1. Introduction
Reducing the use of agrochemicals in the control of weeds, pests and soil pathogens is one of the major challenges in the future of agriculture [
1,
2,
3,
4]. At the same time, increasing interest in environmental protection and human health is leading research towards agronomic strategies that aim to limit the use of fungicides, pesticides and herbicides. In this context, searching for advanced materials and alternative methods for high-efficiency soil treatment, with low costs and low environmental impact, is an urgent requirement for sustainable agriculture.
Soil solarization is a non-chemical method that can provide excellent control of soil-borne pathogens in the open field, greenhouse, nursery and home garden [
5,
6,
7,
8]. This method consists of covering the soil surface for several weeks during summer with a clear thin plastic film with special optical properties so as to increase the accumulation of heat in the soil. The resulting high soil temperatures reduce the presence of most pathogens at different depths, but their action depends profoundly on application time: to be effective, it should exceed 50–60 days. These long timeframes influence and limit the wide spread of this natural method for soil treatment, restricting its great potential.
Due to the lack of alternative eco-friendly methods, soil treatment based on chemical agents (fumigants) is still the most widespread practice to eliminate soil-borne pathogens of fungal, bacterial and nematode origin [
9,
10]. Fumigation totally sterilizes the soil, but it is hazardous for the environment, harmful for human health and extremely aggressive towards soil microorganisms [
11,
12,
13].
Methyl bromide (MB) has been the most widely used fumigant since 1940 because of its broad-spectrum activity. Due to its high toxicity for the environment and human health, its use has been gradually forbidden, being one of the major causes of the depletion of the ozone layer [
14]. In recent years, as an alternative to MB, several fumigants have been adopted for soil disinfection, such as 1,3-dichloropropene (1,3-D), chloropicrin (CP), Metam Sodium, Dazomet (DZ), and dimethyl disulfide [
15,
16]. Nevertheless, chemical fumigation has been recognized as a destructive and dangerous method, non-selective to soil biological targets, resulting in a severe impoverishment of the abundance and diversity of soil microorganisms as well as enzymatic activities.
In the last decades, many studies have shown that the combination of CP and 1,3-D has a significant impact on the prevention and control of soil-borne diseases and insect pests and thus is widely considered a good MB substitute [
17,
18]. On the contrary, some papers reported that 1,3-D leads to environmental pollution, and that the combination of CP and 1,3-D should be impeded. It is well known that the use of a large volume of chemical pesticides is leading to a large number of problems, including environmental damage, soil ecological imbalance, food contamination, pollution in the water basin and hazards for human health [
19,
20,
21].
It has been demonstrated that several harmful chemical elements remain in soil after fumigation; these residues, accumulated year by year, affect the nature of the soil, becoming a real problem for future crops [
22,
23].
Soil solarization is an interesting and ecological alternative to fumigation; it is a very effective natural practice that respects the environment, does not pollute, preserves the quality of crops and is cheaper than fumigation.
Nowadays, it is possible to obtain very efficient soil treatments thanks to plastic films with specific characteristics. Most of the “negative agents” are eradicated just by reaching high temperatures at different depths in the soil. The main limit of this natural practice is its application time, which normally is of eight to nine weeks. This is the time required for a good and satisfactory result when a functionalized plastic film is employed. Moreover, in some areas and in some agricultural production, the available time between two crop cycles is shorter than what is required for a good solarization result. In order to introduce the use of soil solarization even in these contexts, further improvement aiming to reduce times is necessary. Towards this goal, we studied a new approach based on the combination of a solarizing film and a biodegradable black liquid to spray on the soil [
24,
25]. The proposed hybrid system simulates a thermal solar panel (used for home hot water production) and behaves in the same way to increase water temperature in soil. As a consequence, higher soil temperatures are reached in a shorter time with satisfactory disinfecting effects in comparison with those obtained with the traditional method.
We tested this new method over the last decade in different conditions (both pedoclimatic and greenhouse structure types), with several materials (plastic films and black liquid concentration) on different types of soil. Trials were performed with the goal of demonstrating that temperatures registered at different depths in soil were higher for the innovative solarization method compared to the traditional one because of the presence of the black film working as a solar collector. The experimental results confirmed the positive variations in temperature (up to 12 °C to a 5 cm depth) for innovative soil solarization [
26].
