Effects of Elemental Sulfur on Soil pH and Growth of Saskatoon Berry (Amelanchier alnifolia) and Beaked Hazelnut (Corylus cornuta) Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Plant Material and Experimental Set-Up
2.3. Leaf Chlorophyll Concentrations
2.4. Net Photosynthesis (Pn) and Transpiration (E) Rates
2.5. Leaf Elemental Concentrations
2.6. Statistical Analysis
3. Results
3.1. Soil pH
3.2. Chlorophyll Concentrations
3.3. Net Photosynthesis (Pn) and Transpiration (E) Rates
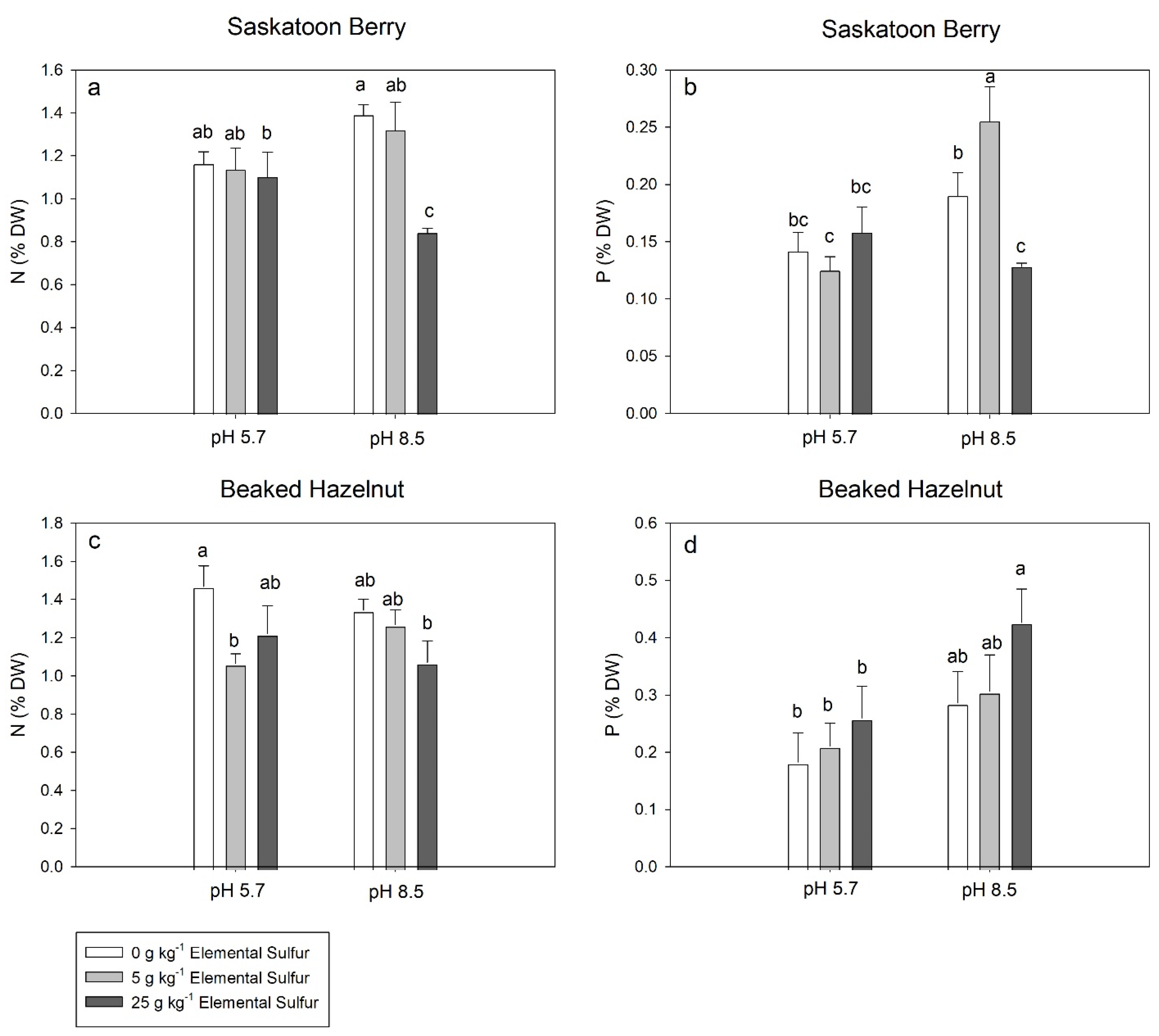
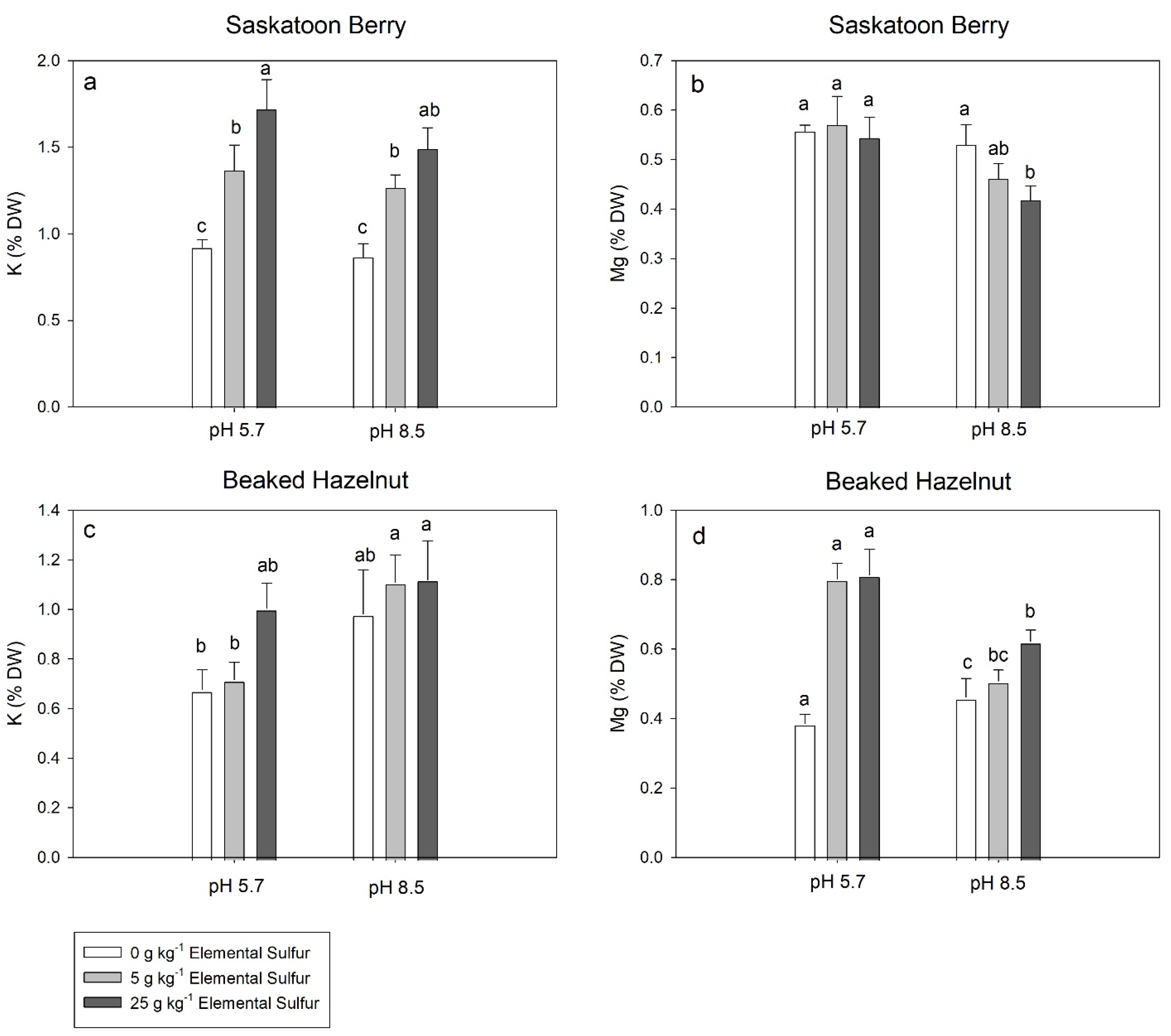
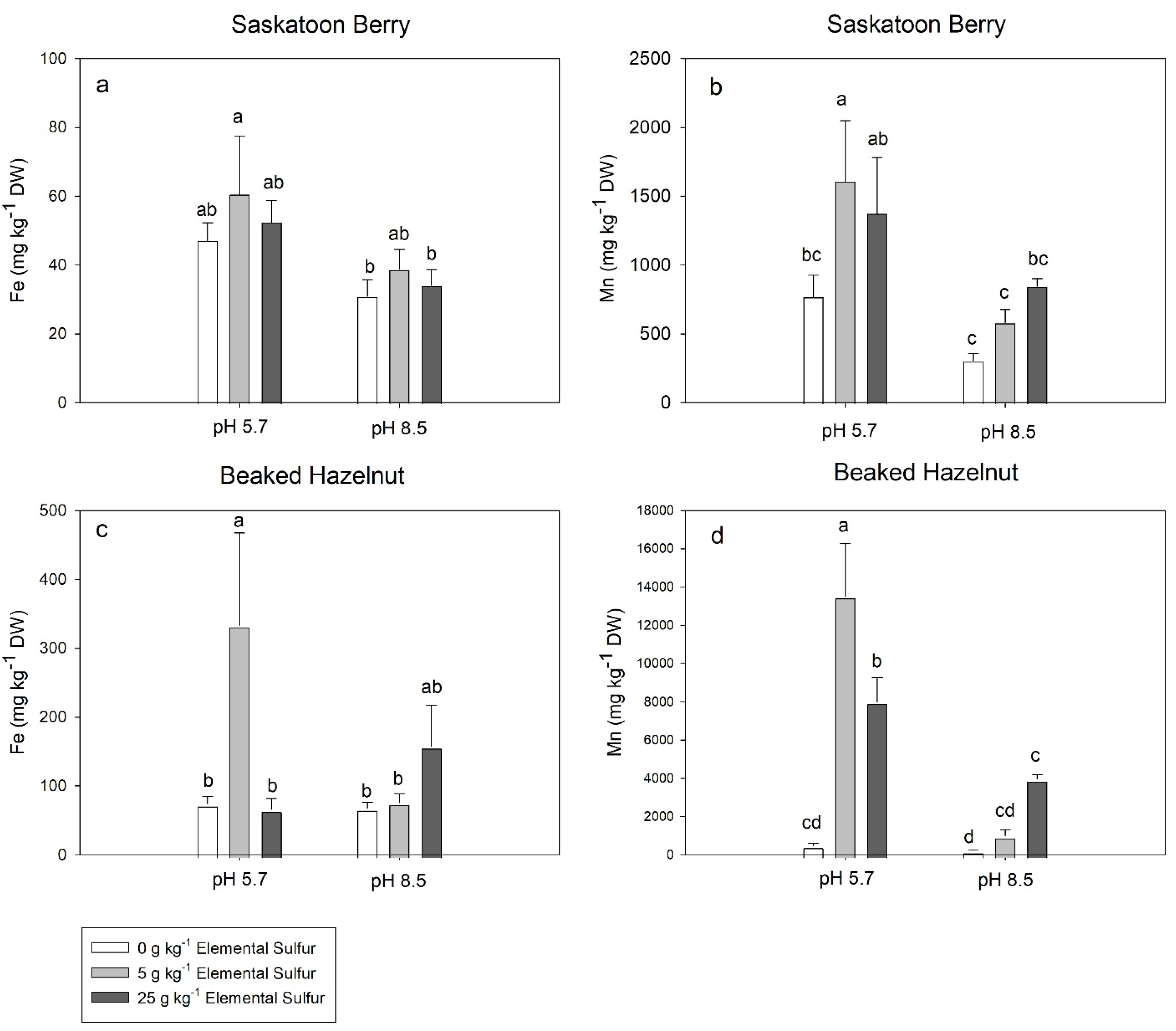
3.4. Leaf Elemental Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowland, S.M.; Prescott, C.E.; Grayston, S.J.; Quideau, S.A.; Bradfield, G.E. Recreating a Functioning Forest Soil in Reclaimed Oil Sands in Northern Alberta: An Approach for Measuring Success in Ecological Restoration. J. Environ. Qual. 2009, 38, 1580–1590. [Google Scholar] [PubMed] [Green Version]
- Zhang, W.; Fleurial, K.; Sherr, I.; Vassov, R.; Zwiazek, J.J. Growth and physiological responses of tree seedlings to oil sands non-segregated tailings. Environ. Pollut. 2020, 259, 113945. [Google Scholar]
- Environment and Parks. Oil Sands Mine Reclamation and Disturbance Tracking by Year. 2017. Available online: http://osip.alberta.ca/library/Dataset/Details/27# (accessed on 5 May 2021).
