significant variation on a local scale, but there is a positive correlation between these three elements and with TOC. This positive correlation is confirmed in vertical cores. However, µ-XRF data reveals no positive correlation on a microscale. The main Se-species in the soil was non-bioavailable Se0, produced by microbial reduction, and organoselenium compounds were the most bioavailable source of selenium. Selenate is not a significant component of the soil Se in these anoxic, organic-rich soils.
The source of these elements was the Carboniferous shales/mudstones that were deposited in anoxic basins in the Carboniferous, where high concentrations of redox-sensitive trace metals accumulated. These elements were remobilised by oxidation into groundwater during post-glaciation weathering and transported into anoxic, organic-rich marshy areas and reprecipitated.
The grasses in the enriched zones have concentrations in the order of 20–80 mg kg−1 Se in the roots, with 5–48 mg kg−1 Se in the shoots with TF values of <1. One site (TL) had the highest concentrations of Se (112.2 mg kg−1 Se in the roots and 78.1 mg kg−1 in the shoots) and a high BAF, which resulted from the relative lack of organic matter in the soil to retain the selenium at this site. White clover proved to be a strong accumulator of Se, with 296.2 mg kg−1 Se in the leaves, 249.5 mg kg−1 Se in the stems and 150.4 mg kg−1 Se in the roots. In sites containing significant soil Mo, the uptake of Mo was higher than Se, with TFs > 1. The uptake of U and As by the plants was restricted.
The hyperaccumulator Brassica oleracea grown over 11 weeks in a small experimental plot on the high Se and Mo soil recorded 971.7 2 mg kg−1 Se and 1000.4 2 mg kg−1 Mo in the leaves of premier kale, with TF factors of 1.70 and 3.24. There were significant differences in uptake between the three varieties examined.
In all the plants examined in this study, selenomethionine is the major selenium species in the plants, with typically 70–90% of the Se budget and approximately 10% selenocysteine and 10% selenite. Thus, the bioavailable selenium system in these West Limerick soils is one dominated by organoselenium species.
The concentrations in the grasses and clover of both Se and Mo are far above the recommended limits for livestock pasture and, as the local farmers well know, the cause of the selenosis and molybdenosis affecting the dairy cattle. The use of Brassica oleracea (var. premier kale) is a route to phytoremediation of the blighted areas, although the removal of the recalcitrant Se0 would be an issue. However, this latter issue could be remedied by the oxidation of the soils and the organic matter they contain by ploughing. This would produce mobile Se-oxyanions and stimulate microbial activity that would also release volatile methylated Se-species [37]. The potential use of this Brassica foliage as a Se-supplement for livestock grazing Se-deficient soils is problematic due to the concomitant high uptake of Mo, although Mo-deficiency also causes health issues for both livestock and humans.
6. Postscript
The farmers of West Limerick are well aware of which fields or areas within fields cause Se and Mo poisoning. The fields often produce a rich crop of grass, but the farmers have to manage access of their dairy herds to these areas. Both sulfur and copper supplements are administered to avoid the toxic effects of selenosis and molybdenosis [95].
Author Contributions
S.L.M. was a research student at the University of Manchester. Conceptualization of project, R.A.D.P.; Methodology, S.L.M., J.F.W.M., R.A.D.P., J.K.; Software, J.F.W.M.; Formal analysis, S.L.M.; Investigation, S.L.M., R.A.D.P., J.K.; Resources, R.A.D.P., B.E.v.D., J.K.; Writing, S.L.M.; Writing—review and editing, R.A.D.P.; Supervision, R.A.D.P., B.E.v.D.; Project administration, R.A.D.P., J.K., B.E.v.D.; Funding acquisition, R.A.D.P., B.E.v.D. All authors have read and agreed to the published version of the manuscript.
Funding
SM was funded by NERC Manchester–Liverpool Doctoral Training Partnership (NERC NE/L002469/1). Additional support was provided by UKRI HEIF Knowledge and Innovation Hub for Environmental Sustainability (RP). This work was carried out with the support of Diamond Light Source at Beamline I18 (proposals 15,475, 15,215 and 12,700).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The field programme was made possible by the generous support of Teagasc, Newcastlewest and Limerick, and by all the farmers who gave permission to sample their agricultural soils—James and John O’Flynn, Tom Larkin, Michael O’Donovan, Patrick Keily and TJ Daley. A special thanks to John and James O’Flynn for very generous logistical support and help growing the Brassica plants. Will Bower, Jon Fellowes, Hazel Waring and Adam Fuller are thanked for their aid with the field agenda. The laboratory programme conducted in the School of Earth and Environmental Sciences received considerable support from Alastair Bewsher for TOC and IC analysis, Paul Lythgoe for XRF and ICP-MS, and Karen Theis for microwave digestions. We acknowledge Diamond Light Source for time on Beamline I18 under proposals 15,475, 15,215 and 12,700 and the generous support of Tina Geraki. Duncan Forster of the Wolfson Molecular Imaging Centre made access to the cryotome possible. We also thank unnamed reviewers for their detailed comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Selenium Uptake from Livestock Pasture Extremely Enriched in Selenium, Molybdenum and Uranium: A Field and X-ray Absorption Study.

Figure A1.
Site FF, looking west. The low ridge in the background is underlain by Clare Shales. The marshy ground to the left is the location of the soils with the highest selenium values. The grassed areas were much lower in selenium, and it decreased to background levels up the slope to the right, by the path.

Figure A2.
Site TL, looking west. A field of rich grass pasture sloping gently right to left. The highest selenium values are in a zone in the centre of the field (Figure 2).

Figure A3.
Site MO, looking east. The marshy foreground includes the highest selenium soil values recorded and high molydenum. The drainage ditch to the left separates site MO from FF. See Figure 3 for contoured Se values.

Figure A4.
Site PK, looking west. The soil in the rough ground in the foreground is high in selenium, but the soils in the field up the slight slope in the background are low in selenium.