Recently, we arranged a trial in China aiming to evaluate the effects of chemical and solar soil disinfection methods on soil bacterial communities [
27]. The experimental results confirmed that one of the main advantages of solarization is to preserve the bacterial communities, the “positive agents” according to Katan theory [
5], which are very useful for soil fertility. With regards to this subject, the use of biochar in the solarization practice has to be considered a further positive aspect due to the important role of this material for soil health and the environment [
28,
29].
We arranged a trial to further implement the state-of-the-art new solarization, comparing, for the first time to our knowledge, the two main methods for soil treatment: innovative solarization and fumigation. The comparison was based on viability control of some of the most common pathogens 30 days after their inoculation in soil. The percentage of active pathogens, verified for each parcel after a month, indicated the efficacy of the different tested methods. Here, we report the experimental results of this trial concerning the control of the presence of pathogens in the parcels treated with the two different methodological approaches.
2. Materials and Methods
2.1. Experimental Setup
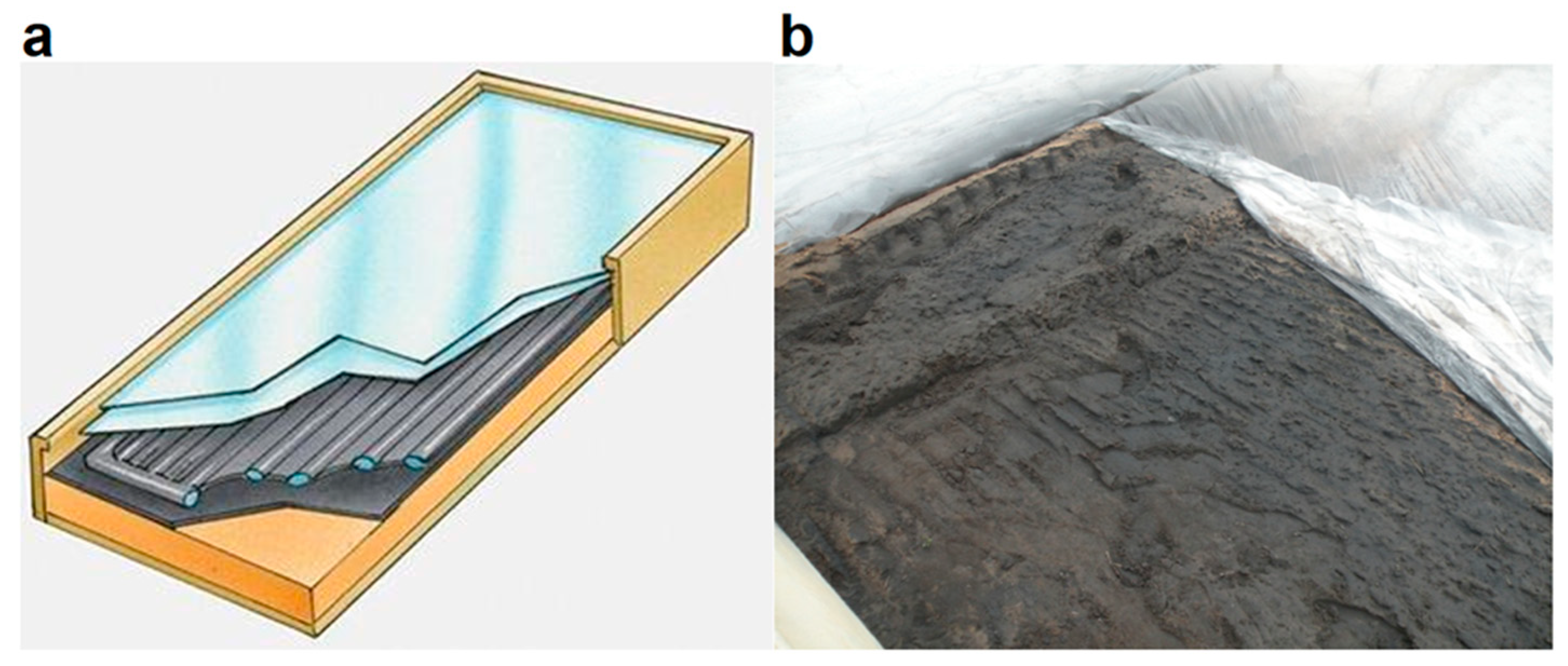
The proposed innovative solarization model consists of a black layer (soil darkened by a biodegradable black liquid) covered by a thermal film (solarization film), namely a specific film capable of transmitting almost all the incident solar radiation and blocking the leak of IR radiation emitted from the soil (
Figure 1b). This setup is a kind of reproduction of a thermal solar panel for hot water (
Figure 1a). Thanks to this hybrid innovative method, patented and trademarked as SOLIN [
30], it is possible to further increase temperatures up to 10–12 °C at different depths in soil.
