Environmental Impact of Sulaimani Steel Plant (Kurdistan Region, Iraq) on Soil Geochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Geologic Setting and the Study Area
2.2. Sampling and Sample Preparation
2.3. Sample Analysis
2.3.1. Grain Size Distribution
2.3.2. Organic Matter Content and pH
2.3.3. Mineral Composition
2.3.4. XRF Measurements
2.3.5. ICP-MS Measurements
2.4. Data Analysis
2.4.1. Statistical Analysis
2.4.2. The Assessment of Soil Pollution
3. Results
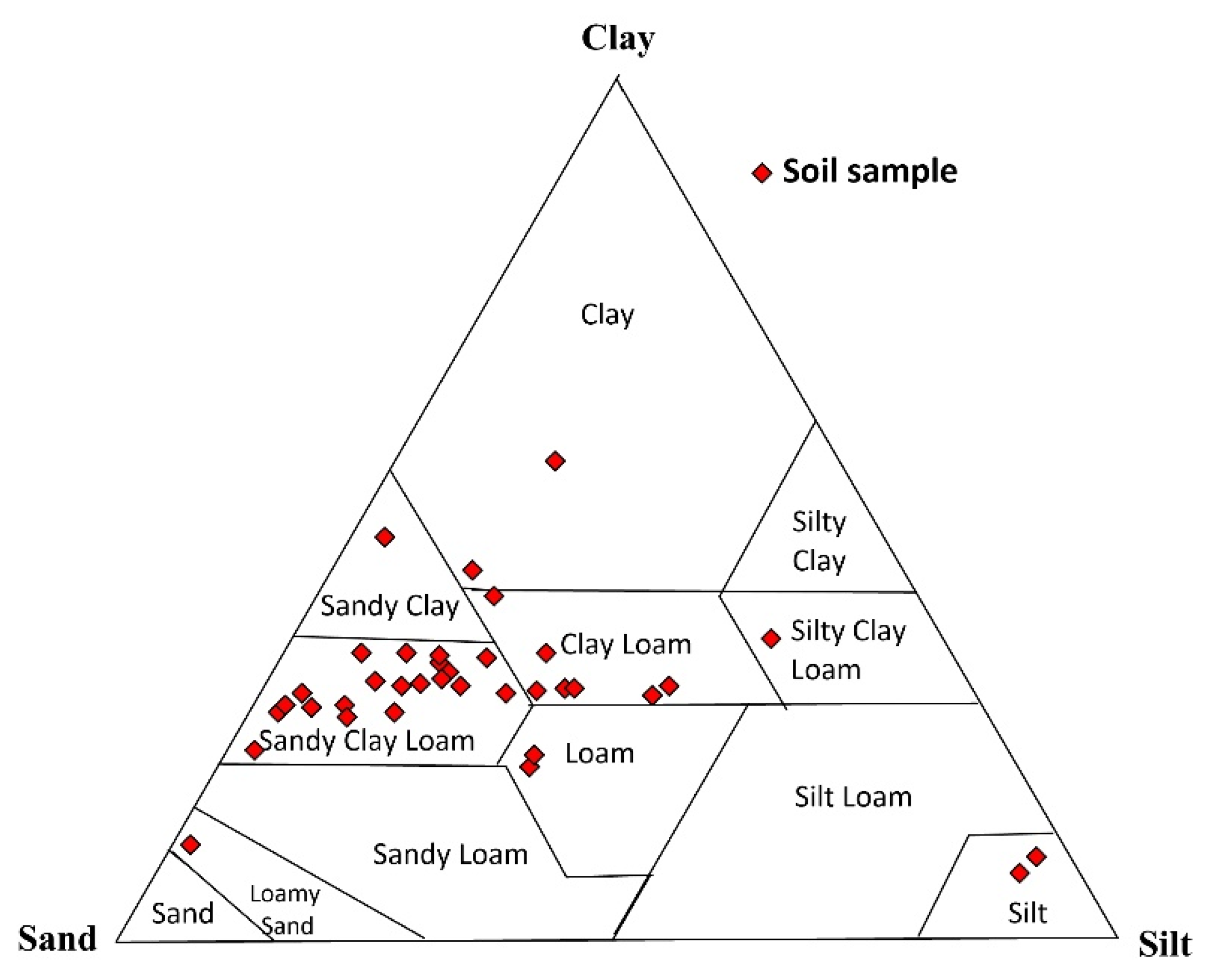
3.1. Grain Size Analysis
3.2. Organic Matter (OM) Content and pH Value
3.3. Mineralogy of Studied Soils
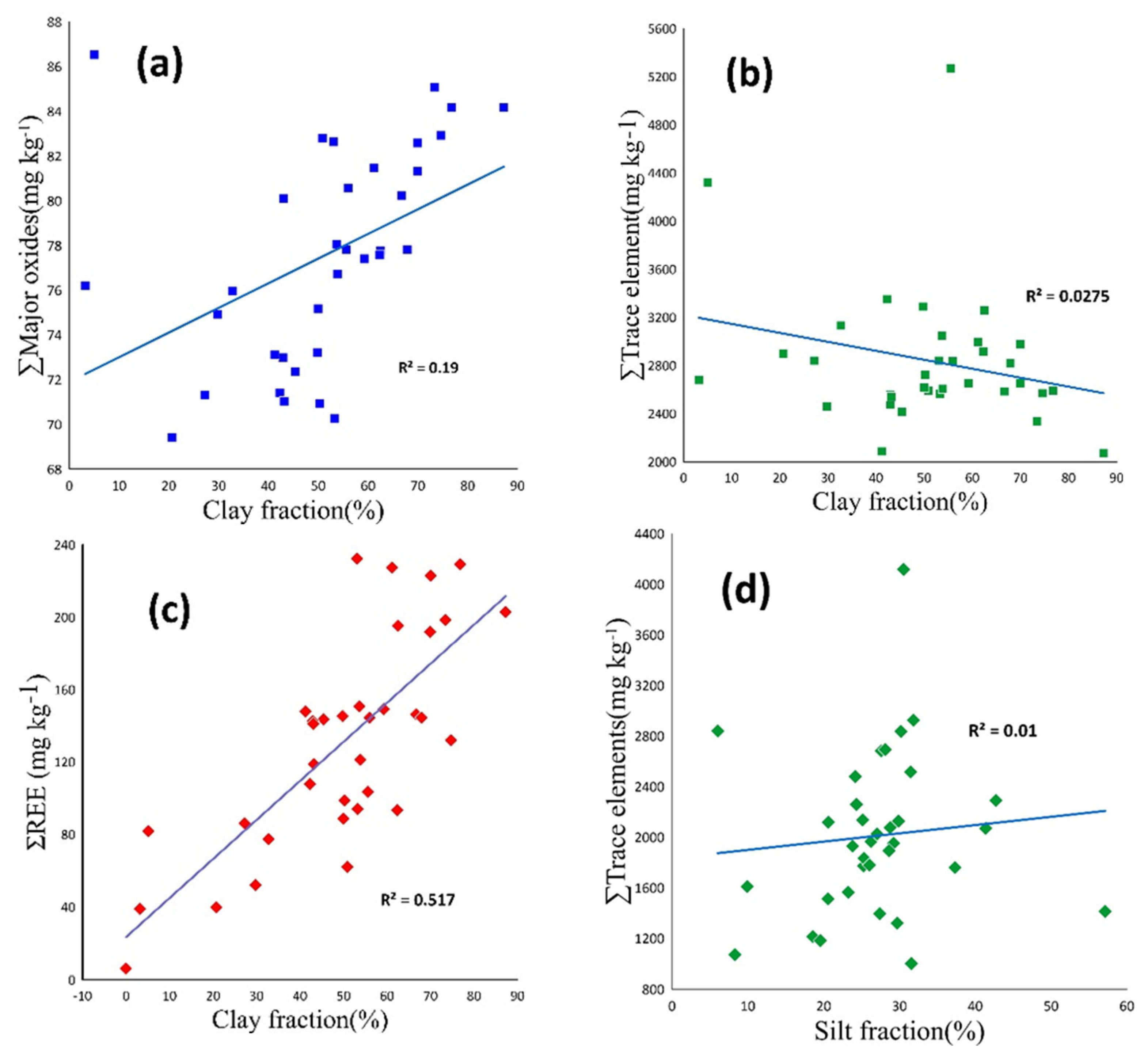
3.4. Geochemical Composition
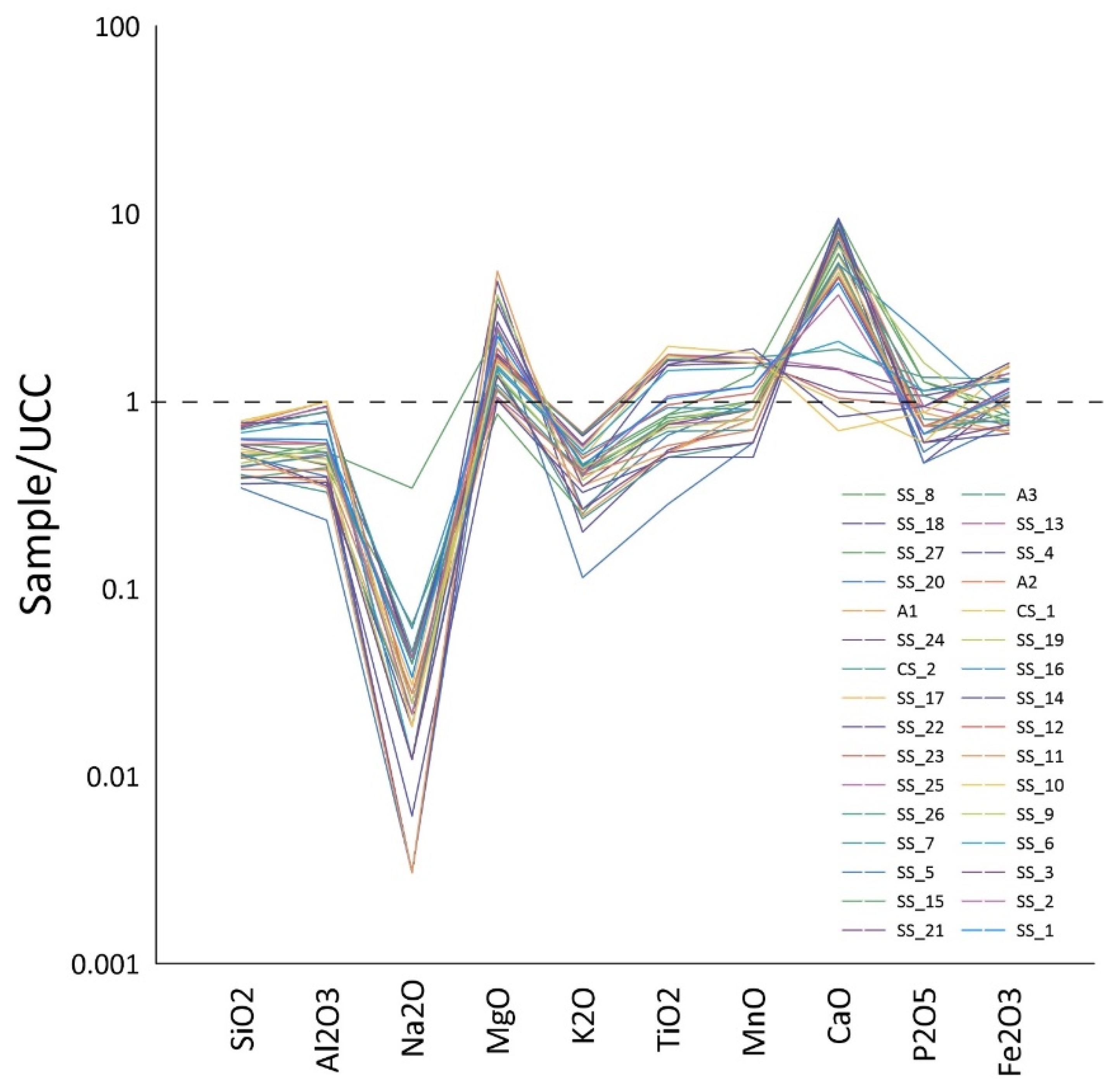
3.4.1. Major Elements
3.4.2. Trace Elements
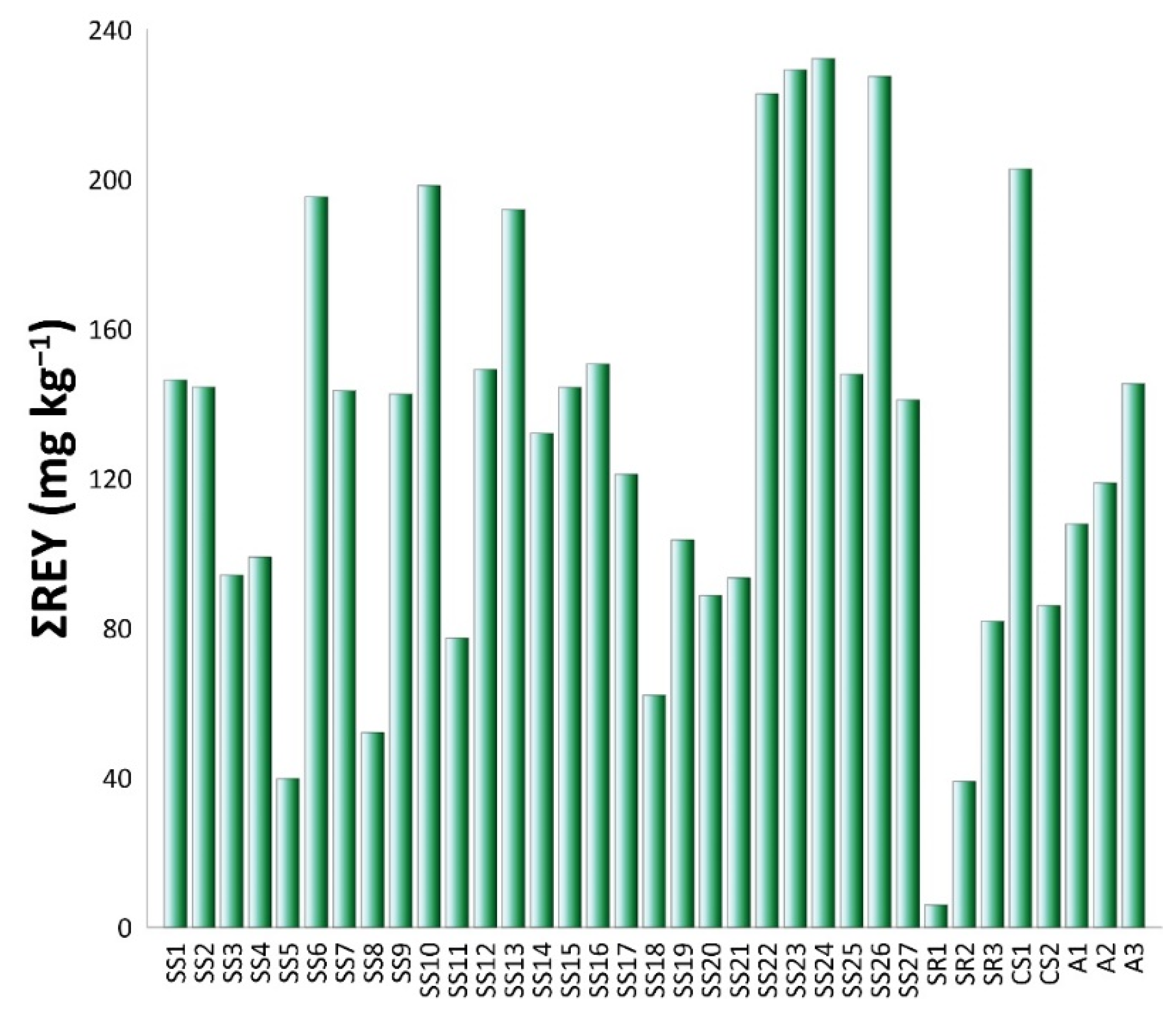
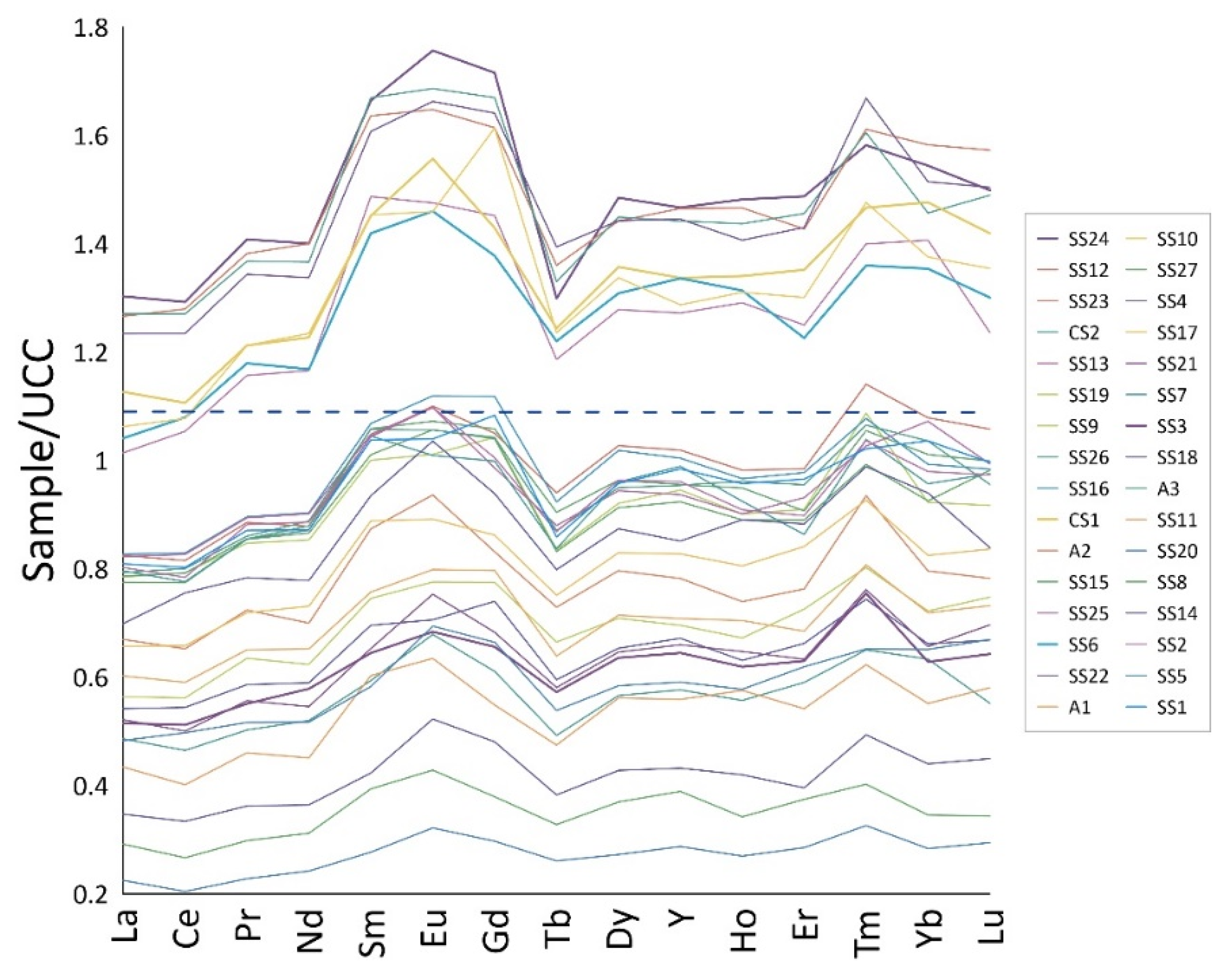
3.4.3. Rare Earth Elements (REEs)
4. Discussion
4.1. Grain Size Distribution of the Soil Samples
4.2. Organic Matter (OM) Distribution and pH in Soils
4.3. Mineral Composition of the Soils
4.4. Distribution of Elements in Studied Samples
4.4.1. Major Oxides
4.4.2. Trace Elements
4.4.3. Rare Earth Elements
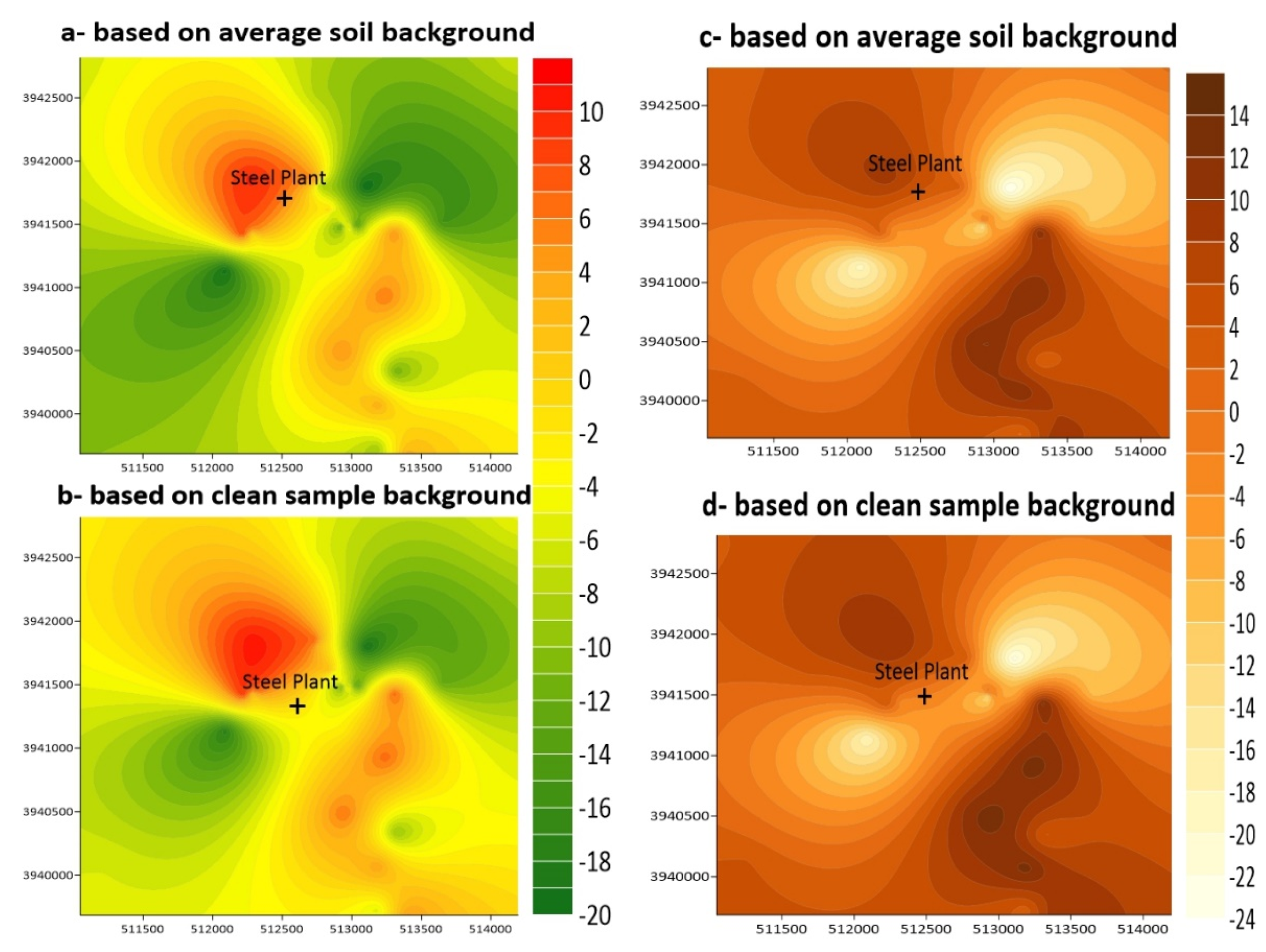
4.5. Contamination Assessment
4.5.1. Enrichment Factor (EF)
4.5.2. Geoaccumulation Index (Igeo)
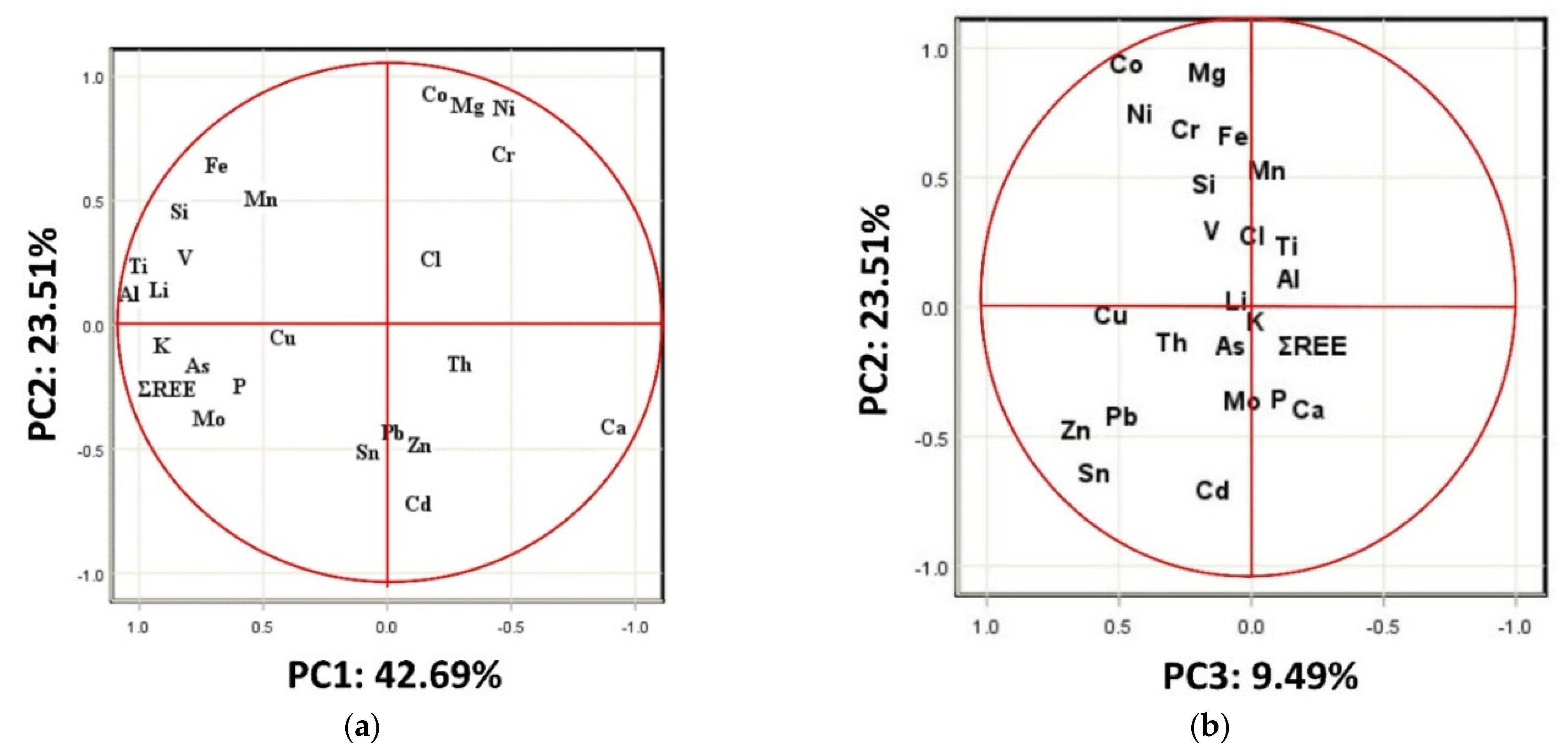
4.6. Principal Component Analysis (PCA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebel, A.; Davitashvili, T. Air, Water and Soil Quality Modelling for Risk and Impact Assessment; Springer: Berlin/Heidelberg, Germany, 2007; pp. 29–44. [Google Scholar]
- Harrison, R.M. Principles of Environmental Chemistry; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Cullen, J.M.; Allwood, J.M.; Bambach, M.D. Mapping the Global Flow of Steel: From Steelmaking to End-Use Goods. Environ. Sci. Technol. 2012, 46, 13048–13055. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.M.; Cullen, J.M.; Milford, R.L. Options for Achieving a 50% Cut in Industrial Carbon Emissions by 2050. Environ. Sci. Technol. 