- Alberta Environment. Guidelines for Reclamation to Forest Vegetation in the Athabasca Oil Sands Region, 2nd ed.; Terrestrial Subgroup of the Reclamation Working Group of the Cumulative Environmental Management Association: Fort McMurray, AB, Canada, 2010. [Google Scholar]
- Howat, D. Acceptable Salinity, Sodicity and pH Values for Boreal Forest Reclamation; Alberta Environment, Environmental Sciences Division: Edmonton, AB, Canada, 2000. [Google Scholar]
- Fung, M.Y.; Macyk, T.M. Reclamation of oil sands mining areas. Reclam. Drastically Disturb. Lands 2000, 41, 755–774. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Rengel, Z. Handbook of Plant Growth pH as the Master Variable; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of plants to adverse chemical soil conditions. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 409–472. [Google Scholar]
- Zhang, W.; Xu, F.; Zwiazek, J.J. Responses of jack pine (Pinus banksiana) seedlings to root zone pH and calcium. Environ. Exp. Bot. 2015, 111, 32–41. [Google Scholar]
- Xu, F.; Tan, X.; Zhang, W.Q.; Zwiazek, J.J. Effects of iron and root zone pH on growth and physiological responses of paper birch (Betula papyrifera), trembling aspen (Populus tremuloides) and red-osier dogwood (Cornus stolonifera) seedlings in a split-root hydroponic system. Acta Physiol. Plant. 2019, 41, 142. [Google Scholar]