Table A1.
X-ray fluorescence data showing the concentration of selected major and trace elements in soil samples collected at 10–20 cm depth at sites MO and PK. TOC by loss on ignition.
Table A1.
X-ray fluorescence data showing the concentration of selected major and trace elements in soil samples collected at 10–20 cm depth at sites MO and PK. TOC by loss on ignition.
| Bulk | Se | Mo | As | U | V | Mn | Cu | CaO | Fe2O3 | SiO2 | Al2O3 | TOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | wt% | wt% | wt% | wt% | wt% |
| MO-1 | 227.2 | 59.3 | 39.4 | 26.0 | 104.6 | 45.4 | 871.8 | 5.4 | 6.1 | 31.5 | 4.9 | 41.7 |
| MO-2 | 763.5 | 431.1 | 89.6 | 407.0 | 77.8 | 236.3 | 111.8 | 4.9 | 12.4 | 21.2 | 3.4 | 48.4 |
| MO-3 | 404.3 | 71.5 | 57.5 | 33.5 | 93.1 | 488.2 | 165.8 | 5.7 | 8.1 | 35.9 | 5.1 | 36.3 |
| MO-4 | 145.9 | 37.6 | 18.6 | 17.0 | 105.7 | 563.5 | 90.0 | 6.5 | 4.8 | 43.7 | 6.4 | 30.3 |
| MO-5 | 840.2 | 193.7 | 72.5 | 295.2 | 94.4 | 152.6 | 144.5 | 5.1 | 9.5 | 10.4 | 1.8 | 61.9 |
| MO-6 | 115.7 | 20.8 | 8.6 | 53.3 | 146.0 | 208.5 | 149.0 | 2.2 | 4.6 | 50.5 | 6.9 | 28.8 |
| MO-7 | 14.7 | 3.2 | 0.0 | 51.8 | 152.8 | 54.4 | 397.9 | 1.4 | 2.3 | 58.2 | 8.3 | 23.5 |
| MO-8 | 1.0 | 6.1 | 0.0 | 17.4 | 195.2 | 210.0 | 924.6 | 1.3 | 3.1 | 62.7 | 9.8 | 17.8 |
| MO-9 | 482.1 | 80.0 | 23.4 | 155.0 | 170.7 | 518.1 | 191.5 | 7.7 | 7.2 | 35.4 | 5.7 | 37.2 |
| MO-10 | 1265.8 | 898.1 | 115.3 | 131.3 | 190.2 | 232.9 | 197.8 | 6.2 | 11.5 | 20.0 | 3.4 | 52.9 |
| PK-1 | 682.0 | 34.9 | 63.1 | 12.4 | 208.6 | 732.2 | 222.8 | 19.1 | 5.7 | 4.5 | 1.1 | 56.5 |
| PK-2 | 5.4 | 1.9 | 0.4 | 10.0 | 184.2 | 78.9 | 51.4 | 1.5 | 1.6 | 65.3 | 8.7 | 15.7 |
| PK-3 | 0.9 | 13.6 | 11.6 | 11.1 | 175.7 | 1101.2 | 28.2 | 0.2 | 3.4 | 72.9 | 9.2 | 8.5 |
| PK-4 | 2.7 | 17.7 | 11.6 | 10.7 | 212.2 | 920.2 | 32.0 | 0.2 | 3.3 | 69.5 | 8.9 | 10.9 |

Table A2.
X-ray fluorescence data showing the concentration of selected major and trace elements in soil samples collected at 10–20 cm depth at site FF TOC by loss on ignition.
Table A2.
X-ray fluorescence data showing the concentration of selected major and trace elements in soil samples collected at 10–20 cm depth at site FF TOC by loss on ignition.
| Bulk | Se | Mo | As | U | V | Mn | Cu | CaO | Fe2O3 | SiO2 | Al2O3 | TOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | wt% | wt% | wt% | wt% | wt% |
| FF-1 | 489.1 | 1627.5 | 99.8 | 599.5 | 163.6 | 113.7 | 762.8 | 4.4 | 5.1 | 46.2 | 7.3 | 28.2 |
| FF-2 | 276.3 | 90.7 | 40.6 | 253.6 | 82.7 | 430.0 | 236.0 | 7.3 | 4.5 | 26.7 | 5.0 | 48.6 |
| FF-3 | 13.4 | 2.8 | 9.9 | 24.0 | 184.9 | 95.6 | 78.0 | 1.3 | 2.6 | 65.2 | 10.7 | 14.8 |
| FF-4 | 56.4 | 9.6 | 47.1 | 37.2 | 115.3 | 262.9 | 46.1 | 2.5 | 4.0 | 46.5 | 7.7 | 32.5 |
| FF-5 | 178.2 | 24.0 | 17.9 | 53.0 | 156.2 | 595.4 | 56.2 | 4.5 | 4.8 | 29.1 | 4.8 | 47.9 |
| FF-6 | 362.2 | 81.6 | 21.9 | 61.8 | 95.4 | 121.0 | 79.8 | 5.5 | 3.5 | 28.4 | 4.6 | 47.8 |
| FF-7 | 31.5 | 30.1 | 20.2 | 24.6 | 109.1 | 466.3 | 74.2 | 1.0 | 4.6 | 67.2 | 10.7 | 10.3 |
| FF-8 | 1.8 | 10.2 | 13.3 | 9.8 | 54.4 | 367.4 | 28.9 | 0.3 | 4.2 | 72.3 | 12.2 | 5.8 |
| FF-9 | 4.4 | 21.4 | 16.4 | 12.5 | 74.3 | 420.5 | 41.3 | 0.3 | 4.1 | 69.8 | 10.8 | 9.1 |
