Investigating Lead Bioavailability in a Former Shooting Range by Soil Microanalyses and Earthworms Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Physicochemical Characterization
2.2. Soil µXRF and SEM EDX Analyses
2.3. Earthworm Testing and Analysis
3. Results and Discussion
3.1. Soil Main Properties
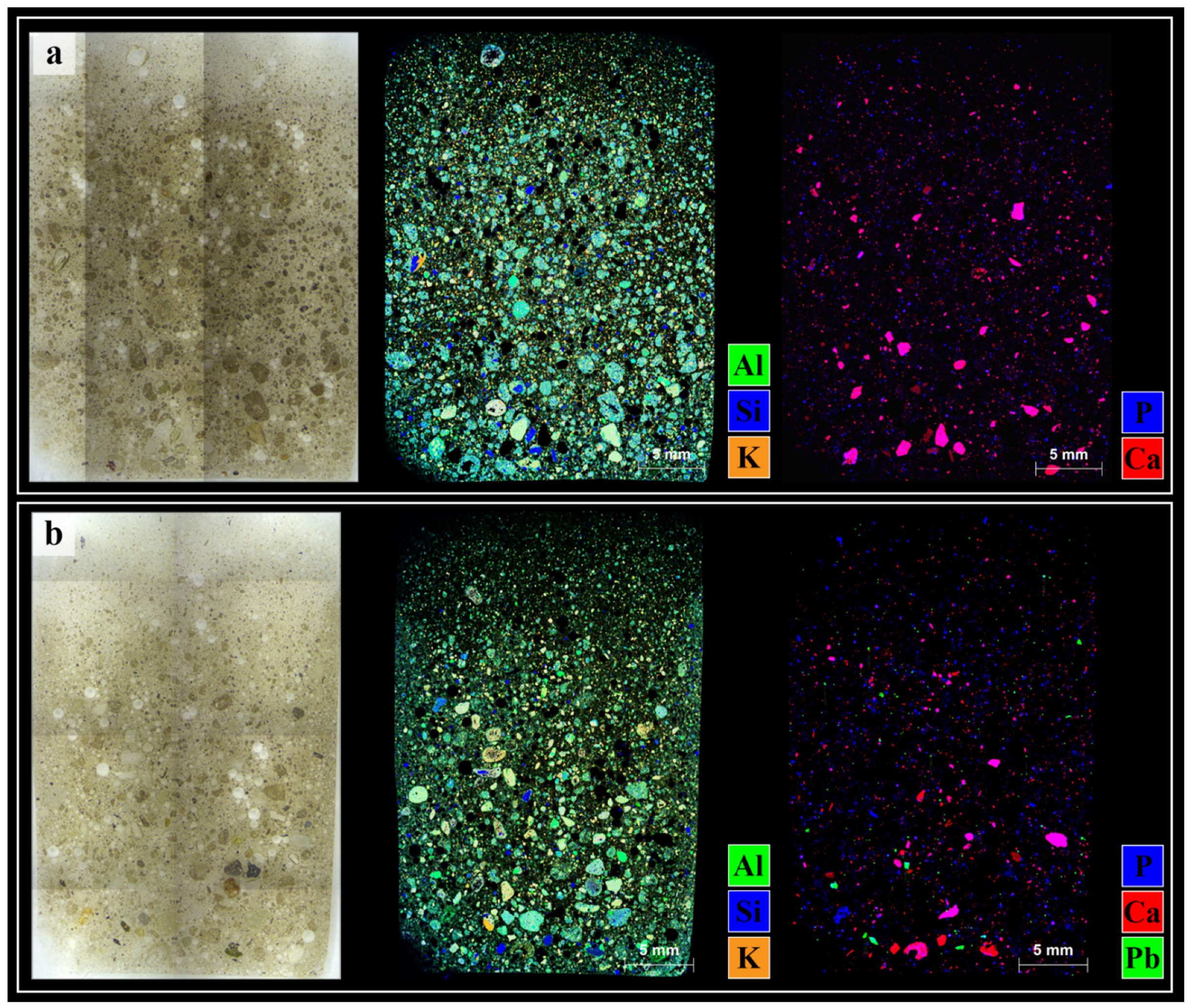
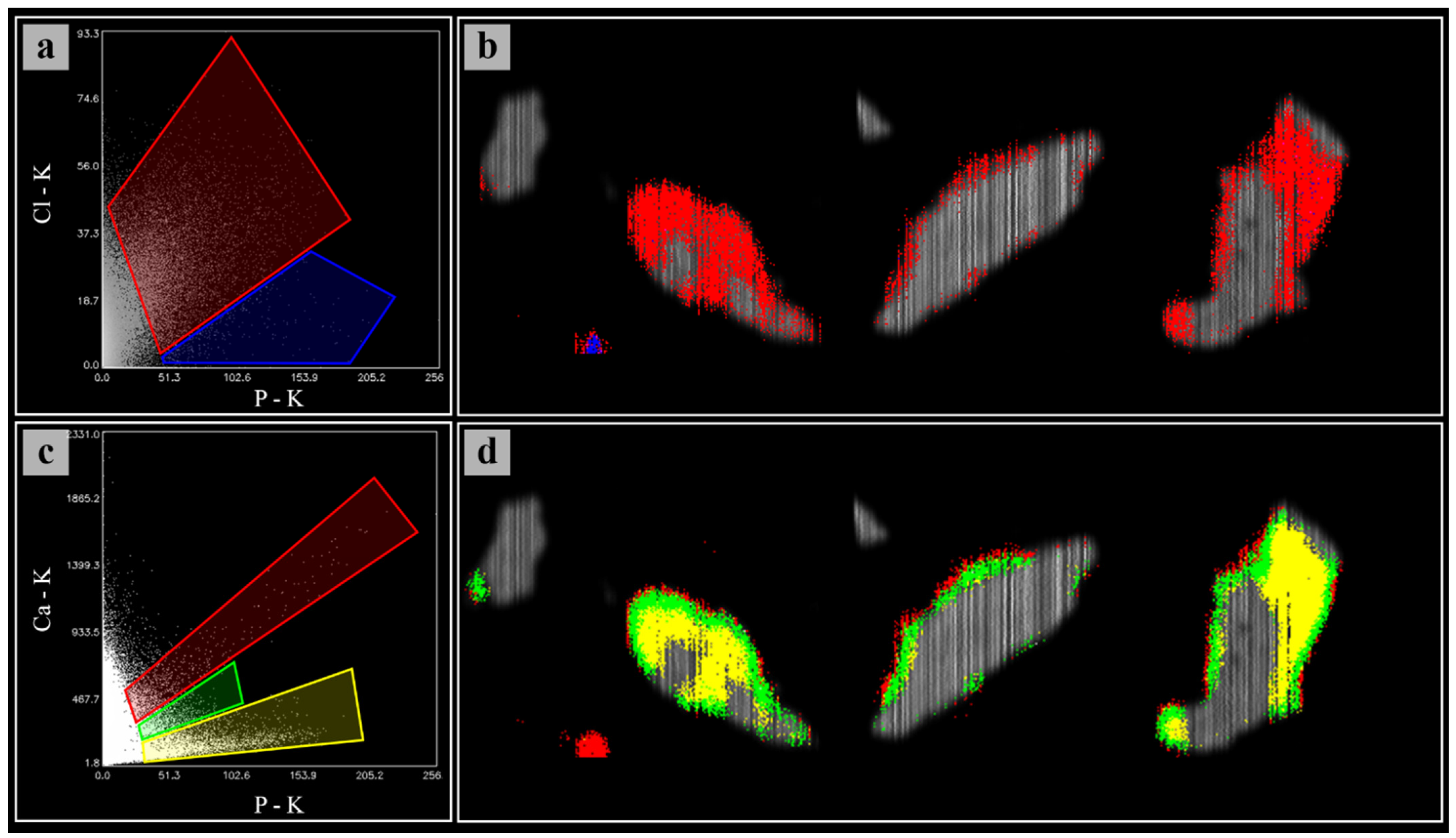
3.2. Soil Microanalysis
3.3. Bioassays with E. andrei
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alloway, B.J. Heavy Metals in Soil; Blackie and Son Ltd.: London, UK, 1990; p. 339. [Google Scholar] [CrossRef]
- Scheckel, K.G.; Diamond, G.L.; Burgess, M.F.; Klotzbach, J.M.; Maddaloni, M.; Miller, B.W.; Partridge, C.R.; Serda, S.M. Amending soils with phosphate as means to mitigate soil lead hazard: A critical review of the state of the science. J. Toxicol. Environ. Health Part B 2013, 16, 337–380. [Google Scholar] [CrossRef] [PubMed]
- Antonovics, J.; Bradshaw, A.; Turner, R. Heavy metal tolerance in plants. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1971; Volume 7, pp. 1–85. [Google Scholar] [CrossRef]
- Česynaitė, J.; Praspaliauskas, M.; Pedišius, N.; Sujetovienė, G. Biological assessment of contaminated shooting range soil using earthworm biomarkers. Ecotoxicology 2021, 30, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.J.; Hodson, M.E.; Arnold, R.E.; Black, S. Survival, Pb-uptake and behaviour of three species of earthworm in Pb treated soils determined using an OECD-style toxicity test and a soil avoidance test. Environ. Pollut. 2005, 138, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Roodbergen, M.; Klok, C.; van der Hout, A. Transfer of heavy metals in the food chain earthworm Black-tailed godwit (Limosa limosa): Comparison of a polluted and a reference site in The Netherlands. Sci. Total Environ. 2008, 406, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Allegretta, I.; Porfido, C.; Martin, M.; Barberis, E.; Terzano, R.; Spagnuolo, M. Characterization of As-polluted soils by laboratory X-ray-based techniques coupled with sequential extractions and electron microscopy: The case of Crocette gold mine in the Monte Rosa mining district. Environ. Sci. Pollut. Res. 2018, 25, 25080–25090. [Google Scholar] [CrossRef] [PubMed]
