Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Soil Characterization and Pb Analysis
2.3. Microcosm Experimental Design
2.4. Test of Phytotoxicity
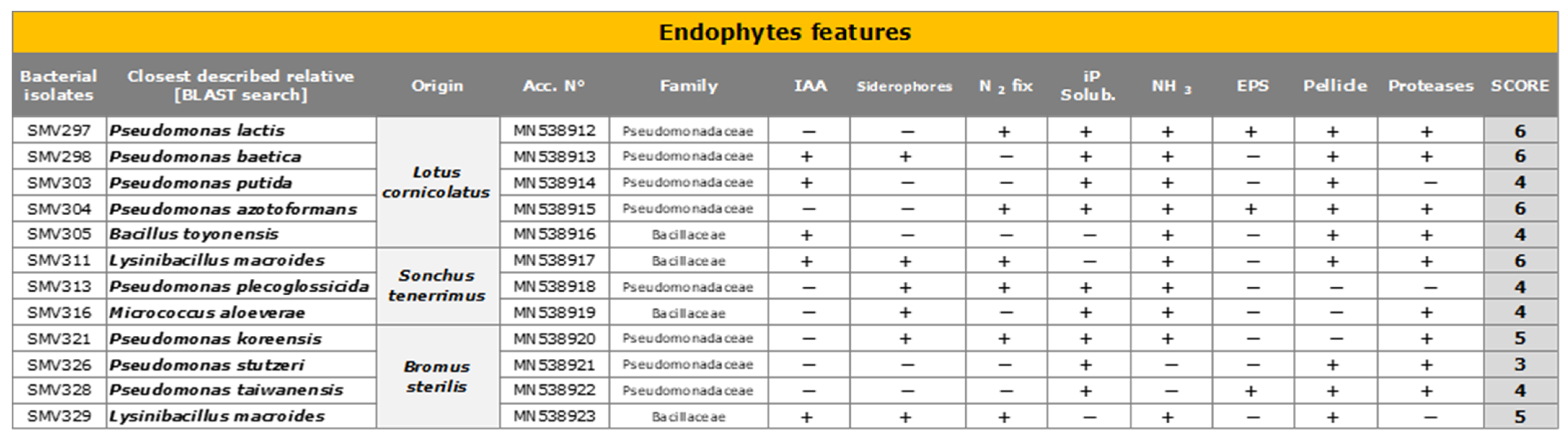
2.5. Endophyte Isolation
2.6. PGPR Characterization
2.7. Next-Generation Ion Torrent Sequencing (NGS)
2.8. Quality Assurance and Quality Control
2.9. Statistical Analysis
3. Results
3.1. Soil Analysis
3.2. Phytotoxicity Test
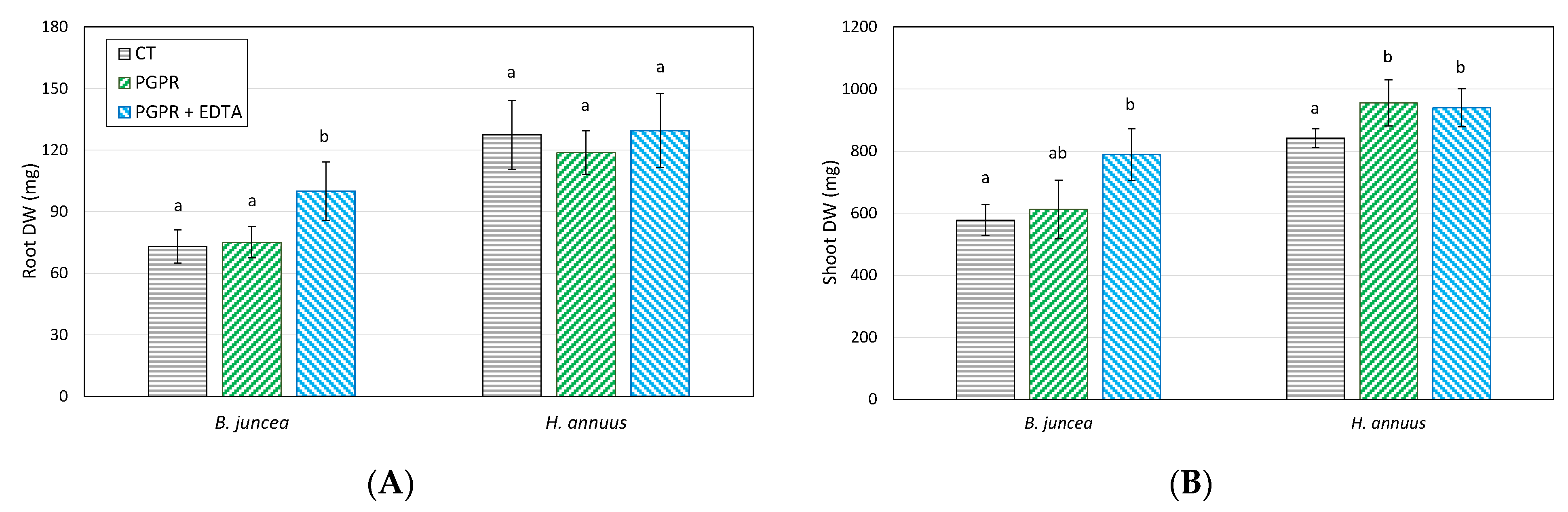
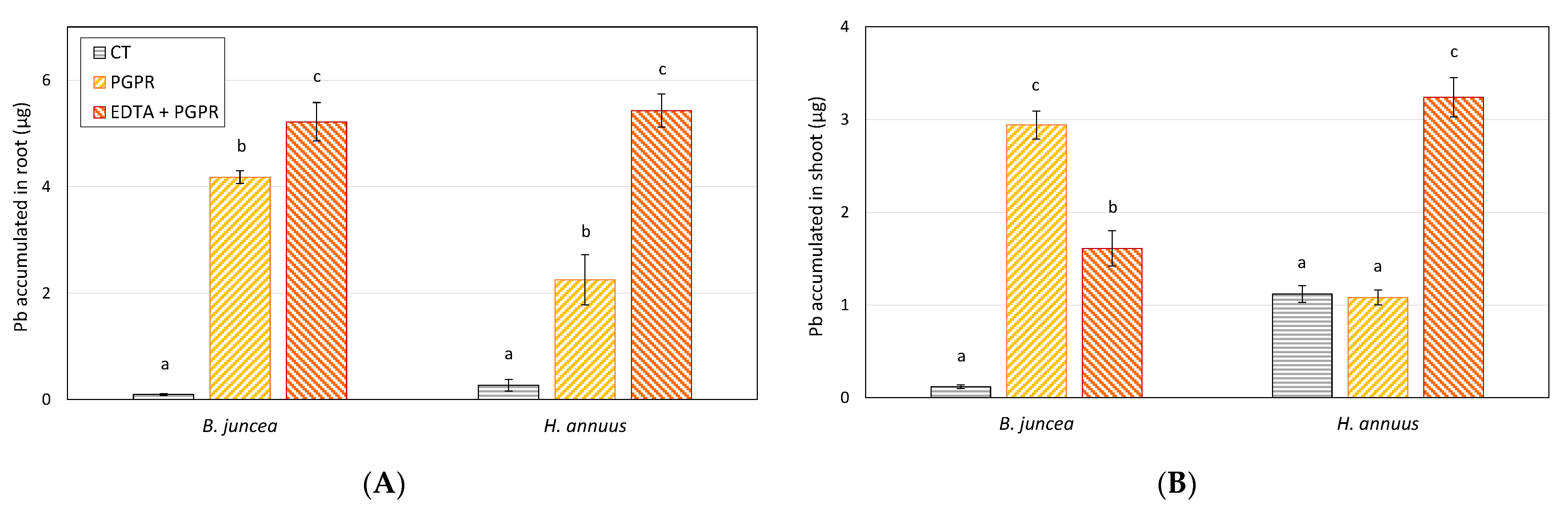
3.3. Effect of PGPR and EDTA on Plant Growth and Pb Phytoextraction Efficiency
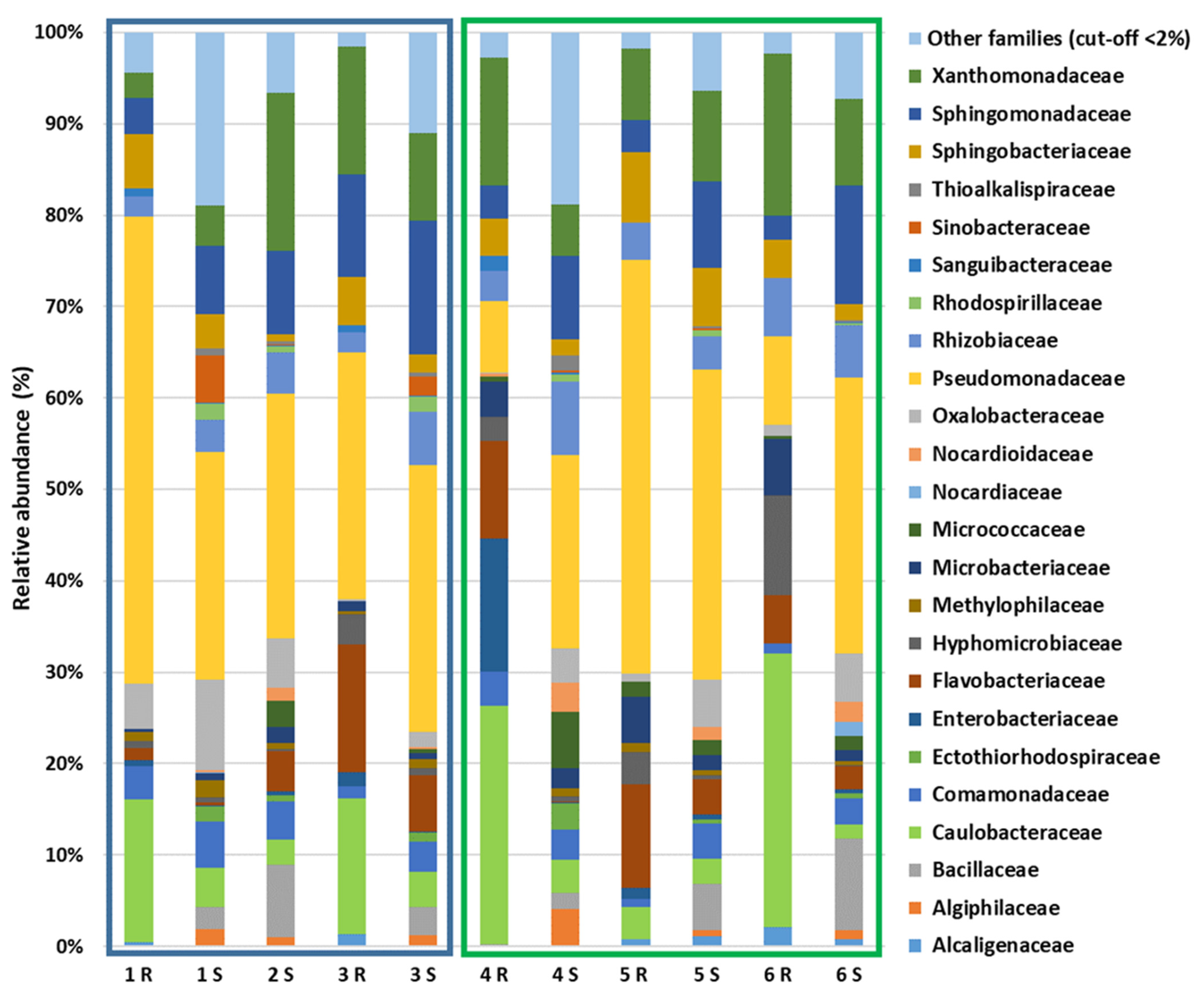
3.4. NGS Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Biores. Technol. 2008, 99, 5296–5308. [Google Scholar]
- Vocciante, M.; Finocchi, A.; De Folly D’Auris, A.; Conte, A.; Tonziello, J.; Pola, A.; Reverberi, A. Enhanced oil spill remediation by adsorption with interlinked multilayered graphene. Materials 2019, 12, 2231. [Google Scholar] [CrossRef] [Green Version]
- Pietrelli, L.; Francolini, I.; Piozzi, A.; Sighicelli, M.; Vocciante, M. Chromium (III) Removal from Wastewater by Chitosan Flakes. Appl. Sci. 2020, 10, 1925. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar]