| FF-10 | 19.1 | 32.1 | 21.0 | 12.8 | 52.4 | 518.7 | 54.3 | 1.9 | 4.8 | 62.1 | 9.4 | 14.6 |
| FF-11 | 8.4 | 8.6 | 9.4 | 19.8 | 48.4 | 113.5 | 45.7 | 2.0 | 3.6 | 64.9 | 9.0 | 13.0 |
| FF-12 | 473.5 | 53.3 | 25.8 | 43.9 | 65.3 | 98.7 | 137.5 | 12.8 | 3.7 | 40.9 | 7.0 | 28.1 |
| FF-13 | 0.0 | 10.5 | 5.3 | 10.2 | 79.7 | 2043.8 | 32.2 | 0.6 | 5.0 | 68.9 | 11.3 | 7.8 |
| FF-14 | 0.0 | 11.9 | 11.9 | 9.2 | 89.1 | 1914.9 | 38.2 | 0.9 | 5.4 | 66.4 | 11.6 | 8.9 |
| FF-15 | 0.0 | 10.0 | 14.0 | 12.9 | 84.8 | 1771.9 | 30.9 | 0.5 | 5.6 | 68.1 | 12.5 | 7.1 |
| FF-16 | 246.0 | 43.2 | 18.4 | 127.9 | 189.7 | 1151.0 | 85.5 | 2.8 | 6.2 | 47.9 | 6.8 | 29.6 |
| FF-17 | 0.0 | 9.4 | 10.3 | 9.1 | 109.7 | 1728.1 | 42.9 | 0.6 | 4.9 | 68.9 | 11.2 | 8.0 |
| FF-18 | 26.3 | 4.6 | 15.2 | 8.8 | 118.4 | 402.4 | 54.8 | 21.8 | 2.8 | 31.1 | 6.2 | 31.5 |
| FF-19 | 0.0 | 3.6 | 0.0 | 14.6 | 93.6 | 381.0 | 17.3 | 1.2 | 3.8 | 67.5 | 11.3 | 10.4 |
| FF-20 | 0.0 | 7.2 | 10.6 | 8.3 | 97.9 | 2051.5 | 40.4 | 0.7 | 5.2 | 67.8 | 12.7 | 7.1 |

Table A3.
X-ray fluorescence data showing the concentration of selected major and trace elements in soil samples collected at 10–20 cm depth at sites TL and TJ. TOC by loss on ignition.
Table A3.
X-ray fluorescence data showing the concentration of selected major and trace elements in soil samples collected at 10–20 cm depth at sites TL and TJ. TOC by loss on ignition.
| Bulk | Se | Mo | As | U | V | Mn | Cu | CaO | Fe2O3 | SiO2 | Al2O3 | TOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | wt% | wt% | wt% | wt% | wt% |
| TL-1 | 1.9 | 0.9 | 13.9 | 6.4 | 79.3 | 602.2 | 54.7 | 1.9 | 3.7 | 62.3 | 11.0 | 13.5 |
| TL-2 | 0.0 | 0.7 | 7.5 | 7.2 | 72.1 | 320.4 | 44.3 | 8.2 | 2.9 | 53.1 | 10.3 | 17.6 |
| TL-3 | 0.0 | 0.4 | 12.2 | 6.5 | 68.5 | 248.0 | 30.1 | 2.5 | 3.5 | 64.6 | 10.7 | 11.8 |
| TL-4 | 0.0 | 0.5 | 12.5 | 7.2 | 85.9 | 498.4 | 43.4 | 2.6 | 3.9 | 65.0 | 12.5 | 9.6 |
| TL-5 | 1.9 | 0.5 | 11.1 | 6.6 | 60.9 | 257.4 | 42.0 | 5.9 | 3.1 | 57.4 | 9.4 | 17.7 |
| TL-6 | 0.0 | 0.4 | 12.5 | 5.2 | 54.3 | 425.4 | 26.4 | 11.1 | 3.4 | 51.6 | 8.4 | 18.8 |
| TL-7 | 3.3 | 0.2 | 13.0 | 6.7 | 77.7 | 240.5 | 42.3 | 2.6 | 4.5 | 54.3 | 12.1 | 18.2 |
| TL-8 | 8.3 | 0.4 | 14.1 | 7.4 | 75.3 | 293.1 | 48.9 | 2.1 | 3.6 | 55.8 | 12.1 | 18.7 |
| TL-9 | 34.2 | 1.9 | 39.8 | 13.0 | 82.1 | 435.8 | 51.4 | 1.9 | 4.8 | 48.1 | 12.1 | 25.4 |
| TL-10 | 88.2 | 1.3 | 32.6 | 6.6 | 78.6 | 436.9 | 56.5 | 7.2 | 3.3 | 54.2 | 11.9 | 16.0 |
| TL-11 | 28.0 | 0.2 | 11.2 | 6.5 | 76.5 | 318.1 | 53.3 | 5.5 | 3.8 | 58.9 | 10.7 | 14.4 |
| TL-12 | 3.4 | 0.6 | 18.8 | 6.0 | 71.2 | 349.2 | 44.8 | 6.6 | 3.6 | 59.2 | 9.3 | 14.3 |
| TL-13 | 0.0 | 0.4 | 9.4 | 5.4 | 58.4 | 477.3 | 31.2 | 2.0 | 3.5 | 55.0 | 12.7 | 18.4 |
| TJ-1 | 0.0 | 1.0 | 15.7 | 6.8 | 65.6 | 1344.3 | 39.8 | 1.6 | 4.6 | 65.8 | 10.9 | 10.0 |
| TJ-2 | 24.9 | 3.7 | 12.1 | 17.0 | 39.7 | 602.7 | 26.7 | 8.9 | 4.7 | 10.2 | 2.2 | 61.9 |
| TJ-3 | 28.4 | 19.0 | 16.5 | 26.6 | 20.7 | 988.5 | 29.2 | 6.3 | 3.3 | 4.5 | 1.2 | 57.2 |
| TJ-4 | 24.1 | 5.8 | 17.1 | 12.6 | 80.9 | 816.4 | 39.1 | 6.9 | 2.9 | 11.5 | 2.5 | 48.5 |
| TJ-5 | 12.7 | 3.5 | 6.2 | 11.3 | 31.1 | 436.3 | 27.0 | 16.2 | 2.2 | 9.6 | 2.3 | 52.8 |

Table A4.