An experimental setup was arranged in July inside a greenhouse constituting four parcels of size 4 × 6 m at Altamura Farm (40°55′79.27″ N; 15°02′56.39″ E) in Southern Italy, with the aim of comparing the innovative soil solarization system with one of the most common fumigation methods based on the use of Dazomet (also called Basamid). The parcels were arranged as following: P1—bare soil; P2—traditional solarization; P3—innovative solarization (
Figure 2); P4—fumigation. T-bags containing the selected pathogens were buried in each parcel at 25 cm depth. P2 and P3 were prepared according to the solarization protocol; on P3, the black liquid was sprayed after irrigation and before covering with solarization film (
Figure 2). P4 was treated with Basamid fumigant with a dose of 70 gr/m
2 and covered with a VIF (virtually impermeable film). During the test, temperature was measured using a thermocouple IEC-RS (K type) powered by a RS Digital Thermometer 1319A. The duration of the trials was 30 days.
2.2. Solarization Film
The solarization effect depends strongly on the temperature reached at different soil layers during the running period. In other words, the higher the temperature, the more efficient the solarization effect. Traditional solarization requires a plastic film with specific optical and thermal properties to cover an adequately wet field as long as necessary to obtain a satisfying result (8–9 weeks). Unsurprisingly, the use of inadequate plastic films could frustrate a good soil solarization effect; this is due to the inability to reach the minimum mean temperature threshold required to eliminate most pathogens, such as nematodes, which have been estimated to not survive temperatures above 42 °C. A highly performing film for solarization has optical characteristics aimed at enhancing the “greenhouse effect” in the soil, trapping the heat associated with solar radiation [
16]. This means that an ideal film should be as transparent to solar radiation as possible (200 nm up to 2500 nm) and opaque to IR radiation emitted by the soil. Under these conditions, heat is accumulated in soil and temperatures at depths up to 35–40 cm increase until a disinfecting effect is achieved: elimination of pathogens of animal (e.g., nematodes) or vegetable (different types of fungi) origin. For our comparative experiment, we selected a solarization plastic film produced in Israel by Ginegar Plastic Products Ltd., having optical properties that we tested in our laboratory as being capable of optimizing solarization effects.
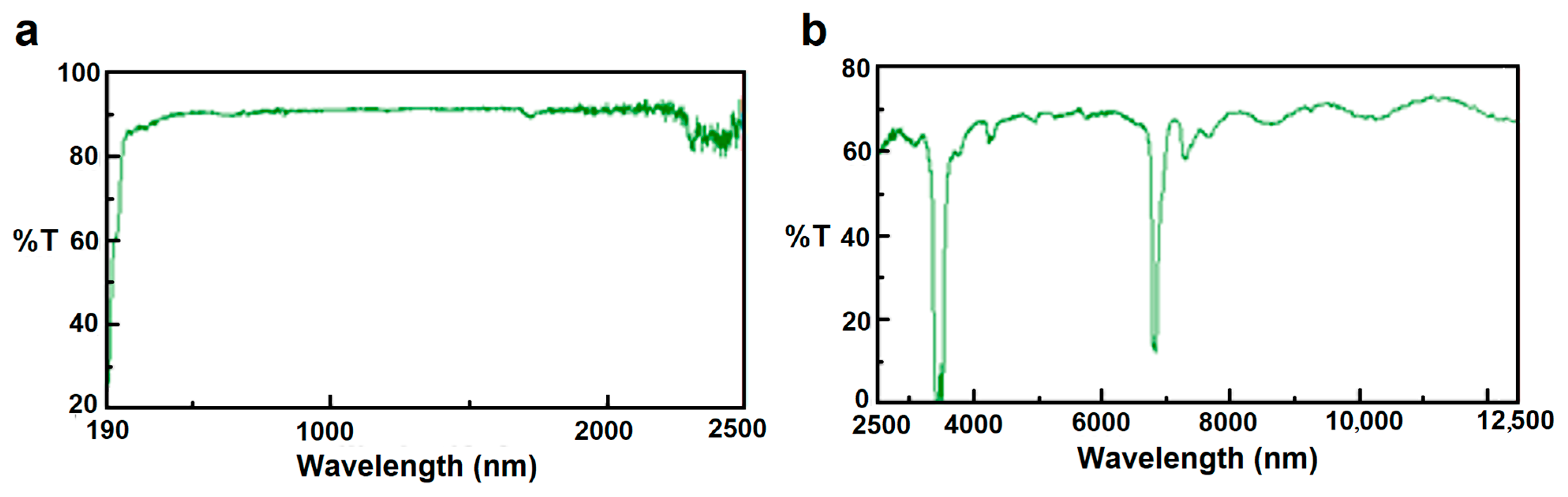
As shown in
Figure 3a,b, the selected plastic film exhibits very high transitivity in the range of solar radiation and a considerable blockage of IR radiation (heat energy) emitted from soil (two absorption peaks in
Figure 3b). These properties induce heat accumulation in the soil that turns into an increase in temperature at different depths.