- Kamaluddin, M.; Zwiazek, J.J. Effects of root medium pH on water transport in paper birch (Betula papyrifera) seedlings in relation to root temperature and abscisic acid treatments. Tree Physiol. 2004, 24, 1173–1180. [Google Scholar] [PubMed] [Green Version]
- Siemens, J.A.; Zwiazek, J.J. Hebeloma crustuliniforme modifies root hydraulic responses of trembling aspen (Populus tremuloides) seedlings to changes in external pH. Plant Soil 2011, 345, 247–256. [Google Scholar]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [PubMed]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.-T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [PubMed]
- Tang, C.; Turner, N.C. The influence of alkalinity and water stress on the stomatal conductance, photosynthetic rate and growth of Lupinus angustifolius L. and Lupinus pilosus Murr. Aust. J. Exp. Agric. 1999, 39, 457–464. [Google Scholar]
- Cai, X.; Ma, Y.; Wang, W.; Wei, C.; Wang, X.; Dun, S. Effects of applying sulphur on alkali soil pH and its consequence on yield and quality of flue-cured tobacco. Acta Table Sin. 2008, 14, 19–22. [Google Scholar]
- Sun, L.; Duan, D.; Peng, C.; He, J.; Shi, J. Influence of sulfur on the speciation transformation and phyto-availability of heavy metals in soil: A review. Chin. J. Appl. Ecol. 2014, 25, 2141–2148. [Google Scholar]