Sample locations, West Limerick. Latitude and longitude.
Table A4.
Sample locations, West Limerick. Latitude and longitude.
| Sample | Lat. | Long. | Sample | Lat. | Long. | Sample | Lat. | Long. |
|---|---|---|---|---|---|---|---|---|
| FF-1 | 52.52260 | −9.06199 | MO-1 | 52.52242 | −9.05856 | TL-1 | 52.51072 | −9.01216 |
| FF-2 | 52.52233 | −9.05963 | MO-2 | 52.52228 | −9.05845 | TL-2 | 52.51088 | −9.01167 |
| FF-3 | 52.52366 | −9.06071 | MO-3 | 52.52225 | −9.05867 | TL-3 | 52.51099 | −9.01104 |
| FF-4 | 52.52317 | −9.05835 | MO-4 | 52.52242 | −9.05890 | TL-4 | 52.51116 | −9.01020 |
| FF-5 | 52.52293 | −9.05842 | MO-5 | 52.52255 | −9.05912 | TL-5 | 52.51111 | −9.00909 |
| FF-6 | 52.52271 | −9.06215 | MO-6 | 52.52167 | −9.05648 | TL-6 | 52.51060 | −9.00967 |
| FF-7 | 52.52247 | −9.06204 | MO-7 | 52.52143 | −9.05757 | TL-7 | 52.51028 | −9.01048 |
| FF-8 | 52.52238 | −9.06208 | MO-8 | 52.52153 | −9.05835 | TL-8 | 52.50988 | −9.01199 |
| FF-9 | 52.52229 | −9.06212 | MO-9 | 52.52212 | −9.05778 | TL-9 | 52.51023 | −9.01207 |
| FF-10 | 52.52230 | −9.06152 | MO-10 | 52.52236 | −9.05875 | TL-10 | 52.51067 | −9.01199 |
| FF-11 | 52.52245 | −9.06139 | TL-11 | 52.51090 | −9.01083 | |||
| FF-12 | 52.52265 | −9.06124 | PK-1 | 52.51888 | −9.06054 | TL-12 | 52.51090 | −9.00883 |
| FF-13 | 52.52334 | −9.05645 | PK-2 | 52.51911 | −9.06244 | TL-13 | 52.51090 | −9.00883 |
| FF-14 | 52.52364 | −9.05577 | PK-3 | 52.51934 | −9.06318 | |||
| FF-15 | 52.52334 | −9.05725 | PK-4 | 52.51940 | −9.06367 | TJ-1 | 52.51633 | −9.00081 |
| FF-16 | 52.52244 | −9.05725 | TJ-2 | 52.51663 | −8.99919 | |||
| FF-17 | 52.52254 | −9.05615 | TL-C1 | 52.51047 | −9.01149 | TJ-3 | 52.51786 | −9.00004 |
| FF-18 | 52.52357 | -9.05893 | FF-C2 | 52.52311 | −9.06078 | TJ-4 | 52.51702 | −9.00111 |
| FF-19 | 52.52387 | −9.05840 | TJ-5 | 52.51663 | −8.99919 | |||
| FF-20 | 52.52397 | −9.05722 |
Figure A5a–e. Representative Linear Combination Fits from Se K-edge XANES for selenium speciation in soils and plants (Table 5, Table 6 and Table 7).
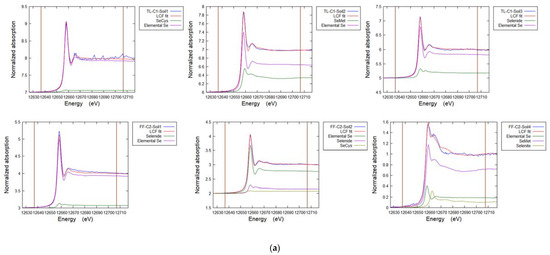
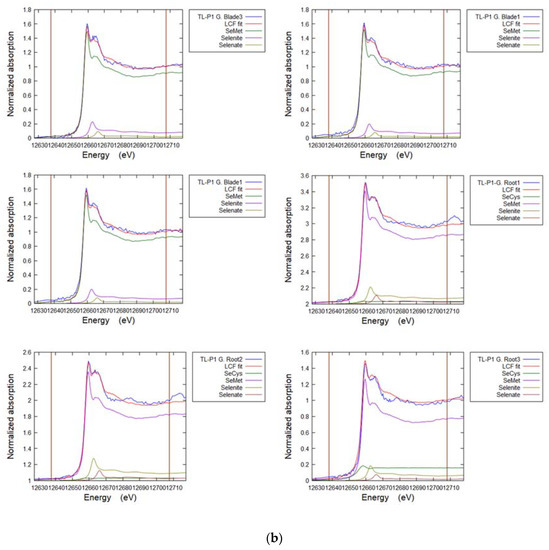
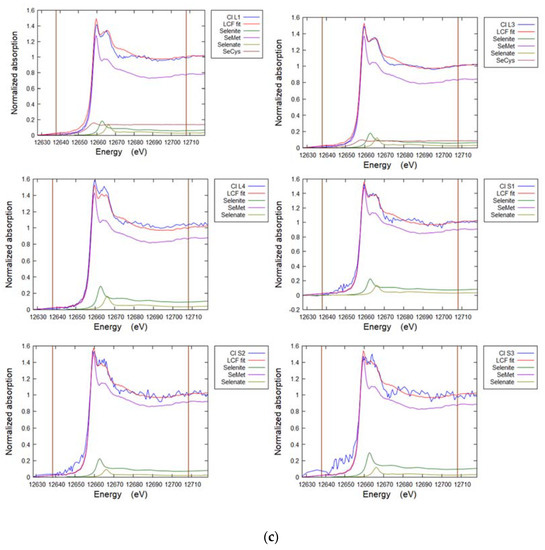
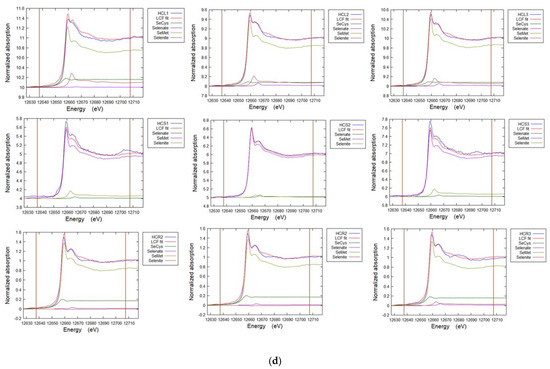
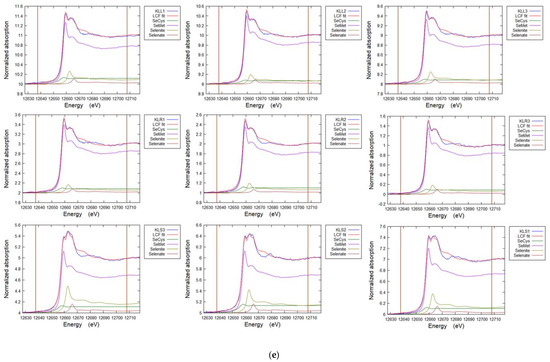
Figure A5.