2010, 44, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, J.; Mercier, G.; Morel, J.L.; Blais, J.F.; Simonnot, M.O. Factors influencing the Zn and Mn extraction from pyrometallurgical sludge in the steel manufacturing industry. J. Environ. Manag. 2015, 158, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Strezov, V.; Chaudhary, C. Impacts of iron and steelmaking facilities on soil quality. J. Environ. Manag. 2017, 203, 1158–1162. [Google Scholar] [CrossRef]
- Lourenco, R.W.; Landim, P.M.B. Risk mapping of public health through geostatistics methods. Public Health Rep. 2005, 21, 150–160. [Google Scholar]
- Sethi, S.; Gupta, P. Soil Contamination: A Menace to Life; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Polizzotto, M.L.; Thompson, A. Bringing soil chemistry to environmental health science to tackle soil contaminants. Front. Environ. Sci. 2022, 10, 981607. [Google Scholar] [CrossRef]
- Schulin, R.; Curchod, F.; Mondeshka, M.; Daskalova, A.; Keller, A. Heavy metal contamination along a soil transect in the vicinity of the iron smelter of Kremikovtzi (Bulgaria). Geoderma 2007, 140, 52–61. [Google Scholar] [CrossRef]
- Yuan, G.L.; Sun, T.H.; Han, P.; Li, J. Environmental geochemical mapping and multivariate geostatistical analysis of heavy metals in top soils of a closed steel smelter: Capital Iron & Steel Factory, Beijing, China. J. Geochem. Explor. 2013, 130, 15–21. [Google Scholar]
- Mohiuddin, K.; Strezov, V.; Nelson, P.F.; Stelcer, E.; Evans, T. Mass and elemental distribution of atmospheric particles nearby blast furnace and electric arc furnace operated industrial areas in Australia. Sci. Total Environ. 2014, 487, 323–334. [Google Scholar] [CrossRef]
- Akporido, O.S.; Agbaire, O.P.; Ipeaiyeda, R.A. Effect of steel production on the quality of soil around udu section of Warri river in the vicinity of a steel plant, Nigeria. Asian J. Appl. Sci. 2014, 7, 552–567. [Google Scholar] [CrossRef][Green Version]
- Namuhani, N.; Kimumwe, C. Soil Contamination with Heavy Metals around Jinja Steel Rolling Mills in Jinja Municipality, Uganda. J. Health Pollut. 2017, 5, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ladonin, V.D. Lanthanides in Soils of the Cherepovets Steel Mill Impact Zone. Eurasian Soil Sci. 2017, 50, 672–680. [Google Scholar] [CrossRef]