- Pavel, L.V.; Gavrilescu, M. Overview of ex situ decontamination techniques for soil cleanup. Environ. Eng. Manag. J. 2008, 7, 815–834. [Google Scholar]
- Vocciante, M.; Reverberi, A.P.; Pietrelli, L.; Dovì, V.G. Improved Remediation Processes through Cost-effective Estimation of Soil Properties from Surface Measurements. J. Clean. Prod. 2017, 167, 680–686. [Google Scholar]
- da Silva, B.M.; Maranho, L.T. Petroleum-contaminated sites: Decision framework for selecting remediation technologies. J. Hazard. Mat. 2019, 378, 120722. [Google Scholar]
- Vocciante, M.; Dovì, V.G.; Ferro, S. Sustainability in ElectroKinetic Remediation Processes: A Critical Analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Song, Y.; Kirkwood, N.; Maksimović, Č.; Zhen, X.; O’Connor, D.; Jin, Y.; Hou, D. Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: A review. Sci. Total Environ. 2019, 663, 568–579. [Google Scholar] [CrossRef]
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I.; Petruzzelli, G.; Franchi, E. Sustainable Valorization of Biomass: From Assisted Phytoremediation to Green Energy Production. In Handbook on Assisted and Amendments Enhanced Sustainable Remediation Technology; Prasad, M.N.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable soil use and management: An interdisciplinary and systematic approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef]
- O’Connor, D.; Zheng, X.; Hou, D.; Shen, Z.; Li, G.; Miao, G.; O’Connell, S.; Guo, M. Phytoremediation: Climate change resilience and sustainability assessment at a coastal brownfield redevelopment. Environ. Int. 2019, 130, 104945. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Franchi, E.; Petruzzelli, G.; Ferro, S. Enhancements in phytoremediation technology: Environmental assessment including different options of biomass disposal and comparison with a consolidated approach. J. Environ. Manag. 2019, 237, 560–568. [Google Scholar] [CrossRef]
- Vocciante, M.; De Follis D’Auris, A.; Franchi, E.; Petruzzelli, G.; Ferro, S. CO2 footprint analysis of consolidated and innovative technologies in remediation activities. J. Clean. Prod. 2021, 126723. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Franchi, E.; Samà, C.; Gila, L.; Zanardi, S.; Palmery, S.; Proto, A.; et al. New Light on Phytoremediation: The Use of Luminescent Solar Concentrators. Appl. Sci. 2021, 11, 1923. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Sheoran, V.; Singh Sheoran, A.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Applicability of a Freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, G.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pedron, F. Sorption: Release processes in soil-the basis of phytoremediation efficiency. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 91–112. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Franchi, E.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci. Total Environ. 2019, 655, 328–336. [Google Scholar] [CrossRef]
- Abbaszadeh-Dahaji, P.; Omidvari, M.; Ghorbanpour, M. Increasing phytoremediation efficiency of heavy metal-contaminated soil using PGPR for sustainable agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Narendra, T., Eds.; Springer: Singapore, 2017; pp. 187–204. [Google Scholar] [CrossRef]
- Legislative Decree 152/2006. Rules in environmental field. In Italian Official Journal; National Legislation: Italy, 2006.