- Gattullo, C.E.; D’Alessandro, C.; Allegretta, I.; Porfido, C.; Spagnuolo, M.; Terzano, R. Alkaline hydrothermal stabilization of Cr(VI) in soil using glass and aluminum from recycled municipal solid wastes. J. Hazard. Mater. 2018, 344, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Sosa, N.N.; Kulkarni, H.; Datta, S.; Beilinson, E.; Porfido, C.; Spagnuolo, M.; Zárate, M.A.; Surber, J. Occurrence and distribution of high arsenic in sediments and groundwater of the Claromecó fluvial basin, southern Pampean plain. Sci. Total Environ. 2019, 695, 133673. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Allegretta, I.; Porfido, C.; Rascio, I.; Spagnuolo, M.; Terzano, R. Assessing chromium pollution and natural stabilization processes in agricultural soils by bulk and micro X-ray analyses. Environ. Sci. Pollut. Res. 2020, 27, 22967–22979. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.C.W.; Beauchemin, S.; Rasmussen, P.E. Lead speciation in house dust from canadian urban homes using EXAFS, Micro-XRF, and Micro-XRD. Environ. Sci. Technol. 2011, 45, 5491–5497. [Google Scholar] [CrossRef]
- McIntosh, K.G.; Cordes, N.L.; Patterson, B.M.; Havrilla, G.J. Laboratory-based characterization of plutonium in soil particles using micro-XRF and 3D confocal XRF. J. Anal. At. Spectrom. 2015, 30, 1511–1517. [Google Scholar] [CrossRef]
- Landrot, G.; Khaokaew, S. Determining the fate of lead (Pb) and phosphorus (P) in alkaline Pb-polluted soils amended with P and acidified using multiple synchrotron-based techniques. J. Hazard. Mater. 2020, 399, 123037. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, X.; Hao, J.; Chen, W.; Cai, P.; Huang, Q. Characterization of Cu distribution in clay-sized soil aggregates by NanoSIMS and micro-XRF. Chemosphere 2020, 249, 126143. [Google Scholar] [CrossRef] [PubMed]