Spectral analysis on the plastic films was performed using an UV/VIS/IR Modular Spectrophotometer (JASCO V-650, accuracy 0.5 nm, range 190–900 nm and 2500–12,500 nm) with an integrating sphere (JASCO ISN-722, inside diameter 60 mm) that allowed estimation of the total light transmission.
2.3. Biochar Mixture
The black surface was obtained by “painting” the soil with a specially prepared biodegradable black liquid that was sprayed before covering with solarization plastic film. The mixture was based on biochar powder, water and a few additives. Just-prepared, this mixture appears as a very viscous blend, but it is easily soluble in water. The biochar used was a high-carbon non-activated charcoal produced from pruning waste through slow pyrolysis. Grain size was also a very important issue to be taken into account in the selection of biochar powder in order to prevent damage in the usual systems used in agriculture for the treatment of soil with liquid products.
The first part of the work focused on defining a suitable concentration of biochar in water to obtain a color as black as possible on the soil while still reaching the proper viscosity and preventing any sediment of biochar in the mixture.
Several tests have been performed with the aim of selecting the best biochar for the job among all the available samples and finding the right balance between the amount of biochar, water and a proper thickening and stabilizing agent (carboxymethyl-cellulose—CMC), including:
Scanning electron microscopy (SEM-FEI Quanta 200 FEG) on different biochar samples;
Metal analysis of different randomly selected samples of biochar through inductively coupled plasma mass spectrometry (ICP-MS);
Dissolution testing in water and dispersion with different amounts of CMC;
Gas chromatography–mass spectrometer (GC-MS) analysis of total hydrocarbons (in the range C10–C40) and polycyclic aromatic hydrocarbons (PAHs), with the aim of excluding the presence of dangerous substances for environment and human health, such as organic pollutants.
2.3.1. SEM Analysis
We analyzed the morphology of various samples of charcoal powder using a SEM, with the goal of selecting the one constituted of particles as homogeneous as possible in shape and with an average grain size smaller than 30 microns so as to avoid the obstruction of irrigation systems employed to spray the black liquid on the soil. Before observation with SEM, the samples were coated with Au–Pd alloy by means of a sputtering device (MED 020. Bal-Tec AG). The coating provided the entire sample surface with a homogeneous layer of metal alloy of 18 ± 0.2 nm. Energy-dispersive X-ray spectrometry was performed by using an Oxford INCA Energy system 250 equipped with an INCAX-act detector. In
Figure 4, a SEM image is shown showing the morphology of the biochar powder selected for the final mixture. As is possible to verify in relation to the size of the picture (100 microns), this sample is made of particles with similar shape and its biggest lump is less than 30 µm big, so it is confirmed to be the most appropriate to be used in dispersion in order to avert the blocking of irrigation systems.
2.3.2. Mass Spectrometry Analysis
Inductively coupled plasma mass spectrometry (ICP-MS) was performed to evaluate the presence of trace elements and heavy metals in the biochar. Analytical results for the chosen sample are reported in
Table 1. The total content of heavy metal was trace amounts or at least within acceptable limits. Mercury (Hg) was not detected. The presence of some metals, such as lead (Pb) or zinc (Zn), may depend on the type of wood used as feedstock and the treatments it underwent before becoming biochar.