(a) Linear combination fit spectra for soil cores sites TL (TL-C1-3) and FF (FF-C1-3). (b) Linear combination fit spectra for grass from site TL. TL-P1 G Blade 1-3, TL-P1 Root 1-3. (c) Linear combination fit spectra for white clover. Cl L1,3,4 Leaf, Cl S1-3 Stem. (d) Linear combination fit spectra for hispi cabbage. HCL1-3 Leaf, HCS1-3 Stem, HCR1-3 Root. (e) Linear combination fit spectra for premier kale. KLL1-3 Leaf, KLR1-3 Root, KLS1-3 Stem.
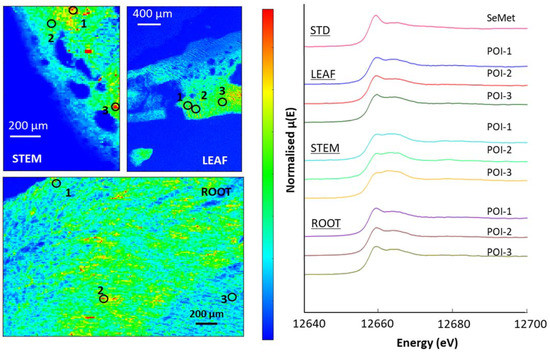
Figure A6.
(Left): µ-XRF maps showing selenium in a cross-section of a premier kale leaf vein, stem and root, with selected points of interest (POIs) for Se K-edge XANES analysis, labelled 1–3. The µ-XRF is on a ‘pseudo-temperature’ scale, with high Se counts in red and areas and no counts in dark blue. (Right): normalised Se K-edge XANES spectra showing the selenium species in selected POIs and the SeMet.

Figure A7.
Photographs of the selenium non-accumulator plants (pasture species) collected for sampling for chemical and XAS analyses. Samples of tall fescue grass with minor necrosis of the leaves and white clover.

Figure A8.
Photographs of the Se accumulator species Brassica oleracea (hispi cabbage, red cabbage (left) and premier kale (right)). Note that the hispi cabbage has minor necrosis of the leaves.
References
- Fordyce, F. Selenium Geochemistry and Health. Ambio A J. Hum. Environ. 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Neal, R.H. Selenium. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie Academic and Professional: London, UK, 1995; pp. 260–283. [Google Scholar]
- Zayed, A.; Lytle, C.M.; Terry, N. Accumulation and volatilization of different chemical species of selenium. Planta 1998, 206, 284–292. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology; Olle, S., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar]
- Guignardi, Z.; Schiavon, M. Biochemistry of Plant Selenium Uptake and Metabolism. In Selenium in Plants; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer: New York, NY, USA, 2017; Chapter 2; pp. 21–34. [Google Scholar]
- Pilon-Smits, E.A.H.; Winkel, L.H.E.; Lin, Z.-Q. (Eds.) Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Plant Ecophysiology; Springer International Publishing: New York, NY, USA, 2017; Volume 11, 340p. [Google Scholar]
- Terry, N.; Zayed, A.M.; de Souza, M.P.A.; Tarun, A.S. Selenium in Higher Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 402–432. [Google Scholar] [CrossRef]
- Rogers, P.A.M.; Arora, S.P.; Fleming, G.A.; Crinion, R.A.; Mclaughlin, J.G. Selenium toxicity in farm animals: Treatment and prevention. Ir. Vet. J. 2000, 43, 151–153. [Google Scholar]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef]
- Ohlendorf, H.M. Kesterson Reservoir: 30 Years of Selenium Risk Assessment and Management. Environ. Manag. 2000, 16, 257–268. [Google Scholar] [CrossRef]
- Dhillon, K.S.; Dhillon, S.K. Selenium toxicity in soils, plants and animals in some parts of Punjab, India. Int. J. Environ. Stud. 1991, 37, 15–24. [Google Scholar] [CrossRef]
- Dhillon, K.S.; Dhillon, S.K. Distribution and management of seleniferous soils. Adv. Agron. 2003, 79, 119–184. [Google Scholar]
- Mills, T.J.; Mast, M.A.; Thomas, J.; Keith, G. Controls on selenium distribution and mobilization in an irrigated shallow groundwater system underlain by Mancos Shale, Uncompahgre River Basin, Colorado, USA. Sci. Total Environ. 2016, 566–567, 1621–1631. [Google Scholar] [CrossRef]
- Teagasc. Crops, Soil and Fertility, Selenium. Available online: https://www.teagasc.ie/crops/soil--soil-fertility/trace-elements/grassland/selenium/ (accessed on 23 September 2022).