- Mohialdeen, I.M.J.; Luqman, O.; Hamasalih, L.O.; Schwark, L. Geochemistry of a crude oil from a shallow well in Bazian Area, Sulaimani, Kurdistan Region, NE Iraq. J. Zankoy Sulaimani Part A 2013, 15, 3. [Google Scholar] [CrossRef]
- Rasheed, R.O.; Faqesaleh, L.I. Evaluation of some heavy metals from water and soil of Bazian Oil Refinery within Sulaimani Governorate, IKR. Marsh Bull 2016, 11, 123. [Google Scholar]
- AL-Taay, A.S.M.; Al-Assie, A.H.A.; Rasheed, O.R. Impact of Bazian Cement Factory on Air, Water, Soil, And Some Green Plants in Sulaimani City-Iraq. Iraqi J. Agric. Sci. 2018, 1027, 354–366. [Google Scholar]
- Ahmed, T.H. Groundwater Potential Mapping and Wellhead Protection Areas in Bazian Sub-Basin, Sulaimani, Kurdistan Region, Iraq. Ph.D. Thesis, University of Sulaimani, Sulaimani, Iraq, 2020. [Google Scholar]
- Hamakarim, B.M. Environmental Evaluation of Some Heavy Metals and Background Radiation Pollution in Soils around the Scrap Metal Recycling Factories in Sulaimani Governorate-Iraq. Ph.D. Thesis, University of Sulaimani, Sulaimani, Iraq, 2020; 137p. [Google Scholar]
- Jassim, S.Z.; Guff, J.C. Geology of Iraq; Jassim, S.Z., Ed.; Directorate General for Geological Survey and Mineral Investigations Publication: Brno, Czech Republic, 2006; 445p. [Google Scholar]
- Hamamin, D.F. Hydrogeological Assessment and Groundwater Vulnerability Map of Basara Basin, Sulaimani Governorate, Iraqi Kurdistan Region. Ph.D. Thesis, University of Sulaimani, Sulaymaniyah, Iraq, 2011; 227p. [Google Scholar]
- Ahmad, K.H.K. Facies Changes Between Kolosh and Sinjar Formations Along Zagros Fold–Thrust Belt in Iraqi Kurdistan Region. J. Geogr. Geol. 2016, 8, 1. [Google Scholar]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Mass Fractions of Forty-Six Major and Trace elements, including Rare Earth Elements, in Sediment and Soil Reference Materials used in Environmental Studies. Geostand. Geoanalytical Res. 2016, 41, 123–135. [Google Scholar] [CrossRef]
- D422–63 ASTM; Standard Test Method for Particle-Size Analysis of Soils. ATSM International: West Conshohocken, PA, USA, 2014.