2.3.3. Precipitation Test of Biochar in Water with CMC
Further tests focused on the identification of the ideal concentration of biochar in water in order to ensure adequate black staining of the soil and make the dispersion stable without sedimentation. Several samples were prepared using different concentrations of CMC in water to analyze the precipitation times and the amount of biochar accumulated on the bottom. CMC is a natural polymer derived from cellulose by substituting some hydroxyl groups (-OH) with carboxyl ones (-CH2-COOH). These groups make CMC water-soluble and therefore usable in many industrial applications where cellulose cannot be exploited because of its insolubility. Thanks to its stabilizing and protective properties, CMC is mainly used as a food additive (E466), thickener, emulsion stabilizer and suspension agent. The many uses of carboxymethyl-cellulose derive from its hydrophilic character, high viscosity of diluted solutions, property of forming grease-resistant films, nontoxicity and excellent behavior as a colloid stabilizer protector. In this work, CMC was used for its stabilizing properties, with the aim of keeping the biochar suspended in water during application in order to have a homogeneous mixture without the risk of biochar precipitating in a very short time. To guarantee the longest possible suspension, several tests were carried out on samples having different concentrations, monitoring biochar precipitation as a function of the time.
Figure 5 shows samples with different behaviors.
2.3.4. GC-FID and GC-MS Analysis of Hydrocarbons and PAHs
The characterization of biochar samples to use in the mixture for the innovative solarization method was completed analyzing both hydrocarbons C
10-C
40 and polycyclic aromatic hydrocarbons (PAHs). The presence of the latter may be due to incomplete combustion during the pyrolysis process. Their characterization was performed with the GC-MS technique, obtaining the results listed in
Table 2.
This outcome excluded the presence of environmentally and health-damaging substances, since PAHs are toxic to aquatic life and are suspected human carcinogens.
2.4. Pathogens Preparation
The trial was performed in order to assess activity against
Rhizoctonia solani,
Fusarium oxysporum lycopersici,
Sclerotinia sclerotiorum and
Phytophthora cactorum by using inoculated kernels and perlite contained in bags (T-bags) that were placed into the soil, as shown in
Figure 6.
These pathogens were chosen because they are largely present in the soil and they represent a serious problem for growers from an economical and agronomical point of view, due their power to affect both the quality and quantity of crops. The concentration of pathogens used to prepare the samples are reported in
Table 3.
An artificial inoculum was made for each pathogen using wheat kernel and letting it grow for 3 weeks. The samples of T-bags were prepared according to the procedure summarized in
Table 4.
2.5. Fumigant
We used Basamid for our experiment (3,5-dimethyl-1,3,5-thiadiazinane-2-thione, also called Dazomet), a fumigant formulated as powder or granules that is largely used as a soil sterilant against a wide range of soil microorganisms, including nematodes, several types of fungi, insects, various bacteria, and weeds. It is stable at normal temperatures when not applied to the soil.
It was applied to the soil by mechanical mixing with a quantity of 70 g/m2. At the end of preparation, the parcel was irrigated and covered by a traditional barrier film. This kind of film works to minimize the leakage of harmful gases that are extremely toxic to the environment and farmers. The most important feature that distinguishes a barrier film is its so-called “permeability”, which is measured in g/m2h. The lower the permeability of a VIF, the better the barrier effect. The permeability of the employed VIF covering the fumigation parcel was 0.02 g/m2h at room temperature (25 °C).
3. Results
The necessary condition for obtaining the best thermal behavior in terms of physical performance (e.g., higher temperatures at different depths in the soil) is to use films with suitable optical properties for greenhouse tests. In fact, as shown in
Figure 3a, the selected film was highly transparent to solar radiation (>90%), which is essential to totally exploit solar incident energy at the soil level. The peaks exhibited in
Figure 3b fall in the thermal emission range of the soil; this means that the heat emitted from the soil outwards was well confined underneath the film and given back to the soil, thus obtaining a remarkable rise in internal temperature.
A further increase in soil temperature was obtained through the innovative biochar-based solarization system, which was found to be efficient enough to reduce the operative time up to 30 days. The selection of the best biochar to use in the formulation was not trivial, due to the stringent requirements regarding both the practical aspects and the impact on agriculture, such as morphology, density and the absence of any pollutants, as shown in
Section 2.3.