- Weltje, L. Mixture toxicity and tissue interactions of Cd, Cu, Pb and Zn in earthworms (Oligochaeta) in laboratory and field soils: A critical evaluation of data. Chemosphere 1998, 36, 2643–2660. [Google Scholar] [CrossRef]
- Martin, M.H.; Coughtrey, P.J. The use of terrestrial animals as monitors and indicators of environmental contamination by heavy metals. In Biological Monitoring of Heavy Metal Pollution; Springer: Dordrecht, The Netherlands, 1982; pp. 221–310. [Google Scholar] [CrossRef]
- Ireland, M.P. Heavy Metal Uptake and Tissue Distribution in Earthworms; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1983; pp. 247–265. [Google Scholar]
- Ministerial Decree. n. 46/2019. Available online: https://www.gazzettaufficiale.it/eli/id/2019/06/07/19G00052/sg (accessed on 29 September 2021).
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Slavich, P.; Petterson, G. Estimating the electrical conductivity of saturated paste extracts from 1:5 soil, water suspensions and texture. Soil Res. 1993, 31, 73–81. [Google Scholar] [CrossRef]
- Bloom, P.R.; Meter, K.; Crum, J.R. Titration method for determination of clay-sized carbonates. Soil Sci. Soc. Am. J. 1985, 49, 1070–1073. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis: Part 3 Chemical Methods 5.3, 1st ed.; Soil Science Society of America Book Series 5; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; p. 1390. [Google Scholar] [CrossRef] [Green Version]
- Duri, L.G.; Visconti, D.; Fiorentino, N.; Adamo, P.; Fagnano, M.; Caporale, A.G. Health risk assessment in agricultural soil potentially contaminated by geogenic Thallium: Influence of plant species on metal mobility in soil-plant system. Agronomy 2020, 10, 890. [Google Scholar] [CrossRef]
- BBodSchV. Bundes-Bodenschutz- und Altlastenverordnung (BBodSchV) vom 12. Juli 1999. Bundesgesetzblatt I 1999, 1554 (English Version) [Federal Soil Protection and Contaminated Sites Ordinance, Dated 12 July 1999]. 1999. Available online: https://www.elaw.org/es/content/germany-federal-soil-protection-and-contaminated-sites-ordinance-bbodschv-12-july-1999 (accessed on 29 September 2021).