- Fleming, G.A. Selenium in Irish Soils and Plants. Soil Sci. 1961, 94, 28–35. [Google Scholar] [CrossRef]
- Fleming, G.A.; Walsh, T. Selenium Occurrence in Certain Irish Soils and Its Toxicity Effects on Animals. Proc. R. Ir. Acad. 1958, 58, 151–166. [Google Scholar]
- McGrath, D.; Fleming, G.A. Trace Elements and Heavy Metal in Irish Soils; Teagasc: Wexford, Ireland, 2007; p. 266. [Google Scholar]
- McLoughlin, S.L. Understanding Selenium Toxicity in the Natural Environment. Ph.D. Thesis, University of Manchester, Manchester, UK, 2018; 200p. [Google Scholar]
- Williams, C.; Brown, G. Uranium content of peaty soils rich in molybdenum and selenium from Co. Limerick, Eire. Geoderma 2017, 6, 223–225. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Banuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Johnson, C.H.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental Selenium Research: From Microscopic Processes to Global Understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, W.; Pang, F. Selenium in Soil–Plant Microbe: A Review. Bull. Environ. Contam. Toxicol. 2022, 108, 167–181. [Google Scholar] [CrossRef]
- Zhao, C.; Ren, J.; Chengze, X.; Lin, E. Study on the Relationship between Soil Selenium and Plant Selenium Uptake. Plant Soil 2005, 277, 197–208. [Google Scholar] [CrossRef]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, D.; Ensley, B.D.E.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Nat. Biotechnol. 1995, 13, 468. [Google Scholar] [CrossRef]
- Monei, N.L.; Veetil, S.K.P.; Gao, J.; Hatch, M. Selective removal of selenium by phytoremediation from post/mining coal wastes: Practicality and implications. Int. J. Min. Reclam. Environ. 2021, 35, 69–77. [Google Scholar] [CrossRef]
- Sun, W.G.; Selim, H.M. Fate and transport of molybdenum in soils: Kinetic modelling. Adv. Agron. 2020, 164, 51–92. [Google Scholar]
- Brogan, J.C.; Fleming, G.A.; Byrne, J.E. Molybdenum and Copper in Irish Pasture Soils. Ir. J. Agric. Res. 1973, 12, 71–81. [Google Scholar]
- McGrath, S.P.; Micó, C.; Zhao, F.J.; Stroud, J.L.; Zhang, H.; Fozard, S. Predicting molybdenum toxicity to higher plants: Estimation of toxicity threshold values. Environ. Pollut. 2010, 158, 3085–3094. [Google Scholar] [CrossRef]
- Axelson, U.; Söderström, M.; Jonsson, A. Risk assessment of high concentrations of molybdenum in forage. Environ. Geochem. Health 2018, 40, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Teagasc Molybdenum. 2017. Available online: https://www.teagasc.ie/crops/soil--soil-fertility/trace-elements/grassland/molybdenum/ (accessed on 31 March 2020).
- Blakley, B.R.; Molybdenum Toxicology in Animals. MDS Manual Veterinary Manual 2022. Available online: https://www.msdvetmanual.com/toxicology/molybdenum-toxicity/molybdenum-toxicity-in-animals (accessed on 15 January 2023).
- Osman, K.T.; Abul Kashem, M. Phytoremediation. In Encyclopaedia of Soil Science, 3rd ed.; Taylor and Francis: Boca Raton, FI, USA, 2017; Volume 1–3, pp. 1717–1724. [Google Scholar]
- Tow, S.W.T.; Eng, Z.X.; Wong, S.P.; Ge, L.; Tan, S.W.; Yong, J.W.H. Axonopus compressus (Sw.) Beauv.: A potential biomonitor for molybdenum in soil pollution. Int. J. Phytoremediation 2018, 20, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.C.; Stephenson, G.L. Ecotoxicity of Aged Uranium in Soil Using Plant, Earthworm and Microarthropod Toxicity Tests. Bull. Environ. Contam. Toxicol. 2012, 88, 43–47. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Z.; Beiyuan, J.; Cui, Y.; Chen, L.; Chen, H.; Fang, L. A critical review of uranium in the soil-plant system: Distribution, bioavailability, toxicity, and bioremediation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 340–365. [Google Scholar] [CrossRef]
- Lai, J.; Liu, Z.; Li, C.; Luo, X. Analysis of accumulation and phytotoxicity mechanism of uranium and cadmium in two sweet potato cultivars. J. Hazard. Mater. 2003, 409, 124997. [Google Scholar] [CrossRef]
- Khan, I.; Afzal Awan, S.; Rizwan, M.; Ali, S.; Zhang, X.; Huang, L. Arsenic behavior in soil-plant system and its detoxification mechanisms in plants: A review. Environ. Pollut. 2021, 286, 117389. [Google Scholar] [CrossRef]
- Atkinson, W.J. Regional Geochemical Studies in County Limerick, Ireland, with Particular Reference to Selenium and Molybdenum. Ph.D. Thesis, University of London, London, UK, 1967; p. 327. [Google Scholar]
- National Research Council. Mineral Tolerance of Animals, 2nd ed.; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Slekovec, M.; Goessier, W. Accumulation of selenium in natural plants and selenium supplemented vegetable and selenium speciation by HPLC-ICPMS. Chem. Speciat. Bioavailab. 2005, 17, 63–73. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; Environmental Protection Agency: Washington, DC, USA, 1996; pp. 7–12. [Google Scholar]
- Nirola, R.; Megharaj, M.; Aryal, R.; Naidu, R. Screening of metal uptake by plant colonizers growing on abandoned copper mine in Kapunda, South Australia. Int. J. Phytoremediat. 2016, 18, 399–405. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Vavpetič, P.; Vogel-Mikuš, K.; Jeromel, L.; Ogrinc Potočnik, N.; Pongrac, P.; Drobne, D.; Pipan Tkalec, Z. Elemental distribution and sample integrity comparison of freeze-dried and frozen-hydrated biological tissue samples with nuclear microprobe. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 348, 147–151. [Google Scholar] [CrossRef]
- Mosselmans, J.F.W.; Quinn, P.D.; Dent, A.J.; Cavill, S.A.; Moreno, S.D.; Peach, A.; Leicester, P.J.; Keylock, S.J.; Gregory, S.R.; Atkinson, K.D.; et al. IUCr. I18—The microfocus spectroscopy beamline at the Diamond Light Source. J. Synchrotron Radiat. 2009, 16, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. IUCr. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Braun, S.; Tuyishime, J.R.M.; Adediran, G.A.; Warrinnier, R.; Hesterberg, D. A probabilistic approach to phosphorus speciation of soils using P K-Edge XANES spectroscopy with linear combination fitting. Soil Syst. 2020, 4, 26. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 4th ed.; CRC Press: New York, NY, USA, 2011; p. 548. [Google Scholar]
- Cavanagh, J. What are “Soil Guideline Values” and Which Should I Use? Manaaki Whenua—Landcare Research: Lincoln, New Zealand, 2019; Available online: https://www.landcareresearch.co.nz/publications/soil-horizons/soil-horizons-articles/soil-guideline-values/ (accessed on 17 January 2023).