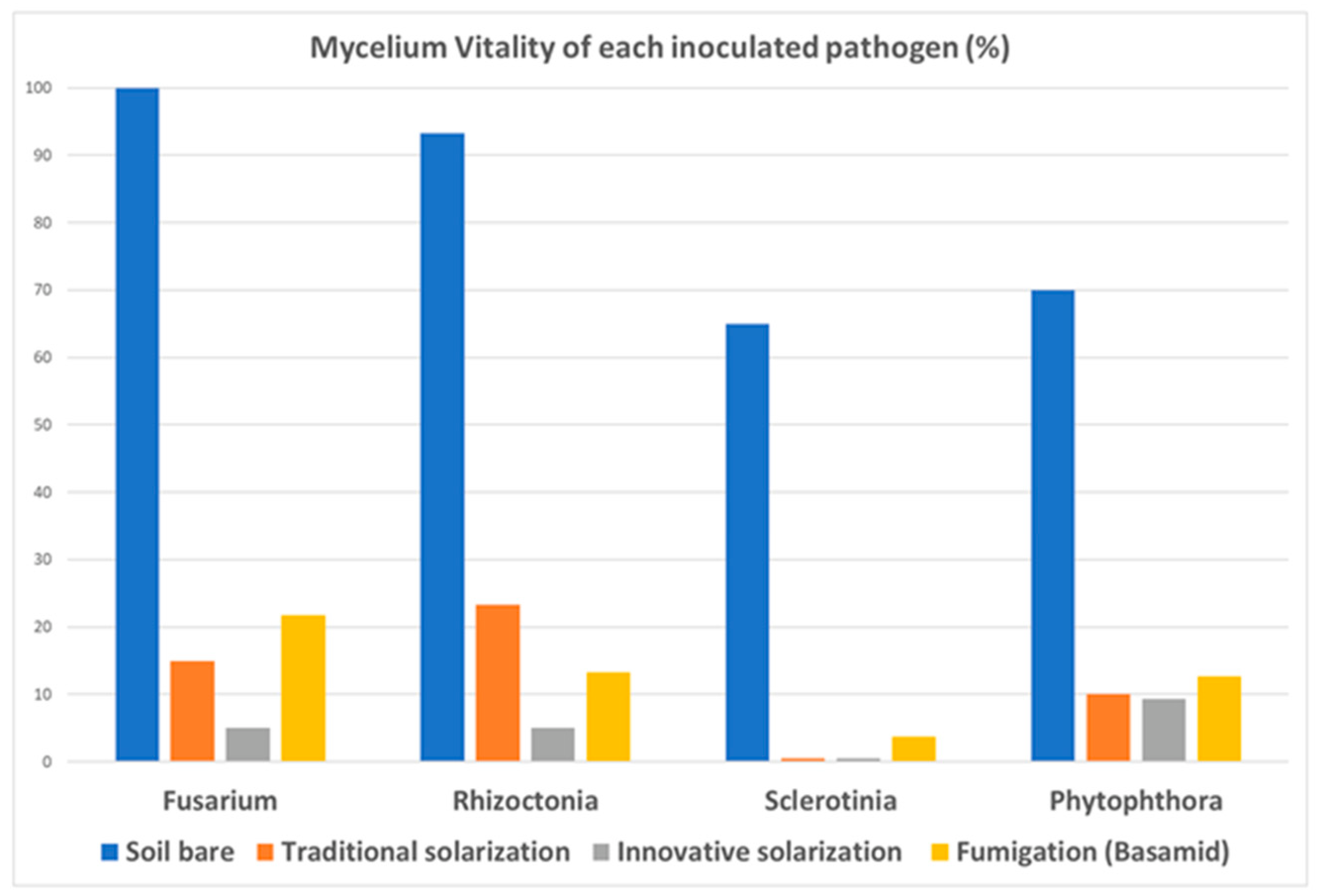
The main experimental results of our trials arose from the vitality control of pathogens in the different parcels, 30 days after exposure. T-bags were recovered from each parcel at the end of the trial and sent to the laboratory for pathogen control. The survival of each pathogen was evaluated by counting the kernels that developed the mycelium of the inoculated pathogen. The percentages of infected kernels are reported in
Table 5.
As expected, pathogen vitality in non-treated parcel P1 was very high (percentages range 65–100%).
Vitality in the P2 parcel considerably decreased in the range of 0–23%, according to the standard results obtained via traditional solarization using appropriate plastic covers.
A further significant decrease occurred for parcel P3, with an estimated vitality in the range 0–9.3%. This huge result is due to the high temperature collected during the tests that, in fact, reached 70 °C at 5 cm depth, a lethal temperature for most common fungal diseases.