- D854–14 ASTM; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ATSM International: West Conshohocken, PA, USA, 2014.
- El-Wakeel, S.K.; Riley, J.P. The Determination of Organic Carbon in Marine Muds. ICES J. Mar. Sci. 1957, 22, 180–183. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region, 3rd ed.; ICARDA (International Center for Agricultural Research in the Dry Areas): Beirut, Lebanon, 2013. [Google Scholar]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geol. J. 1969, 2, 108–118. [Google Scholar]
- Ridgway, J.; Shimmield, G. Estuaries as Repositories of Historical Contamination and their Impact on Shelf Seas. Estuar. Coast. Shelf Sci. 2002, 55, 903–928. [Google Scholar] [CrossRef]
- Khudhur, N.S.; Khudhur, S.H.M.; Ahmad, I.N. An Assessment of Heavy Metal Soil Contamination in a Steel Factory and the Surrounding Area in Erbil. Jordan J. Earth Environ. Sci. 2018, 9, 1–11. [Google Scholar]
- Loska, K.; Wiechuła, D.; Barska, B.; Cebula, E.; Chojnecka, A. Assessment of arsenic enrichment of cultivated soils in Southern Poland. Pol. J. Environ. Stud. 2003, 12, 187–192. [Google Scholar]
- Muller, G. Die Schwermetallbelstung der sediment des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chem. Zeitung. 1981, 105, 156–164. [Google Scholar]
- Kabata-Pendias, H.A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 13 978-3-540-32713-4. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Rudnick, R.L., Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 1–64. [Google Scholar]
- USDA (U.S. Department of Agriculture). Keys to Soil Taxonomy, 10th ed.; USDA: Washington, DC, USA, 2006; p. 332. [Google Scholar]
- Maslennikova, S.; Larina, N.; Larin, S. The effect of sediment grain size on heavy metal content. Lakes Reserv. Ponds 2012, 6, 43–54. [Google Scholar]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef] [PubMed]
- Al-Swadi, H.A.; Usman, A.R.A.; Al-Farraj, A.S.; Al-Wabel, M.I.; Ahmad, M.; Al-Faraj, A. Sources, toxicity potential, and human health risk assessment of heavy metals-laden soil and dust of urban and suburban areas as affected by industrial and mining activities. Sci. Rep. 2022, 12, 8972. [Google Scholar] [CrossRef]
- Mirza, A.T. Composition and phase mineral variation of Portland cement in Mass Factory Sulaimani–Kurdistan Region NE-Iraq. Int. J. Basic Appl. Sci. 2012, 12, 109–118. [Google Scholar]
- Al-Khuzie, D.K.; Al-Hatem, Z.; Shabar, H.A.; Hassan, W.F.; Abdulnabi, Z.A. Heavy metal distribution in grain size fraction in surface sediments: Pollution assessment of Iraqi Coasts. Eco. Env. Cons. 2021, 27, S388–S398. [Google Scholar]
- Horowitz, E.; Elrick, K. The relation of stream sediment surface area, grain size and surface area to trace element chemistry. Appl. Geochem. 1987, 2, 437–451. [Google Scholar] [CrossRef]
- Soureiyatou, F.D.; Chavom, M.B.; Paul-désiré, N. Trace and rare earth elements (REE) distribution in Ngaye alluvial sediments of Mbere-Djerem Basin, North Cameroon: Implications for origin, depositional conditions and paleoclimate reconstitution. SN Appl. Sci. 2020, 2, 2054. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of rare earth elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Rao, W.; Tan, H.; Jiang, S.; Chen, J. Trace element and REE geochemistry of fine- and coarse-grained sands in the Ordos deserts and links with sediments in surrounding areas. Geochemistry 2011, 71, 155–170. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Craigie, N. Principles of Elemental Chemostratigraphy. In Advances in Oil and Gas Exploration & Production; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Wang, X.; Deng, X. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, G.; Liu, M.; Wang, L. Geochemical Characteristics of Rare Earth Elements in Soils from Puding Karst Critical Zone Observatory, Southwest China. Sustainability 2019, 11, 4963. [Google Scholar] [CrossRef]
- Al Obaidy, A.M.J.; Al Mashhadi, A.A.M. Heavy metal contaminations in urban soil within Baghdad City, Iraq. J. Environ. Prot. 2013, 4, 72–82. [Google Scholar] [CrossRef]
- Kazlauskaitė-Jadzevičė, A.; Volungevičius, J.; Gregorauskienė, V.; Marcinkonis, S. The role of PH in heavy metal contamination of urban soil. J. Environ. Eng. Landsc. Manag. 2014, 22, 311–318. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Y.; Pang, H.; Tang, H.; Wang, J.; Zhang, S.; Wang, X. Chapter 1—Novel nanomaterials for environmental remediation of toxic metal ions and radionuclides. In Emerging Nanomaterials for Recovery of Toxic and Radioactive Metal Ions from Environmental Media; Wang, X., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–47. [Google Scholar] [CrossRef]
- Al-Hakari, S.H.S. Geometric Analysis and Structural Evolution of NW Sulaimani Area, Kurdistan Region, Iraq. Ph.D. Thesis, University of Sulaimani, Sulaimanyah, Iraq, 2011; 309p. [Google Scholar]
- Salih, A.L.M. Sedimentology of Sinjar and Khurmala Formations (Paleocene-Lower Eocene) in Northern Iraq. Ph.D. Thesis, University of Baghdad, Baghdad, Iraq, 2013; 215p. [Google Scholar]
- Flugel, E. Microfacies of Carbonate Rocks, 2nd ed.; Springer: Berlin, Germany, 2010; p. 976. [Google Scholar]
- Sadiq, D.M. Geochemistry and Geochronology of Detrital Heavy Minerals in Cretaceous Flysch and Cenozoic Molasse of the Northeastern Iraq. Ph.D. Thesis, University of Sulaimani, Sulaimani, Iraq, 2020; 142p. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill Publishing Company: Austin, TX, USA, 1974; 182p. [Google Scholar]
- Smirnov, P.; Deryagina, O.; Afanasieva, N.; Rudmin, M.; Gursky, H.-J. Clay Minerals and Detrital Material in Paleocene–Eocene Biogenic Siliceous Rocks (Sw Western Siberia): Implications for Volcanic and Depositional Environment Record. Geosciences 2020, 10, 162. [Google Scholar] [CrossRef]
- Mohialdeen, I.M.J.; Merza, T.A. Composition and Origin of White Carbonate Layer in Seramerg-Tagaran area, Sulaimani, Ne Iraq. Zanko 2004, 16, 17–23. [Google Scholar]
- Mohialdeen, I.M.J.; Merza, T.A. Manufacture of Brick Tiles from Local Raw Materials, N & NE Iraq. J. Zankoy Sulaimani 2005, 8, 31–45. [Google Scholar]
- Al-Tamimi, R.A.K.H. Identifying of Clay Minerals in Some Iraqi Soils. Ph.D. Thesis, University of Baghdad, Baghdad, Iraq, 1984. [Google Scholar]