- Cui, Y.; Wang, Q.; Dong, Y.; Li, H.; Christie, P. Enhanced uptake of soil Pb and Zn by Indian mustard and winter wheat following combined soil application of elemental sulphur and EDTA. Plant Soil 2004, 261, 181–188. [Google Scholar]
- Slaton, N.A.; Norman, R.J.; Gilmour, J.T. Oxidation rates of commercial elemental sulfur products applied to an alkaline silt loam from Arkansas. Soil Sci. Soc. Am. J. 2001, 65, 239–243. [Google Scholar]
- Banerjee, M.R.; Chapman, S.J. The significance of microbial biomass sulphur in soil. Biol. Fertil. Soils 1996, 22, 116–125. [Google Scholar]
- Rao, P.S.; Karunasagar Iddya Otta, S.K.; Karunasagar, I. Incidence of bacteria involved in nitrogen and sulphur cycles in tropical shrimp culture ponds. Agric. Int. 2000, 8, 463–472. [Google Scholar]
- D’Aquin, G. Sulphur output from oil sands: Dramatically changing Alberta’s sulphur balance. In Proceedings of the 2008 Oil Sands Heavy Oil Technologies Conference: Stepping Up: Preparation for Growth, Calgary, AB, Canada, 15–17 July 2008; PennWell Corp: Tulsa, OK, USA, 2008; p. 1000. [Google Scholar]
- Lindemann, W.C.; Aburto, J.J.; Haffner, W.M.; Bono, A.A. Effect of sulfur source on sulfur oxidation. Soil Sci. Soc. Am. J. 1991, 55, 85–90. [Google Scholar]