The most interesting results are highlighted by the comparison between parcels P3 and P4: there is a striking difference in performance between the innovative solarization technique and fumigation. This last result represents the main achievement of our experimentation because it demonstrates that the innovative solarization method considered here is capable of eliminating pathogens in soil in a more efficient way than fumigation based on Dazomet, which is a very widespread method.
Experimental data are shown in
Figure 7 to provide a useful graphic representation of the mycelium vitality of the inoculated pathogens and a direct comparison between the adopted methods performed in the trial.
After these first trials, we repeated the same experimental tests, increasing the number of trials and farms, and also considering pedoclimatic aspects across different latitudes (from Southern to Northern Italy) and soil types.
For these trials, we used the same materials and methods as in the former one. The new solarization method was compared to the fumigation approach, inoculating the same four pathogens tested in the previous trial as reported in
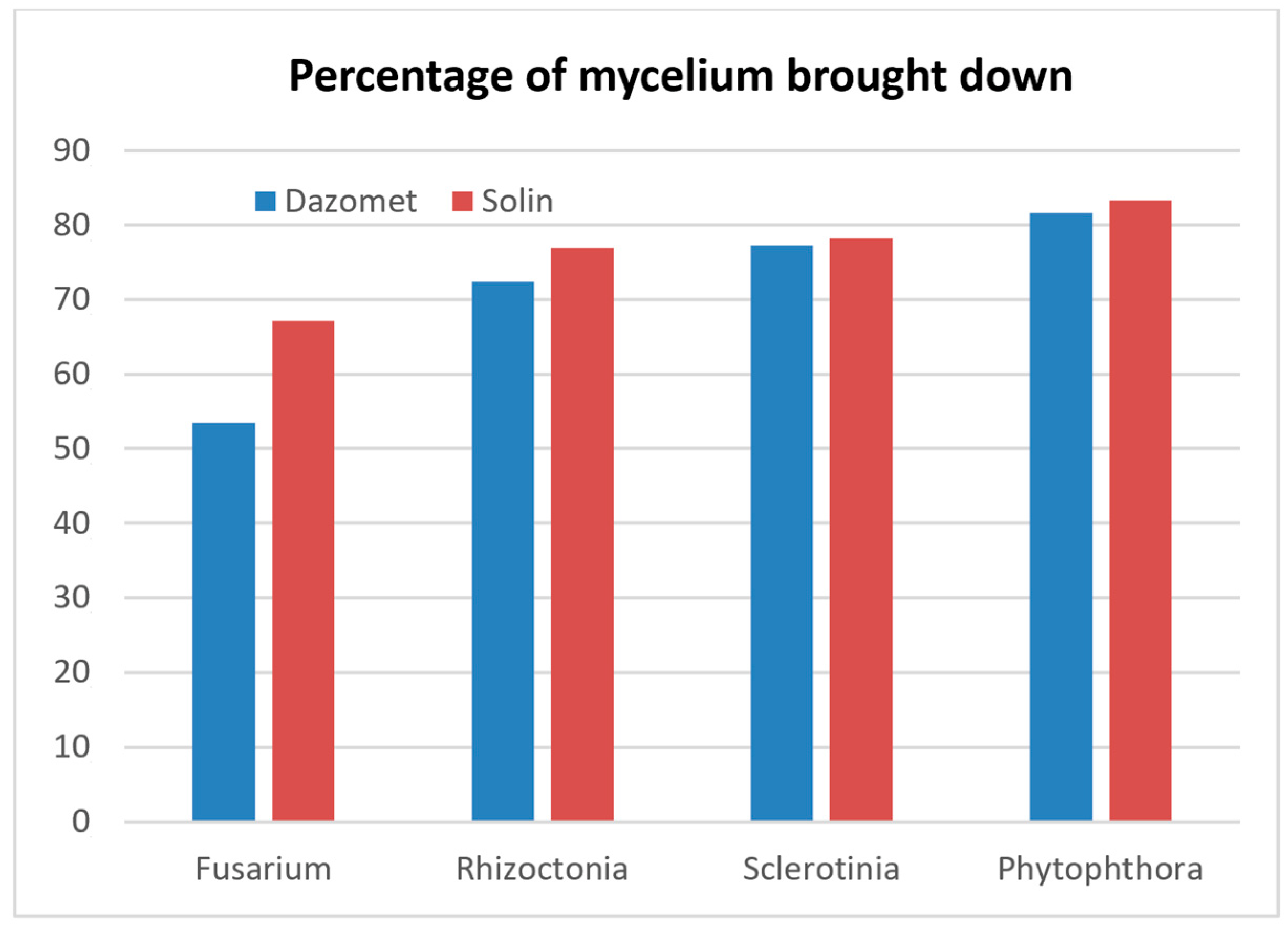
Table 3. The only difference was that this time, just two parcels were considered. Most of the trials were arranged in greenhouses (eight farms) and just one in the open field. According to the experimental protocol adopted in the first year of the trial, after 30 days, T-bags were extracted from the two parcels treated with different methods; then, they were analyzed in the laboratory and the survival of the pathogens was evaluated. The experimental results indicate a better abatement of several pathogens using soil solarization compared to Dazomet fumigation. The same trend was found upon analyzing the data collected from all farms involved in the trials, confirming that innovative solarization is more effective than Dazomet-based fumigation.