- Al-Obaidi, B.S. Natural Occurance of Palygorskite in Some Iraqi Gypsiferous Soils. Ph.D. Thesis, University of Baghdad, Baghdad, Iraq, 2008. [Google Scholar]
- Worden, R.H.; Morad, S. Clay Minerals in Sandstones: Controls on Formation, Distribution and Evolution. Int. Assoc. Sedimentol. Spec. Publ. 2003, 34, 3–4. [Google Scholar] [CrossRef]
- Badawy, W.; Ghanim, E.; Duliu, O.; El Samman, H.; Frontasyeva, M. Major and trace element distribution in soil and sediments from the Egyptian central Nile Valley. J. Afr. Earth Sci. 2017, 131, 53–61. [Google Scholar] [CrossRef]
- Takeda, O.; Ouchi, T.; Okabe, T.H. Recent Progress in Titanium Extraction and Recycling. Met. Mater. Trans. A 2020, 51, 1315–1328. [Google Scholar] [CrossRef]
- Hill, I.; Worden, R.; Meighan, I. Geochemical evolution of a palaeolaterite: The Interbasaltic Formation, Northern Ireland. Chem. Geol. 2000, 166, 65–84. [Google Scholar] [CrossRef]
- Andersson, P.; Worden, R.; Hodgson, D.; Flint, S. Provenance evolution and chemostratigraphy of a Palaeozoic submarine fan-complex: Tanqua Karoo Basin, South Africa. Mar. Pet. Geol. 2004, 21, 555–577. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Chloride in soil: From nutrient to soil pollutant. Environ. Exp. Bot. 2018, 157, 299–309. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Geochemical fractions of rare earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 2015, 5, 12483. [Google Scholar] [CrossRef]
- Fiket, Z.; Medunic, G.; Kniewald, G. Rare earth element distribution in soil nearby thermal power plant. Environ. Earth Sci. 2016, 75, 598. [Google Scholar] [CrossRef]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Influence of the geological setting on the REE geochemistry of estuarine sediments: A case study of the Zrmanja River estuary (eastern Adriatic coast). J. Geochem. Explor. 2017, 182, 70–79. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Insights into the tolerance and phytoremediation potential of Coronopus didymus L. (Sm) grown under zinc stress. Chemosphere 2020, 244, 125350. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.R.; Ugarte, O.M.; Lima, L.H.V.; Silva, J.R.; da Silva, F.B.V.; Lins, S.A.D.S.; Nascimento, C.W.A.D. Risk assessment of heavy metals in soils and edible parts of vegetables grown on sites contaminated by an abandoned steel plant in Havana. Environ. Geochem. Health 2022, 44, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Bo, X.; Qu, J.; Wang, L.; Zhou, J.; Li, S. Characteristics of PCDD/Fs and metals in surface soil around an iron and steel plant in North China Plain. Chemosphere 2018, 216, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Issa, H.M.; Alshatteri, A.H. Source Identification, Ecological Risk and Spatial Analysis of Heavy Metals Contamination in Agricultural Soils of Tanjaro Area, Kurdistan Region, Iraq. UKH J. Sci. Eng. 2021, 5, 18–27. [Google Scholar] [CrossRef]
- Soltani-Gerdefaramarzi, S.; Ghasemi, M.; Ghanbarian, B. Geogenic and anthropogenic sources identification and ecological risk assessment of heavy metals in the urban soil of Yazd, central Iran. PLoS ONE 2021, 16, e0260418. [Google Scholar] [CrossRef]
- Fiket, Z.; Mlakar, M.; Kniewal, G. Distribution of Rare Earth Elements in Sediments of the Marine Lake Mir (Dugi Otok, Croatia). Geosciences 2018, 8, 301. [Google Scholar] [CrossRef]
- Reck, B.K.; Müller, D.B.; Rostkowski, K.; Graedel, T.E. Anthropogenic Nickel Cycle: Insights into Use, Trade, and Recycling. Environ. Sci. Technol. 2008, 42, 3394–3400. [Google Scholar] [CrossRef]


| Parameters | HR ICP-MS Operating Conditions |
|---|---|
| RF power: | 1200 W |
| Coolant Ar flow: | 15.5 L min−1 |
| Auxiliary Ar flow: | 0.85 L min−1 |
| Sample gas flow rate: | 1.063 L min−1 |
| Torch: | Fassel type, 1.5 mm i.d. |
| Nebulizer: | Micro Mist, AR40-1-F02, 0.2 mL min−1 (Glass Expansion) |
| Spray chamber: | Twister, 50 mL, Cyclonic (Glass Expansion) |
| Sample cone: | Ni, 1.1 mm aperture i.d. |
| Skimmer cone: | Ni, 0.8 mm aperture i.d. |
| Acquisition mode: | E-scan |
| No. Scans: | 20 (5 runs, 4 passes) |
| Resolution: | low (LR) = 300 |
| medium (MR) = 4000 | |
| high (HR) = 10,000 | |
| Calibration: | External |
| Sample | % Sand | % Silt | % Clay | OM | pH |
|---|---|---|---|---|---|
| A1 | 25.9 | 31.8 | 42.3 | 7.9 | 7.4 |
| A2 | 27.0 | 29.9 | 43.1 | 9.3 | 7.7 |
| A3 | 20.1 | 30.2 | 49.8 | 3.4 | 7.9 |
| SS1 | 8.1 | 25.3 | 66.7 | 2.2 | 8.9 |
| SS2 | 8.3 | 23.8 | 67.9 | 1.6 | 8.9 |
| SS3 | 17.5 | 29.2 | 53.2 | 2.4 | 8.9 |
| SS2 | 8.3 | 23.8 | 67.9 | 1.6 | 8.9 |
| SS3 | 17.5 | 29.2 | 53.2 | 2.4 | 8.9 |
| SS4 | 25.5 | 24.3 | 50.2 | 1.6 | 8.9 |
| SS5 | 47.7 | 31.6 | 20.7 | 0.3 | 8.7 |
| SS6 | 9.9 | 27.6 | 62.5 | 7.5 | 8.2 |
| SS7 | 28.4 | 26.2 | 45.4 | 8.5 | 8.6 |
| SS8 | 40.5 | 29.7 | 29.8 | 1.8 | 8.7 |
| SS9 | 15.7 | 41.4 | 42.9 | 6.2 | 8.3 |
| SS10 | 1.3 | 25.3 | 73.4 | 5.5 | 8.4 |
| SS11 | 39.9 | 27.4 | 32.7 | 5.1 | 8.5 |
| SS12 | 14.7 | 26.0 | 59.2 | 6.5 | 8.3 |
| SS13 | 4.9 | 25.1 | 69.9 | 7.8 | 8.2 |
| SS14 | 4.8 | 20.6 | 74.6 | 6.3 | 8.3 |
| SS15 | 17.0 | 27.0 | 56.0 | 5.3 | 8.4 |
| SS16 | 18.3 | 28.1 | 53.7 | 4.6 | 8.7 |
| SS17 | 17.6 | 28.6 | 53.8 | 2.3 | 8.9 |
| SS18 | 30.6 | 18.6 | 50.8 | 1.5 | 9.0 |
| SS19 | 13.9 | 30.5 | 55.6 | 1.9 | 8.8 |
| SS20 | 30.5 | 19.6 | 50.0 | 1.2 | 8.7 |
| SS21 | 14.4 | 23.2 | 62.3 | 3.1 | 8.3 |
| SS22 | 5.9 | 24.2 | 70.0 | 9.3 | 8.0 |
| SS23 | 2.6 | 20.6 | 76.8 | 6.5 | 8.0 |
| SS24 | 4.2 | 42.7 | 53.1 | 7.5 | 8.1 |
| SS25 | 21.5 | 37.3 | 41.2 | 5.4 | 8.5 |
| SS26 | 7.4 | 31.5 | 61.1 | 8.6 | 8.3 |
| SS27 | 28.2 | 28.8 | 43.0 | 5.8 | 8.8 |
| CS1 | 2.9 | 9.9 | 87.2 | 4.6 | 7.7 |
| CS2 | 15.7 | 57.1 | 27.2 | 4.4 | 7.9 |
| SR1 | _ | _ | _ | 0.5 | 9.9 |
| SR2 | 88.6 | 8.3 | 3.2 | 0.4 | 9.4 |
| SR3 | 88.9 | 6.0 | 5.1 | 2.7 | 8.4 |
| Average | 22.0 | 27.0 | 51.0 | 4.6 | 8.5 |
| Non Clay Minerals (%) | Clay Minerals (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Cal | Q | Ser | Fel | Clin | Py | He | Gy | Cha | Do | Mag | Mon | Ill | Pal | Chl |
| A1 | 51.8 | 13.5 | 4.4 | - | - | - | - | - | - | - | - | 15.5 | 10.2 | - | - |
| A2 | 48.7 | 17.0 | - | - | - | - | - | - | - | - | - | 20.9 | 12.4 | - | - |
| A3 | 11.7 | 13.5 | - | - | - | - | - | - | - | - | - | 26.6 | 10.7 | - | - |