- Watkinson, J.H.; Blair, G.J. Modelling the oxidation of elemental sulfur in soils. Fertil. Res. 1993, 35, 115–126. [Google Scholar]
- Wildflower Center. Corylus cornuta (Beaked Hazelnut). Native Plants of North America. Available online: https://www.wildflower.org/plants/result.php?id_plant=coco6 (accessed on 25 October 2021).
- Rutter, P.A.; Shepard, M.L. Hybrid Hazelnut Handbook, 1st ed.; Badgersett Research Corporation: Canton, MN, USA, 2002. [Google Scholar]
- Alberta Agriculture and Rural Development (AARD). Saskatoon Berry Production Manual; Alberta Agriculture and Rural Development: Edmonton, AB, Canada, 2013. [Google Scholar]
- Forsch, K.B.C.; Dhar, A.; Naeth, M.A. Effects of woody debris and cover soil types on soil properties and vegetation 4–5 years after oil sands reclamation. Restor. Ecol. 2021, 29, e13420. [Google Scholar]
- Wang, S.; Hu, C.; Cheng, D.; Liao, W.; Li, N.; Chen, L.; Fang, J. Effects of soil pH regulation on indigenous microflora and physiological groups in tea plantations. Soils 2011, 43, 76–80. [Google Scholar]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Sestak, Z.; Catský, J.; Jarvis, P.G. Plant Photosynthetic Production; Manual of Methods; Dr. W. Junk Publishers: The Hague, The Netherlands, 1971. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 25 October 2021).