Figure 8 shows average values concerning the percentage of the eliminated pathogens using the two treatments.
The analysis of the experimental data collected in the second trial campaign highlights that it is possible to use a natural method, based only on solar heat, to obtain very effective control of the most common soil pathogens while still respecting the environment and safeguarding human health.
4. Discussions
The goal of our work was to present an innovative solarization model realized through a thin black layer on the soil that works as a “solar collector”, being a black body able to absorb incident energy. According to the previous results of different trials as well, we know that this new system is able to consistently produce very high temperatures in order to eliminate any pathogens and reduce the required exposure time from 60 to 30 days. The results obtained using this new biochar-based solarization prompt an investigative comparison of its performance with other methods, specifically fumigation.
Here we present the extremely positive experimental results regarding the comparison with the very common fumigant Dazomet, mainly used in horticulture. According to the results reported in
Table 5 and represented in
Figure 7, it seems opportune to us to continue the comparative investigation of this here-proposed natural alternative with other fumigants, such as 1,3-dichloropropene (1,3-D), chloropicrin (CP), Metam Sodium, and dimethyl disulfide.
This further research activity will be aimed not only at validating the new solarization method, but also and most importantly spreading it in contexts where fumigants are still the only solution due to intensive agriculture that requires quick solutions to eliminate pathogens in the soil between one crop and the next. Furthermore, the use of biochar as a main component in the formulation deserves consideration for the important role that it nowadays plays in sustainable agriculture, improving the quality of soils and crops, as widely reported in the literature [
31,
32]. There is evidence that the use of biochar significantly contributes to soil fertility, restoring impoverished land and mitigating the emission of greenhouse gases associated with agriculture. According to this awareness, we believe that this new system, based on the use of biochar, perfectly combines the requirements of sustainable agriculture. Moreover, it is important to underline also that this innovative solarization system has a cost three times less per hectare that Dazomet-based fumigation.
5. Conclusions
The positive results we acquired allow us to propose the introduction of a highly innovative and sustainable method to replace fumigation (which is very toxic) in agriculture. The adopted experimental set-up, consisting of four parcels, aimed to compare different soil treatments, inoculating the parcels with known pathogens with the purpose of evaluating the efficacy of each system as a function of the vitality of surviving pathogens at the end of treatment time.
The results achieved exceed expectations and they confirm that the innovative soil solarization system truly constitutes a valid alternative to the practice of fumigation, mainly in some contexts in which traditional solarization has not been taken into account because farmers cannot wait more than 30 days.
Nowadays, the key challenge for agriculture is to feed an increasing global population while at the same time reducing environmental impact and preserving natural resources for future generations through the adoption of new farming practices [
33,
34]. Our results demonstrate that it is possible to introduce new clean practices with “zero impact” to agriculture for the purpose of environmental sustainability.
The horizons that this opens up could represent a real revolution in agriculture, with dramatic changes that will have a great impact on traditional agronomical practices. Environmental problems such as air, soil and underground water pollution, increasing CO2, climate change, desertification, drought and, last but not least, the safeguarding of human health require clear, concrete and urgent answers. We believe that the innovative solarization here proposed could embody one of the solutions for a sustainable agriculture that must be environmentally respectful and ready to play an important role with respect to these issues, with additional great benefits for the farming economy, crops, food quality and soil health.
About the last aspect, it is important to underline that in 2022, the “Global Forum for Food And Agriculture” addressed this issue with “Sustainable Land Users: Food Security Starts With The Soil” (Berlin, 28 January 2022) [
35]. This is the proof that our work is consistent with the guidelines of the International Committee.