| SS1 | 27.3 | 16.8 | - | - | - | - | - | - | - | - | - | 28.5 | 14.0 | 12.2 | - |
| SS2 | 23.7 | 25.3 | 14.0 | 6.6 | - | - | - | - | - | - | - | 29.0 | - | - | |
| SS3 | 53.0 | 18.9 | - | - | 7.7 | - | - | - | - | - | - | 11.6 | 8.2 | - | - |
| SS4 | 60.4 | 13.6 | - | - | - | - | - | - | - | - | - | 15.2 | 10.0 | - | - |
| SS5 | 57.9 | 13.3 | - | - | - | - | - | - | - | - | - | 24.3 | 3.5 | - | - |
| SS6 | 12.3 | 21.1 | - | - | - | - | - | - | - | - | 9.0 | 23.9 | 17.1 | 19.0 | - |
| SS7 | 41.1 | 16.9 | - | - | - | - | - | - | - | - | - | - | 12.6 | 28.4 | - |
| SS8 | 49.3 | 14.2 | 6.1 | 11.6 | - | - | - | - | - | - | - | 17.7 | - | - | - |
| SS9 | 38.5 | 7.9 | - | - | 17.3 | 11.1 | - | - | - | - | - | 10.3 | 13.6 | - | - |
| SS10 | - | 25.7 | 9.4 | - | - | - | - | - | - | - | - | 42.0 | 16.9 | - | - |
| SS11 | 29.9 | 25.9 | 28.0 | - | - | - | - | - | - | - | - | 8.0 | 7.5 | - | - |
| SS12 | 26.6 | 23.1 | - | - | - | 10.8 | - | - | - | - | - | 23.2 | 15.2 | - | - |
| SS13 | 4.8 | 19.0 | 9.3 | - | - | - | - | - | - | - | - | 43.1 | 17.7 | - | - |
| SS14 | - | 31.2 | - | - | - | - | - | - | - | - | - | 40.2 | 12.3 | 14.6 | - |
| SS15 | 32.1 | 23.7 | - | 16.4 | - | - | - | - | - | - | - | 21.5 | - | - | 4.1 |
| SS16 | 30.9 | 26.7 | - | - | - | 8.1 | - | - | - | - | - | 12.6 | 16.0 | - | - |
| SS17 | 37.1 | 16.5 | 9.8 | - | - | - | - | - | - | - | - | 15.7 | 12.6 | - | - |
| SS18 | 39.9 | 14.0 | - | - | - | - | - | - | - | - | - | 46.0 | - | - | - |
| SS19 | 21.8 | 13.7 | 17.0 | 6.3 | - | - | - | - | - | - | - | 35.9 | - | - | - |
| SS20 | 27.8 | 3.5 | 17.0 | - | - | 12.0 | - | - | 8.2 | - | - | 31.0 | - | - | - |
| SS21 | 31.0 | 20.0 | 14.9 | - | - | - | - | - | - | - | - | 26.0 | 6.8 | - | - |
| SS22 | - | 19.7 | 6.5 | - | - | 18.0 | - | - | - | - | - | 34.1 | 20.1 | - | - |
| SS23 | 4.8 | 17.0 | 4.8 | - | - | - | - | - | - | - | - | 45.1 | 20.8 | - | - |
| SS24 | 14.3 | 21.0 | - | - | - | - | - | - | - | - | - | 42.2 | 18.1 | - | - |
| SS25 | 49.1 | 14.0 | - | - | - | - | - | - | - | - | - | 22.2 | 13.3 | - | - |
| SS26 | 20.5 | 14.5 | - | - | 5.8 | 4.6 | - | - | - | - | - | 32.4 | 20.5 | - | - |
| SS27 | 44.9 | 11.9 | - | 19.6 | - | - | 3.5 | 0.5 | - | - | - | 18.1 | - | - | - |
| CS1 | - | 11.6 | - | 8.2 | 12.3 | - | - | - | - | - | - | 67.0 | - | - | - |
| CS2 | 51.7 | 8.9 | - | - | - | - | - | - | - | - | - | 30.4 | 7.2 | - | - |
| SR1 | 94.7 | 2.5 | - | - | - | - | - | - | - | 1.6 | - | - | - | - | - |
| SR2 | 37.9 | 3.1 | 34.6 | - | - | - | - | - | - | - | - | 20.9 | 2.7 | - | - |
| SR3 | - | 13.6 | 36.3 | - | - | - | - | - | - | - | - | 38.0 | 10.7 | - | - |
| Sample | SiO2 | Al2O3 | Na2O | MgO | K2O | TiO2 | MnO | CaO | P2O5 | Fe2O3 | SO3 | LOI | Total | Al2O3/TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 26.7 | 5.77 | 0.07 | 2.55 | 0.99 | 0.34 | 0.08 | 31.2 | 0.13 | 3.39 | 0.21 | 28.4 | 99.8 | 17.0 |
| A2 | 28.7 | 6.59 | 0.09 | 2.89 | 1.12 | 0.37 | 0.07 | 27.3 | 0.11 | 3.51 | 0.25 | 28.8 | 99.8 | 17.8 |
| A3 | 34.1 | 8.23 | 0.13 | 2.82 | 1.14 | 0.50 | 0.09 | 22.0 | 0.16 | 3.94 | 0.18 | 26.3 | 99.5 | 16.5 |
| SS1 | 41.9 | 9.56 | 0.11 | 5.48 | 1.26 | 0.66 | 0.12 | 15.3 | 0.10 | 5.60 | 0.15 | 19.5 | 99.7 | 14.5 |
| SS2 | 41.3 | 9.13 | 0.07 | 6.11 | 1.12 | 0.68 | 0.12 | 13.2 | 0.10 | 5.80 | 0.21 | 21.7 | 99.5 | 13.4 |
| SS3 | 26.1 | 6.05 | 0.04 | 3.38 | 0.74 | 0.34 | 0.06 | 29.7 | 0.09 | 3.78 | 0.01 | 29.6 | 99.9 | 17.8 |
| SS4 | 24.1 | 5.67 | 0.02 | 2.51 | 0.91 | 0.32 | 0.05 | 33.8 | 0.09 | 3.37 | 0.08 | 28.9 | 99.8 | 17.7 |
| SS5 | 22.8 | 3.56 | 0.01 | 5.95 | 0.32 | 0.18 | 0.06 | 32.4 | 0.07 | 3.82 | 0.16 | 30.4 | 99.8 | 19.8 |
| SS6 | 45.1 | 12.1 | 0.04 | 3.80 | 1.51 | 0.93 | 0.15 | 7.5 | 0.17 | 6.39 | 0.14 | 21.8 | 99.6 | 13.0 |
| SS7 | 29.9 | 7.86 | 0.13 | 3.02 | 1.15 | 0.44 | 0.07 | 25.6 | 0.12 | 3.87 | 0.20 | 27.5 | 99.9 | 17.9 |
| SS8 | 25.6 | 6.80 | 0.21 | 2.11 | 0.70 | 0.54 | 0.14 | 33.6 | 0.19 | 4.69 | 0.28 | 25.0 | 99.9 | 12.6 |
| SS9 | 31.0 | 8.69 | 0.08 | 3.46 | 1.23 | 0.46 | 0.08 | 24.0 | 0.24 | 3.58 | 0.19 | 26.9 | 99.9 | 18.9 |
| SS10 | 52.2 | 15.2 | 0.06 | 4.11 | 1.53 | 1.25 | 0.18 | 2.49 | 0.13 | 7.82 | 0.13 | 14.8 | 99.8 | 12.2 |
| SS11 | 33.6 | 5.31 | 0.01 | 12.2 | 0.68 | 0.34 | 0.09 | 18.6 | 0.10 | 4.94 | 0.11 | 23.7 | 99.7 | 15.6 |
| SS12 | 39.2 | 9.12 | 0.08 | 4.72 | 1.38 | 0.61 | 0.11 | 16.6 | 0.11 | 5.34 | 0.20 | 22.4 | 99.8 | 15.0 |
| SS13 | 48.7 | 14.3 | 0.06 | 4.06 | 1.60 | 1.11 | 0.17 | 5.34 | 0.14 | 7.09 | 0.04 | 17.1 | 99.7 | 12.9 |
| SS14 | 51.2 | 11.6 | 0.04 | 6.56 | 1.11 | 1.00 | 0.19 | 2.95 | 0.14 | 8.03 | 0.10 | 16.8 | 99.8 | 11.6 |
| SS15 | 39.2 | 8.22 | 1.12 | 5.84 | 1.29 | 0.54 | 0.10 | 19.7 | 0.10 | 4.36 | 0.19 | 22.1 | 102.6 | 15.2 |
| SS16 | 38.8 | 9.16 | 0.20 | 3.69 | 1.45 | 0.59 | 0.09 | 19.2 | 0.33 | 4.36 | 0.14 | 21.8 | 99.9 | 15.5 |
| SS17 | 35.6 | 7.54 | 0.10 | 5.16 | 1.17 | 0.48 | 0.09 | 21.7 | 0.11 | 4.73 | 0.07 | 23.1 | 99.8 | 15.7 |
| SS18 | 34.9 | 5.44 | 0.06 | 10.8 | 0.56 | 0.35 | 0.08 | 25.1 | 0.07 | 5.37 | 0.08 | 16.9 | 99.7 | 15.5 |
| SS19 | 36.9 | 7.11 | 0.04 | 9.12 | 1.06 | 0.49 | 0.10 | 17.4 | 0.10 | 5.44 | 0.04 | 21.7 | 99.5 | 14.5 |
| SS20 | 34.1 | 6.09 | 0.01 | 8.90 | 0.74 | 0.42 | 0.10 | 19.4 | 0.08 | 5.30 | 0.07 | 24.6 | 99.8 | 14.5 |
| SS21 | 38.4 | 6.97 | 0.01 | 8.14 | 0.98 | 0.48 | 0.09 | 16.3 | 0.10 | 5.90 | 0.14 | 22.2 | 99.8 | 14.5 |
| SS22 | 49.3 | 13.4 | 0.14 | 4.42 | 1.82 | 0.99 | 0.16 | 4.04 | 0.16 | 6.54 | 0.32 | 18.5 | 99.8 | 13.6 |
| SS23 | 49.8 | 15.3 | 0.08 | 4.18 | 1.89 | 1.13 | 0.17 | 3.72 | 0.14 | 7.64 | 0.15 | 15.7 | 99.8 | 13.6 |
| SS24 | 48.0 | 14.5 | 0.13 | 4.31 | 1.64 | 1.05 | 0.16 | 5.28 | 0.17 | 7.04 | 0.45 | 17.1 | 99.8 | 13.8 |
| SS25 | 29.5 | 8.08 | 0.14 | 2.60 | 1.20 | 0.48 | 0.08 | 27.1 | 0.14 | 3.72 | 0.03 | 26.8 | 99.9 | 16.8 |
| SS26 | 47.0 | 13.5 | 0.15 | 4.04 | 1.86 | 1.07 | 0.17 | 6.76 | 0.20 | 6.62 | 0.07 | 18.4 | 99.8 | 12.7 |
| SS27 | 33.0 | 9.04 | 0.13 | 3.33 | 1.25 | 0.52 | 0.09 | 28.1 | 0.19 | 4.17 | 0.22 | 19.8 | 99.9 | 17.4 |
| CS1 | 50.6 | 15.4 | 0.06 | 3.88 | 1.39 | 1.11 | 0.16 | 3.54 | 0.09 | 7.88 | 0.07 | 15.5 | 99.7 | 13.9 |
| CS2 | 26.9 | 5.02 | 0.07 | 3.60 | 0.66 | 0.32 | 0.06 | 30.3 | 0.11 | 3.99 | 0.22 | 28.5 | 99.8 | 15.7 |
| SR1 | 2.50 | 0.04 | 0.01 | 0.35 | 0.01 | 0.02 | 0.01 | 53.5 | 0.01 | 0.83 | 0.13 | 42.5 | 99.9 | 2.00 |