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis Part 3—Chemical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar] [CrossRef] [Green Version]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1d—Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, MI, USA, 1986; pp. 383–411. [Google Scholar]
- EPA—United State Environmental Protection Agency. Method 3051A Microwave assisted acid digestion of sediments, sludges, soils, and oils. In Test Methods for Evaluating Solid Waste: Physical/Chemical Methods (SW-846); U.S. Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- Grifoni, M.; Petruzzelli, G.; Barbafieri, M.; Rosellini, I.; Pedron, F. Soil quality protection at heavy metal-contaminated manufactured gas plant sites: Role of biological remediation. In Enhancing Cleanup of Environmental Pollutants; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 231–260. [Google Scholar] [CrossRef]
- Pedron, F.; Petruzzelli, G.; Barbafieri, M.; Tassi, E. Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere 2009, 75, 808–814. [Google Scholar] [CrossRef]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted phytoremediation of a multi-contaminated soil: Investigation on arsenic and lead combined mobilization and removal. J. Environ. Manag. 2017, 203, 316–329. [Google Scholar] [CrossRef]
- Jez, E.; Lestan, D. EDTA retention and emissions from remediated soil. Chemosphere 2016, 151, 202–209. [Google Scholar] [CrossRef]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef]
- Li, F.L.; Qiu, Y.; Xu, X.; Yang, F.; Wang, Z.; Feng, J.; Wang, J. EDTA-enhanced phytoremediation of heavy metals from sludge soil by Italian ryegrass (Lolium perenne L.). Ecotox. Environ. Safe. 2020, 191, 110185. [Google Scholar] [CrossRef]
- Grifoni, M.; Rosellini, I.; Petruzzelli, G.; Pedron, F.; Franchi, E.; Barbafieri, M. Application of sulphate and cytokinin in assisted arsenic phytoextraction by industrial Cannabis sativa L. Environ. Sci. Pollut. Res. 2021, 28, 47294–47305. [Google Scholar] [CrossRef]
- Tassi, E.; Pouget, J.; Petruzzelli, G.; Barbafieri, M. The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 2008, 71, 66–73. [Google Scholar] [CrossRef]
- EPA—United State Environmental Protection Agency. Method 3052 Microwave assisted acid digestion of siliceous and organically based matrices. In Test Methods for Evaluating Soild Waste: Physical/Chemical Methods (SW-846); U.S. Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- ISO—International Organization for Standardization. ISO 18763:2016 Soil Quality–Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Shahab, S.; Ahmed, N.; Khan, N.S. Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. J. Plant Pathol. 2009, 91, 61–63. [Google Scholar]
- Milagres, A.M.F.; Machuca, A.; Napoleão, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Santaella, C.; Schue, M.; Berge, O.; Heulin, T.; Achouak, W. The exopolysaccharide of Rhizobium sp. YAS34 is not necessary for biofilm formation on Arabidopsis thaliana and Brassica napus roots but contributes to root colonization. Environ. Microbiol. 2008, 10, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, P.; Sørensen, J. Multi-target and medium-independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol. Ecol. 1997, 22, 183–192. [Google Scholar] [CrossRef]
- Kifle, M.H.; Laing, M.D. Isolation and screening of bacteria for their diazotrophic potential and their influence on growth promotion of maize seedlings in greenhouses. Front. Plant Sci. 2015, 6, 1225. [Google Scholar] [CrossRef] [Green Version]
- Liba, C.M.; Ferrara, F.I.S.; Manfio, G.P.; Fantinatti-Garboggini, F.; Albuquerque, R.C.; Pavan, C.; Ramos, P.L.; Moreira-Filho, C.A.; Barbosa, H.R. Nitrogen-fixing chemo-organotrophic bacteria isolated from Cyanobacteria-deprived lichens and their ability to solubilize phosphate and to release amino acids and phytohormones. J. Appl. Microbiol. 2006, 101, 1076–1086. [Google Scholar] [CrossRef]