- Natural Regions Committee. Natural Regions and Subregions of Alberta; Downing, D.J., Pettapiece, W.W., Eds.; Government of Alberta: Edmonton, AB, Canada, 2006. [Google Scholar]
- Calvo-Polanco, M.; Zhang, W.; Macdonald, S.E.; Señorans, J.; Zwiazek, J.J. Boreal forest plant species responses to pH: Ecological interpretation and application to reclamation. Plant Soil 2017, 420, 195–208. [Google Scholar]
- Chalaturnyk, R.J.; Don Scott, J.; Özüm, B. Management of oil sands tailings. Pet. Sci. Technol. 2002, 20, 1025–1046. [Google Scholar]
- Ramos-Padrón, E.; Bordenave, S.; Lin, S.; Bhaskar, I.M.; Dong, X.; Sensen, C.W.; Fournier, J.; Voordouw, G.; Gieg, L.M. Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond. Environ. Sci. Technol. 2011, 45, 439–446. [Google Scholar] [PubMed]
- Engelhardt, R.; Todirescu, M. An Introduction to Development in Alberta’s Oil Sands; University of Alberta: Edmonton, AB, Canada, 2005. [Google Scholar]
- Zhang, C.; Zhang, M.; Zeng, Y. Effects of sulphur on chemical properties of calcareous soil. Chin. J. Appl. Ecol. 2007, 7, 1453–1458. [Google Scholar]
- Zhao, X.; Li, Y.; Mai, W.; Tian, X.; Lai, H.; Lv, J. A preliminary study on amendment of saline soil through addition of sulphur. Agric. Res. Arid. Areas 2008, 4, 74–78. [Google Scholar]
- Cao, Y.; Zhao, Y.; Zhao, W.; Lv, J. Research on effect of sulfur application on saline-alkaline soil pH and availability of trace elements in Huanghe River irrigation district in Ningxia. Agric. Res. Arid. Areas 2010, 28, 199–201. [Google Scholar]
- Perry, L. pH for the Garden. 2003. Available online: http://pss.uvm.edu/ppp/pubs/oh34.htm (accessed on 25 October 2021).
- Soaud, A.A.; Al Darwish, F.H.; Saleh, M.E.; El-Tarabily, K.A.; Sofian-Azirun, M.; Rahman, M.M. Effects of elemental sulfur, phosphorus, micronutrients and ‘Paracoccus versutus’ on nutrient availability of calcareous soils. Aust. J. Crop Sci. 2011, 5, 554. [Google Scholar]
- CNPS. Saskatoon Serviceberry, Amelanchier Alnifolia. 2021. Available online: https://calscape.org/Amelanchier-alnifolia-() (accessed on 25 October 2021).
- Gill, J.D.; Healy, W.M. Shrubs and Vines for Northeastern Wildlife; USDA Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1974.
- Tang, K.; Zhu, W.; Zhou, W.; Yi, Z.; Tu, N. Research progress on effects of soil pH on plant growth and development. Crop Res. 2013, 27, 207–212. [Google Scholar]
- Rout, G.; Samantaray, S.; Das, P. Aluminium toxicity in plants: A review. Agronomie 2001, 21, 4–5. [Google Scholar]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; Blamey, F.P.C. Kinetics and nature of aluminium rhizotoxic effects: A review. J. Exp. Bot. 2016, 67, 4451–4467. [Google Scholar] [PubMed]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar]
- Karimizarchi, M.; Aminuddin, H.; Khanif, M.Y.; Radziah, O. Elemental sulphur application effects on nutrient availability and sweet maize (Zea mays L.) response in a high pH soil of Malaysia. Malays. J. Soil Sci. 2014, 18, 75–86. [Google Scholar]
- Janzen, H.H.; Bettany, J.R. Sulfur nutrition of rapeseed: I. Influence of fertilizer nitrogen and sulfur rates. Soil Sci. Soc. Am. J. 1984, 48, 100–107. [Google Scholar]