| SR2 | 28.2 | 2.96 | 0.04 | 18.5 | 0.25 | 0.19 | 0.26 | 21.2 | 0.04 | 4.49 | 0.01 | 23.5 | 99.7 | 15.6 |
| SR3 | 45.6 | 7.64 | 0.01 | 16.3 | 0.97 | 0.71 | 0.08 | 6.56 | 0.09 | 8.53 | 0.09 | 13.2 | 99.8 | 10.8 |
| Average | 36.3 | 8.60 | 0.11 | 5.51 | 1.11 | 0.60 | 0.11 | 19.2 | 0.13 | 5.20 | 0.15 | 22.9 | 99.8 | 14.3 |
| Sample | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS1 | 20.7 | 25.1 | 50.6 | 6.18 | 23.5 | 4.88 | 1.04 | 4.33 | 0.60 | 3.73 | 0.79 | 2.22 | 0.31 | 2.07 | 0.31 |
| SS2 | 20.2 | 24.9 | 49.4 | 6.26 | 23.9 | 4.90 | 1.10 | 4.02 | 0.61 | 3.76 | 0.75 | 2.07 | 0.31 | 1.96 | 0.30 |
| SS3 | 13.5 | 16.0 | 32.2 | 3.91 | 15.6 | 3.03 | 0.68 | 2.62 | 0.40 | 2.48 | 0.51 | 1.45 | 0.23 | 1.26 | 0.20 |
| SS4 | 14.1 | 16.8 | 34.3 | 4.16 | 15.9 | 3.27 | 0.71 | 2.96 | 0.42 | 2.55 | 0.52 | 1.52 | 0.22 | 1.32 | 0.21 |
| SS5 | 6.03 | 6.97 | 12.9 | 1.62 | 6.54 | 1.30 | 0.32 | 1.19 | 0.18 | 1.06 | 0.22 | 0.66 | 0.10 | 0.57 | 0.09 |
| SS6 | 28.1 | 32.3 | 68.0 | 8.37 | 31.5 | 6.67 | 1.46 | 5.51 | 0.85 | 5.10 | 1.09 | 2.82 | 0.41 | 2.71 | 0.40 |
| SS7 | 20.8 | 24.7 | 48.9 | 6.07 | 23.5 | 4.91 | 1.01 | 4.00 | 0.59 | 3.74 | 0.77 | 1.99 | 0.31 | 1.91 | 0.30 |
| SS8 | 8.17 | 9.05 | 16.8 | 2.12 | 8.42 | 1.85 | 0.43 | 1.52 | 0.23 | 1.44 | 0.28 | 0.86 | 0.12 | 0.69 | 0.11 |
| SS9 | 19.9 | 24.3 | 49.9 | 6.02 | 23.0 | 4.70 | 1.01 | 4.17 | 0.58 | 3.59 | 0.75 | 2.09 | 0.33 | 1.85 | 0.28 |
| SS10 | 27.0 | 32.9 | 67.9 | 8.61 | 33.3 | 6.83 | 1.46 | 6.45 | 0.87 | 5.22 | 1.09 | 2.99 | 0.44 | 2.75 | 0.42 |
| SS11 | 11.7 | 13.5 | 25.3 | 3.27 | 12.2 | 2.83 | 0.63 | 2.19 | 0.33 | 2.19 | 0.48 | 1.25 | 0.19 | 1.10 | 0.18 |
| SS12 | 21.4 | 25.6 | 51.4 | 6.29 | 23.7 | 4.91 | 1.10 | 4.20 | 0.66 | 4.01 | 0.82 | 2.26 | 0.34 | 2.16 | 0.33 |
| SS13 | 26.7 | 31.4 | 66.4 | 8.21 | 31.5 | 6.99 | 1.48 | 5.81 | 0.83 | 4.99 | 1.07 | 2.87 | 0.42 | 2.81 | 0.38 |
| SS14 | 17.9 | 21.7 | 47.6 | 5.56 | 21.0 | 4.39 | 1.04 | 3.76 | 0.56 | 3.41 | 0.74 | 2.03 | 0.30 | 1.88 | 0.26 |
| SS15 | 20.1 | 24.4 | 49.9 | 6.07 | 23.7 | 4.98 | 1.07 | 4.23 | 0.63 | 3.76 | 0.79 | 2.09 | 0.32 | 2.02 | 0.31 |
| SS16 | 21.1 | 25.6 | 52.2 | 6.36 | 24.4 | 5.02 | 1.12 | 4.47 | 0.65 | 3.97 | 0.80 | 2.25 | 0.32 | 1.99 | 0.31 |
| SS17 | 17.4 | 20.4 | 41.5 | 5.11 | 19.7 | 4.17 | 0.89 | 3.45 | 0.53 | 3.24 | 0.67 | 1.93 | 0.28 | 1.65 | 0.26 |
| SS18 | 9.1 | 10.8 | 21.0 | 2.57 | 9.84 | 1.99 | 0.52 | 1.92 | 0.27 | 1.67 | 0.35 | 0.91 | 0.15 | 0.88 | 0.14 |
| SS19 | 14.6 | 17.5 | 35.4 | 4.51 | 16.8 | 3.50 | 0.78 | 3.10 | 0.47 | 2.76 | 0.56 | 1.67 | 0.24 | 1.44 | 0.23 |
| SS20 | 12.4 | 15.0 | 31.3 | 3.67 | 14.0 | 2.74 | 0.69 | 2.66 | 0.38 | 2.28 | 0.48 | 1.42 | 0.20 | 1.30 | 0.21 |
| SS21 | 13.9 | 16.2 | 31.5 | 3.95 | 14.7 | 3.07 | 0.75 | 2.73 | 0.41 | 2.52 | 0.54 | 1.46 | 0.23 | 1.31 | 0.22 |
| SS22 | 30.4 | 38.3 | 77.8 | 9.54 | 36.1 | 7.55 | 1.66 | 6.56 | 0.98 | 5.63 | 1.17 | 3.29 | 0.50 | 3.03 | 0.47 |
| SS23 | 30.8 | 39.3 | 80.6 | 9.81 | 37.8 | 7.69 | 1.65 | 6.46 | 0.95 | 5.62 | 1.22 | 3.28 | 0.48 | 3.16 | 0.49 |
| SS24 | 30.8 | 40.4 | 81.4 | 10.0 | 37.8 | 7.82 | 1.76 | 6.86 | 0.91 | 5.79 | 1.23 | 3.42 | 0.47 | 3.09 | 0.46 |
| SS25 | 19.7 | 25.5 | 52.1 | 6.35 | 24.3 | 4.93 | 1.10 | 3.94 | 0.62 | 3.68 | 0.75 | 2.14 | 0.31 | 2.14 | 0.31 |
| SS26 | 30.3 | 39.4 | 80.0 | 9.71 | 36.9 | 7.85 | 1.69 | 6.68 | 0.93 | 5.65 | 1.19 | 3.35 | 0.48 | 2.91 | 0.46 |
| SS27 | 19.4 | 24.0 | 48.8 | 6.07 | 23.4 | 4.75 | 1.06 | 4.16 | 0.58 | 3.56 | 0.74 | 2.05 | 0.30 | 1.85 | 0.30 |
| SR1 | 1.59 | 1.00 | 1.45 | 0.20 | 0.85 | 0.16 | 0.04 | 0.19 | 0.03 | 0.19 | 0.04 | 0.12 | 0.02 | 0.06 | 0.01 |
| SR2 | 7.42 | 7.54 | 12.0 | 1.36 | 5.38 | 1.06 | 0.33 | 1.09 | 0.15 | 1.10 | 0.24 | 0.63 | 0.09 | 0.54 | 0.08 |
| SR3 | 11.7 | 15.1 | 27.6 | 3.27 | 12.9 | 2.64 | 0.67 | 2.19 | 0.35 | 2.12 | 0.45 | 1.30 | 0.20 | 1.32 | 0.19 |
| CS1 | 28.1 | 34.9 | 69.7 | 8.61 | 33.1 | 6.82 | 1.56 | 5.72 | 0.87 | 5.29 | 1.11 | 3.11 | 0.44 | 2.95 | 0.44 |
| CS2 | 12.1 | 15.1 | 29.3 | 3.57 | 14.0 | 2.79 | 0.68 | 2.44 | 0.34 | 2.21 | 0.46 | 1.36 | 0.20 | 1.27 | 0.17 |
| A1 | 14.9 | 18.7 | 37.2 | 4.62 | 17.6 | 3.56 | 0.80 | 3.19 | 0.45 | 2.79 | 0.58 | 1.58 | 0.24 | 1.44 | 0.23 |
| A2 | 16.4 | 20.8 | 41.1 | 5.14 | 18.9 | 4.11 | 0.94 | 3.33 | 0.51 | 3.11 | 0.61 | 1.75 | 0.28 | 1.59 | 0.24 |
| A3 | 20.0 | 24.6 | 50.4 | 6.11 | 24.0 | 4.97 | 1.06 | 4.17 | 0.61 | 3.70 | 0.80 | 2.20 | 0.32 | 2.07 | 0.30 |
| Min | 1.59 | 1.00 | 1.45 | 0.20 | 0.85 | 0.16 | 0.04 | 0.19 | 0.03 | 0.19 | 0.04 | 0.12 | 0.02 | 0.06 | 0.01 |
| Max | 30.8 | 40.4 | 81.4 | 10.0 | 37.8 | 7.85 | 1.76 | 6.86 | 0.98 | 5.79 | 1.23 | 3.42 | 0.50 | 3.16 | 0.49 |
| Average | 18.2 | 22.3 | 45.0 | 5.52 | 21.2 | 4.39 | 0.98 | 3.78 | 0.55 | 3.37 | 0.70 | 1.95 | 0.29 | 1.80 | 0.27 |
| SD | 7.61 | 9.83 | 20.63 | 2.54 | 9.63 | 2.02 | 0.42 | 1.72 | 0.24 | 1.45 | 0.31 | 0.83 | 0.12 | 0.79 | 0.12 |
| RSD | 41.7 | 44.2 | 45.9 | 45.9 | 45.6 | 46.1 | 43.2 | 45.5 | 44.2 | 43.0 | 43.5 | 42.7 | 41.7 | 44.1 | 43.4 |
| Sample | ∑REEs | ∑REY | ∑LREE | ∑HREE | ∑LREE/∑HREE | Eu/Eu * | Ce/Ce * | (La/Yb)UCC | (La/Nd)UCC | (Er/Nd)UCC |
|---|---|---|---|---|---|---|---|---|---|---|
| SS1 | 101 | 146 | 116 | 10.0 | 11.5 | 0.98 | 0.96 | 0.78 | 0.93 | 1.11 |
| SS2 | 99.4 | 144 | 114 | 9.8 | 11.7 | 1.07 | 0.93 | 0.82 | 0.91 | 1.01 |
| SS3 | 64.7 | 94.2 | 74.1 | 6.5 | 11.4 | 1.05 | 0.96 | 0.82 | 0.89 | 1.09 |
| SS4 | 68.1 | 99.0 | 78.1 | 6.8 | 11.5 | 0.98 | 0.97 | 0.82 | 0.92 | 1.12 |
| SS5 | 26.7 | 39.8 | 30.8 | 2.9 | 10.7 | 1.12 | 0.90 | 0.79 | 0.93 | 1.18 |
| SS6 | 135 | 195 | 154 | 13.4 | 11.5 | 1.04 | 0.97 | 0.77 | 0.89 | 1.05 |
| SS7 | 98.0 | 143 | 113 | 9.6 | 11.8 | 0.99 | 0.94 | 0.83 | 0.91 | 0.99 |
| SS8 | 34.9 | 52.1 | 40.2 | 3.7 | 10.8 | 1.11 | 0.90 | 0.84 | 0.94 | 1.20 |
| SS9 | 98.4 | 143 | 113 | 9.5 | 12.0 | 0.99 | 0.97 | 0.85 | 0.92 | 1.07 |
| SS10 | 138 | 198 | 158 | 13.8 | 11.4 | 0.95 | 0.95 | 0.77 | 0.86 | 1.05 |
| SS11 | 52.1 | 77.3 | 59.9 | 5.7 | 10.5 | 1.10 | 0.90 | 0.79 | 0.96 | 1.20 |
| SS12 | 102 | 149 | 117 | 10.6 | 11.1 | 1.05 | 0.95 | 0.76 | 0.94 | 1.12 |
| SS13 | 134 | 192 | 152 | 13.4 | 11.4 | 1.00 | 0.97 | 0.72 | 0.87 | 1.07 |
| SS14 | 92.5 | 132 | 105 | 9.2 | 11.5 | 1.11 | 1.02 | 0.74 | 0.90 | 1.13 |
| SS15 | 99.9 | 144 | 114 | 9.9 | 11.5 | 1.01 | 0.97 | 0.78 | 0.89 | 1.03 |