- Conte, A.; Chiaberge, S.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M.; Franchi, E.; Pietrini, I. Dealing with complex contamination: A novel approach with a combined bio-phytoremediation strategy and effective analytical techniques. J. Environ. Manag. 2021, 288, 112381. [Google Scholar] [CrossRef]
- Saifullah; Meers, E.; Qadir, M.; de Caritat, P.; Tack, F.M.; Du Laing, G.; Zia, M.H. EDTA-assisted Pb phytoextraction. Chemosphere 2009, 74, 1279–1291. [Google Scholar] [CrossRef]
- Xu, Y.; Yamaji, N.; Shen, R.; Ma, J.F. Sorghum roots are inefficient in uptake of EDTA-chelated lead. Ann. Bot. 2007, 99, 869–875. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Wen, D.; Fu, R.; Feng, L. Application of alkyl polyglycosides for enhanced bioremediation of petroleum hydrocarbon-contaminated soil using Sphingomonas changbaiensis and Pseudomonas stutzeri. Sci. Total Environ. 2020, 719, 137456. [Google Scholar] [CrossRef]
- Rathi, M.; Yogalakshmi, K.N. Brevundimonas diminuta MYS6 associated Helianthus annuus L. for enhanced copper phytoremediation. Chemosphere 2021, 263, 128195. [Google Scholar] [CrossRef] [PubMed]
- Nazli, F.; Mustafa, A.; Ahmad, M.; Hussain, A.; Jamil, M.; Wang, X.; Shakeel, Q.; Imtiaz, M.; El-Esawi, M.A. A Review on Practical Application and Potentials of Phytohormone-Producing Plant Growth-Promoting Rhizobacteria for Inducing Heavy Metal Tolerance in Crops. Sustainability 2020, 12, 9056. [Google Scholar] [CrossRef]
- Barbafieri, M.; Morelli, E.; Tassi, E.; Pedron, F.; Remorini, D.; Petruzzelli, G. Overcoming limitation of “recalcitrant areas” to phytoextraction process: The synergistic effects of exogenous cytokinins and nitrogen treatments. Sci. Total Environ. 2018, 639, 1520–1529. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Bassegio, C.; Campagnolo, M.A.; Schwantes, D.; Gonçalves Junior, A.C.; Manfrin, J.; Schiller, A.D.P.; Bassegio, D. Growth and accumulation of Pb by roots and shoots of Brassica juncea L. Int. J. Phytoremediat. 2020, 22, 134–139. [Google Scholar] [CrossRef]
- Chauhan, P.; Rajguru, A.B.; Dudhe, M.Y.; Mathur, J. Efficacy of lead (Pb) phytoextraction of five varieties of Helianthus annuus L. from contaminated soil. Environ. Technol. Innov. 2020, 18, 100718. [Google Scholar] [CrossRef]
- Gurajala, H.K.; Cao, X.; Tang, L.; Ramesh, T.M.; Lu, M.; Yang, X. Comparative assessment of Indian mustard (Brassica juncea L.) genotypes for phytoremediation of Cd and Pb contaminated soils. Environ. Pollut. 2019, 254, 113085. [Google Scholar] [CrossRef]
- Rathika, R.; Srinivasan, P.; Alkahtani, J.; Al-Humaid, L.A.; Alwahibi, M.S.; Mythili, R.; Selvankumar, T. Influence of biochar and EDTA on enhanced phytoremediation of lead contaminated soil by Brassica juncea. Chemosphere 2021, 271, 129513. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Hassan, M.H.; Kalam, M.A.; Ashrafur Rahman, S.M.; Abedin, M.J.; Shahir, A. An experimental investigation of biodiesel production, characterization, engine performance, emission and noise of Brassica juncea methyl ester and its blends. J. Clean. Prod. 2014, 79, 74–81. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy production potential of phytoremediation plant biomass: Helianthus annuus and Silybum marianum. Ind. Crops Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Iram, S.; Tariq, I.; Ahmad, K.S.; Jaffri, S.B. Helianthus annuus based biodiesel production from seed oil garnered from a phytoremediated terrain. Int. J. Ambient Energy 2020. [Google Scholar] [CrossRef]
- Pant, S.; Ritika; Komesu, A.; Penteado, E.D.; Diniz, A.A.R.; Rahman, M.A.; Kuila, A. NaOH pretreatment and enzymatic hydrolysis of Brassica juncea using mixture of cellulases. Environ. Technol. Innov. 2021, 21, 101324. [Google Scholar] [CrossRef]