- Land Management. Sewage Sludge in Agriculture: Code of Practice. Department for Environment Food & Rural Affairs, Environment Agency Sewage Sludge in Agriculture: Code of Practice for England, Wales and Northern Ireland. 2019. Available online: https://www.gov.uk/government/publications/sewage-sludge-in-agriculture-code-of-practice/sewage-sludge-in-agriculture-code-of-practice-for-england-wales-and-northern-ireland#producers-test-sewage-sludge (accessed on 20 January 2023).
- Tolu, J.; Bouchet, S.; Helfenstein, J.; Hausheer, O.; Chékifi, S.; Frossard, E.; Tamburini, F.; Chadwick, O.A.; Winkel, L.H.E. Understanding soil selenium accumulation and bioavailability through size resolved and elemental characterization of soil extracts. Nat. Commun. 2022, 13, 6974. [Google Scholar] [CrossRef]
- Jacobs, L.W. Selenium in Agriculture and the Environment; Soil Science of Society of America Special Publication: Madison, WI, USA, 1989; p. 233. [Google Scholar]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Bio/Technol. 2009, 8, 88–110. [Google Scholar] [CrossRef]
- Fay, D.; Kramers, G.; Zhang, C.; McGrath, D.; Grennan, E. Soil Geochemical Atlas of Ireland; Teagasc and The Environment Protection Agency: Washington, DC, USA, 2007; pp. 88–89. [Google Scholar]
- Wignall, P.B.; Best, J.L. The Western Irish Namurian Basin reassessed. Basin Res. 2000, 12, 59–78. [Google Scholar] [CrossRef]
- Knight, J.; Coxon, P.; McCabe, M.A.; McCarron, S.G. Pleistocene glaciations in Ireland. Dev. Quat. Sci. 2004, 2, 183–191. [Google Scholar]
- Oremland, R.S.; Hollibaugh, J.T.; Maest, A.S.; Presser, T.S.; Miller, L.G.; Culbertson, C.W. Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: Biogeochemical significance of a novel, sulfate-independent respiration. Appl. Environ. Microbiol. 1989, 55, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.I.; Coker, V.S.; Charnock, J.M.; Pattrick, R.A.D.; Mosselmans, J.F.W.; Law, N.; Beveridge, T.J.; Lloyd, J.R. Microbial manufacture of chalcogenide-based nanoparticles via the reduction of selenite using Veillonella atypica: An in situ EXAFS study. Nanotechnology 2008, 19, 155603. [Google Scholar] [CrossRef]
- Pearce, C.I.; Pattrick, R.A.D.; Law, N.; Charnock, J.M.; Coker, V.S.; Fellowes, J.W.; Oremland, R.S.; Lloyd, J.R. Investigating different mechanisms for biogenic selenite transformations: Geobacter sulfurreducens, Shewanella oneidensis and Veillonella atypica. Environ. Technol. 2009, 30, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Fellowes, J.W.; Pattrick, R.A.D.; Boothman, C.; Al Lawati, W.M.M.; van Dongen, B.E.; Charnock, J.M.; Lloyd, J.R.; Pearce, C.I. Microbial selenium transformations in seleniferous soils. Eur. J. Soil Sci. 2013, 64, 629–638. [Google Scholar] [CrossRef]
- Cipriano, P.E.; Fonseca da Silva, R.; Dias de Lima, F.R.; de Oliveira, C.; de Lima, A.B.; Celante, G.; Dos Santos, A.A.; Archilha, M.V.L.R.; Pinatto-Botelho, M.F.; Faquin, V.; et al. Selenium biofortification via soil and its effect on plant metabolism and mineral content of sorghum plants. J. Food Compos. Anal. 2002, 109, 104505. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Johnsson, L. The association between selenium and humic substances in forested ecosystems—Laboratory evidence. Appl. Organomet. Chem. 1994, 8, 141–147. [Google Scholar] [CrossRef]
- Trippe, R.C., III; Pilon-Smit, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium-rich dissolved organic matter determines selenium uptake in wheat grown on Low-selenium arable land soils. Plant Soil 2015, 408, 73–94. [Google Scholar] [CrossRef]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shao, H.; Huang, H.; Shen, Y.; Wang, L.; Wu, F.; Han, D.; Song, J.; Jia, H. Overexpression of the phosphate transporter gene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum. Environ. Exp. Bot. 2017, 137, 158–165. [Google Scholar] [CrossRef]
- Ren, H.Z.; Li, X.M.; Guo, L.N.; Wang, L.; Hao, X.Y.; Zeng, J.M. Integrative Transcriptome and Proteome Analysis Reveals the Absorption and Metabolism of Selenium in Tea Plants [Camellia sinensis (L.) O. Kuntze]. Front. Plant Sci. 2022, 13, 848349. [Google Scholar] [CrossRef]