| SS16 | 104 | 151 | 119 | 10.3 | 11.6 | 1.02 | 0.96 | 0.83 | 0.92 | 1.08 |
| SS17 | 83.4 | 121 | 95.2 | 8.55 | 11.1 | 1.02 | 0.96 | 0.80 | 0.90 | 1.15 |
| SS18 | 42.2 | 62.1 | 48.6 | 4.36 | 11.2 | 1.16 | 0.94 | 0.79 | 0.95 | 1.09 |
| SS19 | 71.5 | 104 | 81.6 | 7.37 | 11.1 | 1.02 | 0.94 | 0.78 | 0.90 | 1.16 |
| SS20 | 61.3 | 88.7 | 70.0 | 6.26 | 11.2 | 1.12 | 1.00 | 0.74 | 0.93 | 1.20 |
| SS21 | 63.4 | 93.5 | 72.9 | 6.68 | 10.9 | 1.13 | 0.93 | 0.79 | 0.96 | 1.16 |
| SS22 | 154 | 223 | 177 | 15.1 | 11.8 | 1.02 | 0.96 | 0.81 | 0.92 | 1.07 |
| SS23 | 159 | 229 | 183 | 15.2 | 12.1 | 1.01 | 0.97 | 0.80 | 0.90 | 1.02 |
| SS24 | 161 | 232 | 186 | 15.4 | 12.1 | 1.04 | 0.95 | 0.84 | 0.93 | 1.06 |
| SS25 | 103 | 148 | 118 | 10.0 | 11.9 | 1.08 | 0.96 | 0.77 | 0.91 | 1.03 |
| SS26 | 158 | 228 | 182 | 15.0 | 12.2 | 1.01 | 0.96 | 0.87 | 0.93 | 1.07 |
| SS27 | 97.6 | 141 | 112 | 9.38 | 12.0 | 1.03 | 0.95 | 0.84 | 0.90 | 1.03 |
| SR1 | 3.36 | 5.96 | 3.89 | 0.47 | 8.28 | 0.99 | 0.76 | 1.16 | 1.02 | 1.69 |
| SR2 | 24.1 | 39.0 | 28.8 | 2.82 | 10.2 | 1.34 | 0.89 | 0.90 | 1.22 | 1.37 |
| SR3 | 55.1 | 81.9 | 64.3 | 5.92 | 10.9 | 1.21 | 0.92 | 0.74 | 1.02 | 1.18 |
| CS1 | 140 | 203 | 160 | 14.2 | 11.3 | 1.08 | 0.95 | 0.76 | 0.92 | 1.10 |
| CS2 | 58.8 | 86.0 | 67.9 | 6.01 | 11.3 | 1.13 | 0.94 | 0.77 | 0.93 | 1.14 |
| A1 | 74.3 | 108 | 85.7 | 7.30 | 11.7 | 1.03 | 0.94 | 0.84 | 0.92 | 1.05 |
| A2 | 81.6 | 119 | 94.3 | 8.10 | 11.6 | 1.10 | 0.94 | 0.84 | 0.96 | 1.09 |
| A3 | 101 | 145 | 115 | 10.0 | 11.5 | 1.01 | 0.97 | 0.77 | 0.89 | 1.08 |
| min | 3.36 | 5.96 | 3.89 | 0.47 | 8.28 | 0.95 | 0.76 | 0.72 | 0.86 | 0.99 |
| max | 161 | 232 | 186 | 15.4 | 12.2 | 1.34 | 1.02 | 1.16 | 1.22 | 1.22 |
| avg | 89.7 | 130 | 103 | 8.94 | 11.3 | 1.06 | 0.94 | 0.81 | 0.93 | 0.93 |
| stdev | 40.8 | 58.2 | 46.8 | 3.86 | 0.70 | 0.08 | 0.04 | 0.07 | 0.06 | 0.06 |
| RSD | 45.5 | 44.7 | 45.4 | 43.2 | 6.20 | 7.08 | 4.38 | 9.02 | 6.51 | 6.51 |
| N = 32 | SiO2 | Al2O3 | Na2O | MgO | K2O | TiO2 | MnO | CaO | P2O5 | Fe2O3 | SO3 | LOI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OM | 0.39 | 0.53 | 0.10 | −0.37 | 0.67 | 0.47 | 0.38 | −0.40 | 0.38 | 0.22 | 0.35 | −0.22 | |||
| PH | −0.23 | −0.33 | −0.01 | 0.33 | −0.34 | −0.30 | −0.25 | 0.26 | −0.09 | −0.17 | −0.35 | 0.04 | |||
| N = 32 | Rb | Ba | Li | Cs | Be | Sr | Th | U | Zr | P | Sc | V | Cr | Co | |
| OM | 0.69 | 0.70 | 0.58 | 0.67 | 0.69 | −0.43 | −0.04 | 0.65 | 0.64 | 0.63 | 0.43 | 0.49 | −0.46 | −0.35 | |
| PH | −0.54 | −0.52 | −0.49 | −0.53 | −0.50 | 0.63 | −0.25 | −0.45 | −0.49 | −0.41 | −0.52 | −0.54 | 0.13 | 0.02 | |
| N = 32 | Ni | Cu | Cd | Zn | Mo | Pb | TI | Bi | Sn | Sb | As | S | Cl | ||
| OM | −0.53 | 0.33 | 0.09 | −0.08 | 0.71 | 0.12 | 0.10 | 0.21 | 0.13 | 0.24 | 0.56 | 0.26 | −0.21 | ||
| PH | 0.22 | −0.55 | 0.06 | −0.05 | −0.59 | −0.44 | −0.06 | −0.28 | −0.34 | −0.51 | −0.50 | −0.28 | 0.19 | ||
| N = 32 | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu |
| OM | 0.66 | 0.66 | 0.66 | 0.65 | 0.67 | 0.66 | 0.64 | 0.65 | 0.66 | 0.65 | 0.65 | 0.63 | 0.67 | 0.63 | 0.63 |
| PH | −0.40 | −0.39 | −0.39 | −0.38 | −0.39 | −0.41 | −0.37 | −0.38 | −0.38 | −0.38 | −0.39 | −0.39 | −0.41 | −0.41 | −0.38 |
| SiO2 | Al2O3 | Na2O | MgO | K2O | TiO2 | MnO | CaO | P2O5 | Fe2O3 | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1 | ||||||||||
| Al2O3 | 0.889 ** | 1 | |||||||||
| Na2O | 0.086 | 0.080 | 1 | ||||||||
| MgO | 0.164 | −0.232 | −0.114 | 1 | |||||||
| K2O | 0.794 | 0.909 ** | 0.205 | −0.319 | 1 | ||||||
| TiO2 | 0.906 | 0.973 ** | 0.039 | −0.135 | 0.837 ** | 1 | |||||
| MnO | 0.671 ** | 0.612 ** | 0.009 | 0.306 | 0.426 * | 0.669 ** | 1 | ||||
| CaO | −0.980 ** | −0.855 ** | −0.006 | −0.255 | −0.740 ** | −0.887 ** | −0.72 ** | 1 | |||
| P2O5 | 0.335 * | 0.467 ** | 0.132 | −0.365 * | 0.599 ** | 0.382 * | 0.151 | −0.262 | 1 | ||
| Fe2O3 | 0.925 ** | 0.777 ** | −0.096 | 0.323 | 0.585 ** | 0.857 ** | 0.675 ** | −0.936 ** | 0.131 | 1 | |
| SO3 | 0.063 | 0.191 | 0.206 | −0.357 * | 0.257 | 0.139 | −0.026 | −0.019 | 0.317 | −0.026 | 1 |
| LOI | −0.927 ** | −0.759 ** | −0.065 | −0.361 * | −0.637 ** | −0.790 ** | −0.65 ** | 0.890 ** | −0.268 | −0.91 ** | 0.003 |
| Components | ||||
|---|---|---|---|---|
| Metals | 1 | 2 | 3 | 4 |
| Li | 0.97 | 0.09 | 0.01 | −0.07 |
| Th | −0.23 | −0.22 | 0.38 | 0.77 |
| P | 0.63 | −0.28 | −0.15 | 0.23 |
| V | 0.86 | 0.23 | 0.11 | 0.27 |
| Cr | −0.41 | 0.76 | 0.32 | 0.26 |
| Co | −0.13 | 0.87 | 0.40 | 0.07 |
| Ni | −0.42 | 0.82 | 0.36 | 0.00 |
| Cu | 0.49 | −0.11 | 0.61 | −0.01 |
| Cd | −0.06 | −0.79 | 0.23 | −0.39 |
| Zn | −0.07 | −0.55 | 0.60 | −0.43 |
| Mo | 0.78 | −0.44 | 0.12 | 0.05 |
| Pb | −0.02 | −0.50 | 0.57 | 0.23 |
| Sn | 0.14 | −0.57 | 0.67 | −0.12 |
| As | 0.83 | −0.22 | 0.01 | 0.22 |
| Cl | −0.12 | 0.21 | 0.07 | −0.38 |
| Si | 0.89 | 0.41 | 0.12 | −0.05 |
| Al | 0.98 | 0.06 | −0.08 | −0.02 |
| Mg | −0.26 | 0.82 | 0.25 | −0.20 |
| K | 0.96 | −0.13 | −0.06 | 0.02 |
| Ti | 0.95 | 0.17 | −0.07 | −0.08 |
| Mn | 0.57 | 0.45 | 0.02 | −0.20 |
| Ca | −0.85 | −0.47 | −0.13 | 0.08 |
| Fe | 0.74 | 0.58 | 0.15 | −0.13 |
| ΣREEs | 0.97 | −0.15 | −0.09 | 0.04 |
| Eigenvalue | 10.25 | 5.65 | 2.28 | 1.51 |
| % Total Variance | 42.7 | 23.5 | 9.49 | 6.27 |
| Cumulative % Variance | 42.7 | 66.2 | 75.7 | 82.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamarashid, R.A.; Fiket, Ž.; Mohialdeen, I.M.J. Environmental Impact of Sulaimani Steel Plant (Kurdistan Region, Iraq) on Soil Geochemistry. Soil Syst. 2022, 6, 86. https://doi.org/10.3390/soilsystems6040086
Hamarashid RA, Fiket Ž, Mohialdeen IMJ. Environmental Impact of Sulaimani Steel Plant (Kurdistan Region, Iraq) on Soil Geochemistry. Soil Systems. 2022; 6(4):86. https://doi.org/10.3390/soilsystems6040086
Chicago/Turabian StyleHamarashid, Roshna A., Željka Fiket, and Ibrahim M. J. Mohialdeen. 2022. "Environmental Impact of Sulaimani Steel Plant (Kurdistan Region, Iraq) on Soil Geochemistry" Soil Systems 6, no. 4: 86. https://doi.org/10.3390/soilsystems6040086
APA StyleHamarashid, R. A., Fiket, Ž., & Mohialdeen, I. M. J. (2022). Environmental Impact of Sulaimani Steel Plant (Kurdistan Region, Iraq) on Soil Geochemistry. Soil Systems, 6(4), 86. https://doi.org/10.3390/soilsystems6040086