- Orman, S. Effects of elemental sulphur and farmyard manure applications to calcareous saline clay loam soil on growth and some nutrient concentrations of tomato plants. J. Food Agric. Environ. 2012, 10, 720–725. [Google Scholar]
- Lambers, H.; Chapin III, F.S.; Pons, T.L. Plant Physiological Ecology; Springer Science & Business Media: New York, NY, USA, 2008. [Google Scholar]
- Viani, R.A.; Rodrigues, R.R.; Dawson, T.E.; Lambers, H.; Oliveira, R.S. Soil pH accounts for differences in species distribution and leaf nutrient concentrations of Brazilian woodland savannah and seasonally dry forest species. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 64–74. [Google Scholar]
- Starast, M.; Karp, K.; Vool, E. Effect of NPK fertilization and elemental sulphur on growth and yield of lowbush blueberry. Agric. Food Sci. 2007, 16, 34–45. [Google Scholar]
- Modaihsh, A.S.; Al-Mustafa, W.A.; Metwally, A.I. Effect of elemental sulphur on chemical changes and nutrient availability in calcareous soils. Plant Soil 1989, 116, 95–101. [Google Scholar]




| p-Value | Chl-a:Chl-b | Total Chl | Pn | E | N | P | K | Mg | Ca | S | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0.0268 * | 0.5765 | 0.8920 | 0.0147 * | 0.0792 | 0.0879 | 0.1772 | 0.0085 ** | 0.1126 | 0.7828 | 0.0087 ** | 0.0029 ** |
| S | 0.0871 | 0.2335 | 0.0104 * | 0.0014 ** | 0.8907 | 0.4855 | 2.36 × 10−6 *** | 0.2691 | 0.7618 | 1.90 × 10−6 *** | 0.4370 | 0.0519 |
| pH × S | 0.0046 ** | 0.3489 | 0.0056 ** | 0.0615 | 0.0171 * | 0.0012 ** | 0.7452 | 0.3932 | 0.047 * | 0.0279 * | 0.9464 | 0.5005 |
| p-Value | Chl-a:Chl-b | Total Chl | Pn | E | N | P | K | Mg | Ca | S | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0.9739 | 0.1801 | 0.0055 ** | 0.8289 | 0.7742 | 0.0082 ** | 0.0105 * | 0.2672 | 0.2413 | 0.2032 | 0.9411 | 0.8827 |
| S | 0.0001 *** | 0.0001 *** | 0.5872 | 9.17 × 10−5 *** | 0.0261 * | 0.1151 | 0.1694 | 2.07 × 10−7 *** | 0.0111 * | 3.27 × 10−12 *** | 0.0054 ** | 1.88 × 10−7 *** |
| pH × S | 0.0345 * | 0.2034 | 0.1579 | 0.0438 * | 0.1546 | 0.7711 | 0.5302 | 0.0014 ** | 0.0191 * | 0.0202 * | 0.0214 * | 0.0001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, W.; Vassov, R.; Sherr, I.; Du, N.; Zwiazek, J.J. Effects of Elemental Sulfur on Soil pH and Growth of Saskatoon Berry (Amelanchier alnifolia) and Beaked Hazelnut (Corylus cornuta) Seedlings. Soil Syst. 2022, 6, 31. https://doi.org/10.3390/soilsystems6020031
Sun X, Zhang W, Vassov R, Sherr I, Du N, Zwiazek JJ. Effects of Elemental Sulfur on Soil pH and Growth of Saskatoon Berry (Amelanchier alnifolia) and Beaked Hazelnut (Corylus cornuta) Seedlings. Soil Systems. 2022; 6(2):31. https://doi.org/10.3390/soilsystems6020031
Chicago/Turabian StyleSun, Xuehui, Wenqing Zhang, Robert Vassov, Ira Sherr, Ning Du, and Janusz J. Zwiazek. 2022. "Effects of Elemental Sulfur on Soil pH and Growth of Saskatoon Berry (Amelanchier alnifolia) and Beaked Hazelnut (Corylus cornuta) Seedlings" Soil Systems 6, no. 2: 31. https://doi.org/10.3390/soilsystems6020031
APA StyleSun, X., Zhang, W., Vassov, R., Sherr, I., Du, N., & Zwiazek, J. J. (2022). Effects of Elemental Sulfur on Soil pH and Growth of Saskatoon Berry (Amelanchier alnifolia) and Beaked Hazelnut (Corylus cornuta) Seedlings. Soil Systems, 6(2), 31. https://doi.org/10.3390/soilsystems6020031