- Gupta, S.; Vollmer, M.; Krebs, R. The importance of mobile, mobilisable and pseudo total heavy metal fractions in soil for three-level risk assessment and risk management. Sci. Total Environ. 1996, 178, 11–20. [Google Scholar] [CrossRef]
- Kingston, H.M.; Haswell, S.J. Microwave-Enhanced Chemistry: Fundamentals, Sample Preparation, and Applications; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Solé, V.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Alfeld, M.; Janssens, K. Strategies for processing mega-pixel X-ray fluorescence hyperspectral data: A case study on a version of Caravaggio’s painting Supper at Emmaus. J. Anal. At. Spectrom. 2015, 30, 777–789. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals no. 207. Earthworm, Acute Toxicity Test. 1984. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948293.pdf (accessed on 3 January 2021).
- Allegretta, I.; Porfido, C.; Panzarino, O.; Fontanella, M.C.; Beone, G.M.; Spagnuolo, M.; Terzano, R. Determination of as concentration in earthworm coelomic fluid extracts by total-reflection X-ray fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2017, 130, 21–25. [Google Scholar] [CrossRef]
- Porfido, C.; Allegretta, I.; Panzarino, O.; Laforce, B.; Vekemans, B.; Vincze, L.; de Lillo, E.; Terzano, R.; Spagnuolo, M. Correlations between as in earthworms’ coelomic fluid and as bioavailability in highly polluted soils as revealed by combined laboratory X-ray techniques. Environ. Sci. Technol. 2019, 53, 10961–10968. [Google Scholar] [CrossRef] [PubMed]
- USDA Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/geo/?cid=nrcs142p2_053587 (accessed on 3 January 2021).
- Jowett, M.; Price, H.I. Solubilities of the phosphates of lead. Trans. Faraday Soc. 1932, 28, 668–681. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Scheckel, K.G.; Ryan, J.A. Effects of aging and pH on dissolution Kinetics and stability of Chloropyromorphite. Environ. Sci. Technol. 2002, 36, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O. Lead orthophosphates—IV Formation and stability in the environment. Geochim. Cosmochim. Acta 1974, 38, 887–898. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Rossi, A.M.; Costa, A.M.; Perez, C.A.C.; Moreira, J.C.; Saldanha, M. Studies on the mechanisms of lead immobilization by hydroxyapatite. Environ. Sci. Technol. 2002, 36, 1625–1629. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Rocha, N.C.; Moreira, J.C.; Rossi, A.M.; Soares, G.A. Characterization of phase evolution during lead immobilization by synthetic hydroxyapatite. Mater. Charact. 2004, 53, 71–78. [Google Scholar] [CrossRef]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D.; Grubb, D.G. Phosphate application to firing range soils for Pb immobilization: The unclear role of phosphate. J. Hazard. Mater. 2007, 144, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.K.; Scheckel, K.G.; Impellitteri, C.A.; Ryan, J.A. Toxic metals in the environment: Thermodynamic considerations for possible immobilization strategies for Pb, Cd, As, and Hg. Crit. Rev. Environ. Sci. Technol. 2004, 34, 495–604. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Chen, M.; Hardison, D.W.; Harris, W.G. Lead transformation and distribution in the soils of shooting ranges in Florida, USA. Sci. Total Environ. 2003, 307, 179–189. [Google Scholar] [CrossRef]
- Cotter-Howells, J. Lead phosphate formation in soils. Environ. Pollut. 1996, 93, 9–16. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Morgan, J.; Morgan, A. Earthworms as biological monitors of cadmium, copper, lead and zinc in metalliferous soils. Environ. Pollut. 1988, 54, 123–138. [Google Scholar] [CrossRef]