- Van Hoewyk, D.; Garifullina, G.F.; Ackley, A.R.; Abdel-Ghany, S.E.; Marcus, M.A.; Fakra, S.; Ishiyama, K.; Inoue, E.; Pilon, M.; Takahashi, H.; et al. Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiol. 2005, 139, 1518–1528. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of silicon influx transporter OsNIP2; 1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Weres, O.; Jaouni, A.-R.; Tsao, L. The distribution, speciation and geochemical cycling of selenium in a sedimentary environment, Kesterson Reservoir, California, U.S.A. Appl. Geochem. 1989, 4, 543–563. [Google Scholar] [CrossRef]
- Presser, T.S.; Swain, W.C. Geochemical evidence for Se mobilization by the weathering of pyritic shale, San Joaquin Valley, California, U.S.A. Appl. Geochem. 1990, 5, 703–717. [Google Scholar] [CrossRef]
- Wu, L.; Van Mantgem, P.J.; Guo, X. Effects of forage plant and field legume species on soil selenium redistribution, leaching, and bioextraction in soils. Arch. Environ. Contam. Toxicol. 1996, 31, 329–338. [Google Scholar] [CrossRef]
- Wu, L. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol. Environ. Saf. 2004, 57, 257–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Moore, J.N. Selenium Fractionation and Speciation in a Wetland System. Environ. Sci. Technol. 1996, 30, 2613–2619. [Google Scholar] [CrossRef]
- Johnsson, L. Trends and annual fluctuations in selenium concentrations in wheat grain. Plant Soil 1991, 138, 67–73. [Google Scholar] [CrossRef]
- Lloyd, J.R. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 2003, 27, 411–425. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Luo, K.; Li, H. Environmental behaviours of selenium in soil of typical selenosis area in China. J. Environ. Sci. 2008, 20, 859–864. [Google Scholar] [CrossRef]
- Davies, E.B.; Watkinson, J.H. Uptake of native and applied selenium by pasture species. N. Z. J. Agric. Res. 1966, 9, 317–327. [Google Scholar] [CrossRef]
- Yuan, L.; Yin, X.; Zhu, Y.; Li, F.; Huang, Y.; Liu, Y.; Lin, Z. Selenium in Plants and Soils, and Selenosis in Enshi, China: Implications for Selenium Biofortification. In Phytoremediation and Biofortification Two sides of One Coin; Sharma, K., Ed.; Springer Briefs in Molecular Science: Green Chemistry for Sustainability; Springer: Berlin/Heidelberg, Germany, 2012; Chapter 2; pp. 7–31. [Google Scholar]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef]
- Dhillon, K.; Banuelos, G. Overview and Prospects of Selenium Phytoremediation Approaches. In Selenium in Plants; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer-Link: New York, NY, USA, 2017; pp. 277–322. [Google Scholar]
- Shinmachi, F.; Buchner, P.; Stroud, J.L.; Parmar, S.; Zhao, F.J. Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol. 2010, 153, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.; Pratas, J.; Mayank, V.; Rohan, D.; Paul, M. Accumulation of uranium by aquatic plants in field conditions: Prospects for phytoremediation. Sci. Total Environ. 2014, 470–471, 993–1002. [Google Scholar] [CrossRef]
- Merkel, B.; Hasche-Berger, A. (Eds.) Uranium in the Environment; Springer: Berlin/Heidelberg, Germany, 2006; p. 897. [Google Scholar]
- Sheppard, S.C.; Evenden, W.G.; Anderson, A.J. Multiple assays of uranium toxicity in soil. Environ. Toxicol. Water Qual. 1992, 7, 275–294. [Google Scholar] [CrossRef]
- Teagasc. Teagasc Dairy Manual. Available online: https://www.teagasc.ie/publications/2016/teagasc-dairy-manual.php (accessed on 1 December 2021).
- Brown, T.A.; Shrift, A. Selenium: Toxicity and Tolerance in Higher Plants. Biol. Rev. 1982, 57, 59–84. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Pilon-Smits, E.A.H. Ecological aspects of plant selenium hyperaccumulation. Plant Biol. 2011, 14, 1–10. [Google Scholar] [CrossRef]
- Renkema, H.; Koopmans, A.; Kersbergen, L.; Kikkert, J.; Hale, B.; Berkelaar, B. The effect of transpiration on selenium uptake and mobility in durum wheat and spring canola. Plant Soil 2012, 354, 239–250. [Google Scholar] [CrossRef]
- Hale, K.l.; McGrath, S.P.; Lombi, E.; Stack, S.M.; Terry, N.; Pickering, I.J.; George, G.N.; Pilon-Smits, E.A.H. Molybdenum sequestration in Brassica species. A Role for Anthocyanins? Plant Physiol. 2001, 126, 1391–1402. [Google Scholar] [CrossRef]
- Neunhauserer, C.; Berreck, M.; Insam, H. Remediation of soils contaminated with molybdenum using soil amendments and phytoremediation. Water Soil Pollut. 2001, 128, 85–96. [Google Scholar] [CrossRef]
- WHO. Selenium. In Trace Elements in Human Nutrition and Health; World Health Organisation: Geneva, Switzerland, 1996; Chapter 6; pp. 105–120. [Google Scholar]
- O’Flynn, J.; (Farmer of Ballyegny Beg). Personal Communications, 2014–2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).