- Luo, J.; Qi, S.; Gu, X.W.S.; Wang, J.; Xie, X. An evaluation of EDTA additions for improving the phytoremediation efficiency of different plants under various cultivation systems. Ecotoxicology 2016, 25, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Mahmood-ul-Hassan, M.; Suthar, V.; Ahmad, R.; Yousra, M. Heavy metal phytoextraction—natural and EDTA-assisted remediation of contaminated calcareous soils by sorghum and oat. Environ. Monit. Assess. 2017, 189, 1–10. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M.; et al. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, A.K.; Udayan, A.; Kumar, S. Role of microbial community and metal-binding proteins in phytoremediation of heavy metals from industrial wastewater. Bioresour. Technol. 2021, 326, 124750. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
- Franchi, E.; Fusini, D. Plant Growth-Promoting Rhizobacteria (PGPR) Assisted Phytoremediation of Inorganic and Organic Contaminants Including Amelioration of Perturbed Marginal Soils. In Handbook of Assisted and Amendment-Enhanced Sustainable Remediation Technology; Prasad, M.N.V., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 477–500. [Google Scholar]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- He, X.; Xu, M.; Wei, Q.; Tang, M.; Guan, L.; Lou, L.; Xu, X.; Hu, Z.; Chen, Y.; Shen, Z.; et al. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2020, 205, 111333. [Google Scholar] [CrossRef] [PubMed]
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | |
|---|---|---|---|---|---|---|
| pH | 8.22 ± 0.1 | 8.44 ± 0.2 | 8.42 ± 0.1 | 8.51 ± 0.2 | 8.38 ± 0.1 | 8.12 ± 0.1 |
| EC (μS cm−1) | 596 ± 12 | 644 ± 10 | 548 ± 13 | 621 ± 8.5 | 495 ± 11 | 607 ± 6.1 |
| Clay (%) | 14.4 ± 0.2 | 8.74 ± 0.2 | 12.9 ± 0.4 | 9.78 ± 0.3 | 10.2 ± 1.0 | 13.1 ± 1.1 |
| Silt (%) | 18.5 ± 1.5 | 13.2 ± 0.9 | 17.4 ± 1.4 | 15.6 ± 1.1 | 16.2 ± 0.9 | 14.8 ± 1.3 |
| Sand (%) | 67.1 ± 1.1 | 78.1 ± 0.1 | 70.1 ± 0.3 | 67.8 ± 1.3 | 71.5 ± 0.4 | 72.3 ± 0.5 |
| CEC (Cmol(+) kg−1) | 18.7 ± 0.2 | 18.7 ± 1.2 | 20.5 ± 0.4 | 17.5 ± 1.2 | 16.2 ± 0.8 | 19.4 ± 0.5 |
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | Pb-Soil | |
|---|---|---|---|---|---|---|---|
| Total | 106 ± 2.2 | 112 ± 8.1 | 106 ± 3.5 | 111 ± 6.9 | 107 ± 6.1 | 137 ± 3.5 | 112 ± 4.8 |
| H2O | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| KNO3 1 M | 1.30 ± 0.2 | 0.77 ± 0.1 | 1.70 ± 0.6 | 0.92 ± 0.3 | 1.34 ± 0.6 | 1.04 ± 0.1 | 1.15 ± 0.2 |
| EDTA 1% | 21.5 ± 2.3 | 21.4 ± 3.1 | 20.3 ± 2.0 | 20.5 ± 1.9 | 20.6 ± 2.5 | 20.9 ± 1.7 | 20.8 ± 2.8 |
| EDTA 2 mM | 6.89 ± 1.8 | 7.27 ± 0.9 | 7.66 ± 1.3 | 6.55 ± 1.3 | 7.47 ± 1.1 | 7.72 ± 2.4 | 7.68 ± 2.1 |
| Treatment | B. juncea | H. annuus | ||
|---|---|---|---|---|
| Root | Shoot | Root | Shoot | |
| CT | 1.33 ± 0.51 a | 0.20 ± 0.05 a | 2.14 ± 0.58 a | 1.34 ± 0.25 a |
| PGPR | 55.8 ± 4.89 c | 4.82 ± 0.77 c | 19.0 ± 3.46 b | 1.14 ± 0.13 a |
| PGPR + EDTA | 52.3 ± 4.74 c | 2.04 ± 0.54 b | 42.0 ± 5.10 c | 3.46 ± 0.69 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrini, I.; Grifoni, M.; Franchi, E.; Cardaci, A.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants. Soil Syst. 2021, 5, 55. https://doi.org/10.3390/soilsystems5030055
Pietrini I, Grifoni M, Franchi E, Cardaci A, Pedron F, Barbafieri M, Petruzzelli G, Vocciante M. Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants. Soil Systems. 2021; 5(3):55. https://doi.org/10.3390/soilsystems5030055
Chicago/Turabian StylePietrini, Ilaria, Martina Grifoni, Elisabetta Franchi, Anna Cardaci, Francesca Pedron, Meri Barbafieri, Gianniantonio Petruzzelli, and Marco Vocciante. 2021. "Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants" Soil Systems 5, no. 3: 55. https://doi.org/10.3390/soilsystems5030055
APA StylePietrini, I., Grifoni, M., Franchi, E., Cardaci, A., Pedron, F., Barbafieri, M., Petruzzelli, G., & Vocciante, M. (2021). Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants. Soil Systems, 5(3), 55. https://doi.org/10.3390/soilsystems5030055