- Zimdahl, R.L.; Skogerboe, R.K. Behavior of lead in soil. Environ. Sci. Technol. 1977, 11, 1202–1207. [Google Scholar] [CrossRef]
- Tan, Q.-G.; Ke, C.; Wang, W.-X. Rapid Assessments of metal bioavailability in marine sediments using coelomic fluid of sipunculan worms. Environ. Sci. Technol. 2013, 47, 7499–7505. [Google Scholar] [CrossRef]
- Chapman, P.M. Effects of gut sediment contents on measurements of metal levels in benthic invertebrates—A Cautionary Note. Bull. Environ. Contam. Toxicol. 1985, 35, 345–347. [Google Scholar] [CrossRef]
- Adamowicz, A. Morphology and ultrastructure of the earthworm Dendrobaena veneta (Lumbricidae) coelomocytes. Tissue Cell 2005, 37, 125–133. [Google Scholar] [CrossRef]
- Kägi, J.H. Overview of metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar] [CrossRef]
- Morgan, A.; Stürzenbaum, S.; Winters, C.; Grime, G.; Aziz, N.A.A.; Kille, P. Differential metallothionein expression in earthworm (Lumbricus rubellus) tissues. Ecotoxicol. Environ. Saf. 2004, 57, 11–19. [Google Scholar] [CrossRef]
- Dai, J.; Becquer, T.; Rouiller, J.H.; Reversat, G.; Bernhard-Reversat, F.; Nahmani, J.; Lavelle, P. Heavy metal accumulation by two earthworm species and its relationship to total and DTPA-extractable metals in soils. Soil Biol. Biochem. 2003, 36, 91–98. [Google Scholar] [CrossRef]


| Sand | Silt | Clay | pH (H2O) | EC | OC a | OM b | N | C/N | CaCO3 | P2O5 | |
| g kg−1 | dS m−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | |||||
| S1 | 600 | 240 | 160 | 7.8 | 0.21 | 18 | 31 | 2.2 | 8 | 155 | 0.29 |
| S2 | 590 | 265 | 145 | 7.4 | 0.21 | 30 | 53 | 2.5 | 12 | 70 | 0.60 |
| Cd | Sb | Pb | Zn | Cd * | Sb * | Pb * | Zn * | ||||
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | ||||
| S1 | 0.3 | 0.3 | 17 | 209 | 0.03 | 0.61 | 0.01 | 0.3 | |||
| S2 | 3.3 | 22.7 | 1575 | 328 | 0.15 | 6.36 | 0.02 | 1.3 | |||
| SV | 5 | 10 | 100 | 300 | n.a. | n.a. | n.a. | n.a. |
| Pb Concentration in Soil | Mortality | Mean Earthworm Weight | Pb Mean Earthworm Tissue Concentration | Bioconcentration Factor | Pb Mean Earthworm Fluid Concentration | ||
|---|---|---|---|---|---|---|---|
| mg kg−1 | g | mg kg−1 | µg L−1 | ||||
| Day 0 | Day 28 | ||||||
| S1 | 17 | 0 | 0.36 ± 0.04 ns | 0.43 ± 0.04 ns | 11.1 ± 2.2 b | - | 251.8 ± 55.4 b |
| S2 | 1575 | 0 | 0.35 ± 0.06 ns | 0.44 ± 0.07 ns | 94.9 ± 19.8 a | 0.06 | 794.4 ± 232.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porfido, C.; Gattullo, C.E.; Allegretta, I.; Fiorentino, N.; Terzano, R.; Fagnano, M.; Spagnuolo, M. Investigating Lead Bioavailability in a Former Shooting Range by Soil Microanalyses and Earthworms Tests. Soil Syst. 2022, 6, 25. https://doi.org/10.3390/soilsystems6010025
Porfido C, Gattullo CE, Allegretta I, Fiorentino N, Terzano R, Fagnano M, Spagnuolo M. Investigating Lead Bioavailability in a Former Shooting Range by Soil Microanalyses and Earthworms Tests. Soil Systems. 2022; 6(1):25. https://doi.org/10.3390/soilsystems6010025
Chicago/Turabian StylePorfido, Carlo, Concetta Eliana Gattullo, Ignazio Allegretta, Nunzio Fiorentino, Roberto Terzano, Massimo Fagnano, and Matteo Spagnuolo. 2022. "Investigating Lead Bioavailability in a Former Shooting Range by Soil Microanalyses and Earthworms Tests" Soil Systems 6, no. 1: 25. https://doi.org/10.3390/soilsystems6010025
APA StylePorfido, C., Gattullo, C. E., Allegretta, I., Fiorentino, N., Terzano, R., Fagnano, M., & Spagnuolo, M. (2022). Investigating Lead Bioavailability in a Former Shooting Range by Soil Microanalyses and Earthworms Tests. Soil Systems, 6(1), 25. https://doi.org/10.3390/soilsystems6010025










