Soil Sulfur Sources Differentially Enhance Cadmium Tolerance in Indian Mustard (Brassica juncea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Experimental Layout
2.2. Growth Parameters
2.3. Estimation of Cadmium and Sulfur Content
2.4. Gas Exchange and Photosynthetic Parameters
2.5. Assessment of Oxidative Damage
2.6. Antioxidant Enzymes and Non-Enzymatic Antioxidants
2.7. Non-Protein Thiols and Total Phytochelatins Content
2.8. S-Assimilating Enzymes and S-Containing Amino Acids
2.9. Confocal Laser Microscopy to Study Root Cell Viability
2.10. Physiological Measurements of the Guard Cells
2.11. Statistical Analysis
3. Results
3.1. Screening of Cultivars for Cd Tolerance
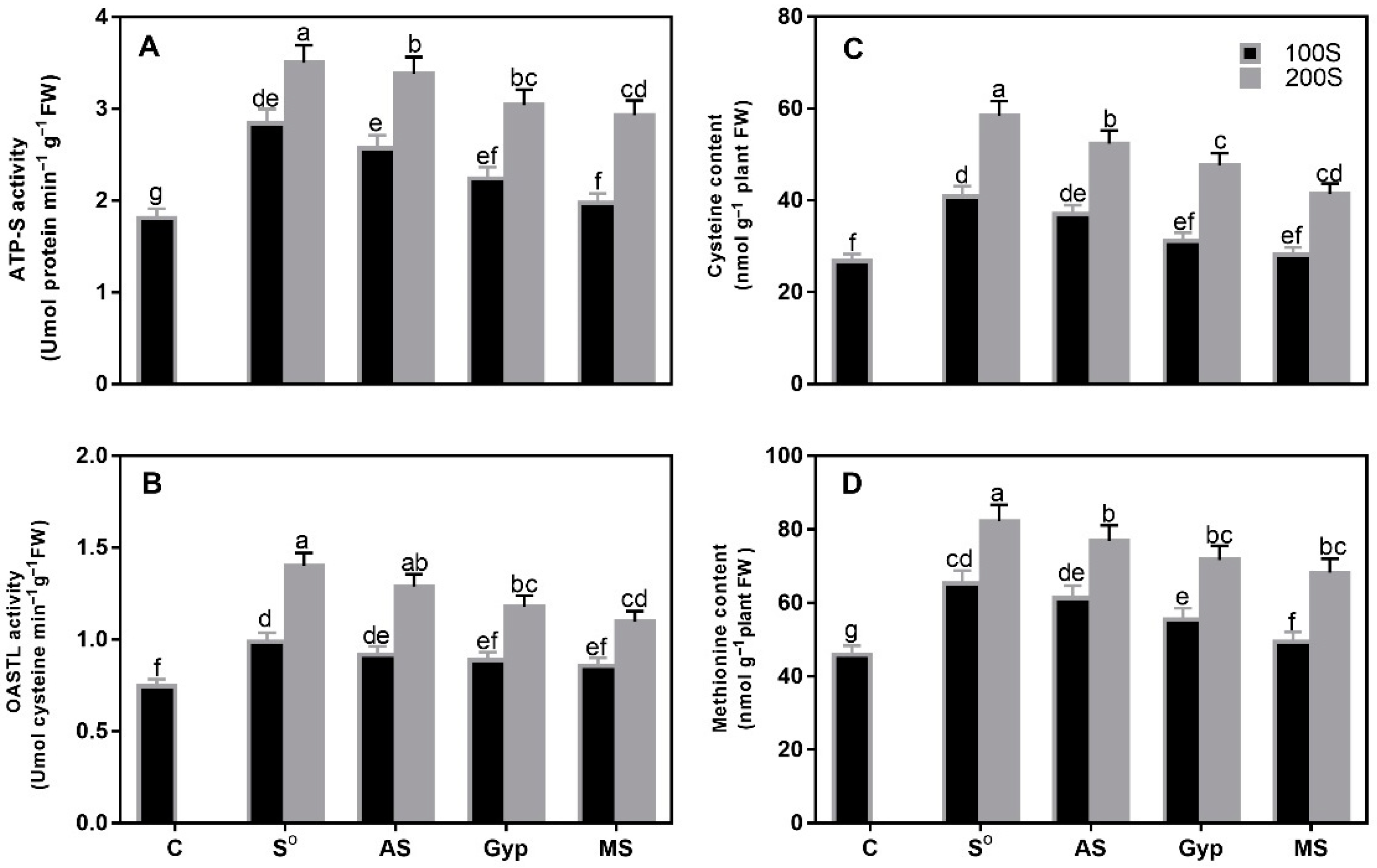
3.2. Response of Plants to Different S Sources and S Levels
3.3. Effects of Different S Sources on Cd and S Accumulation
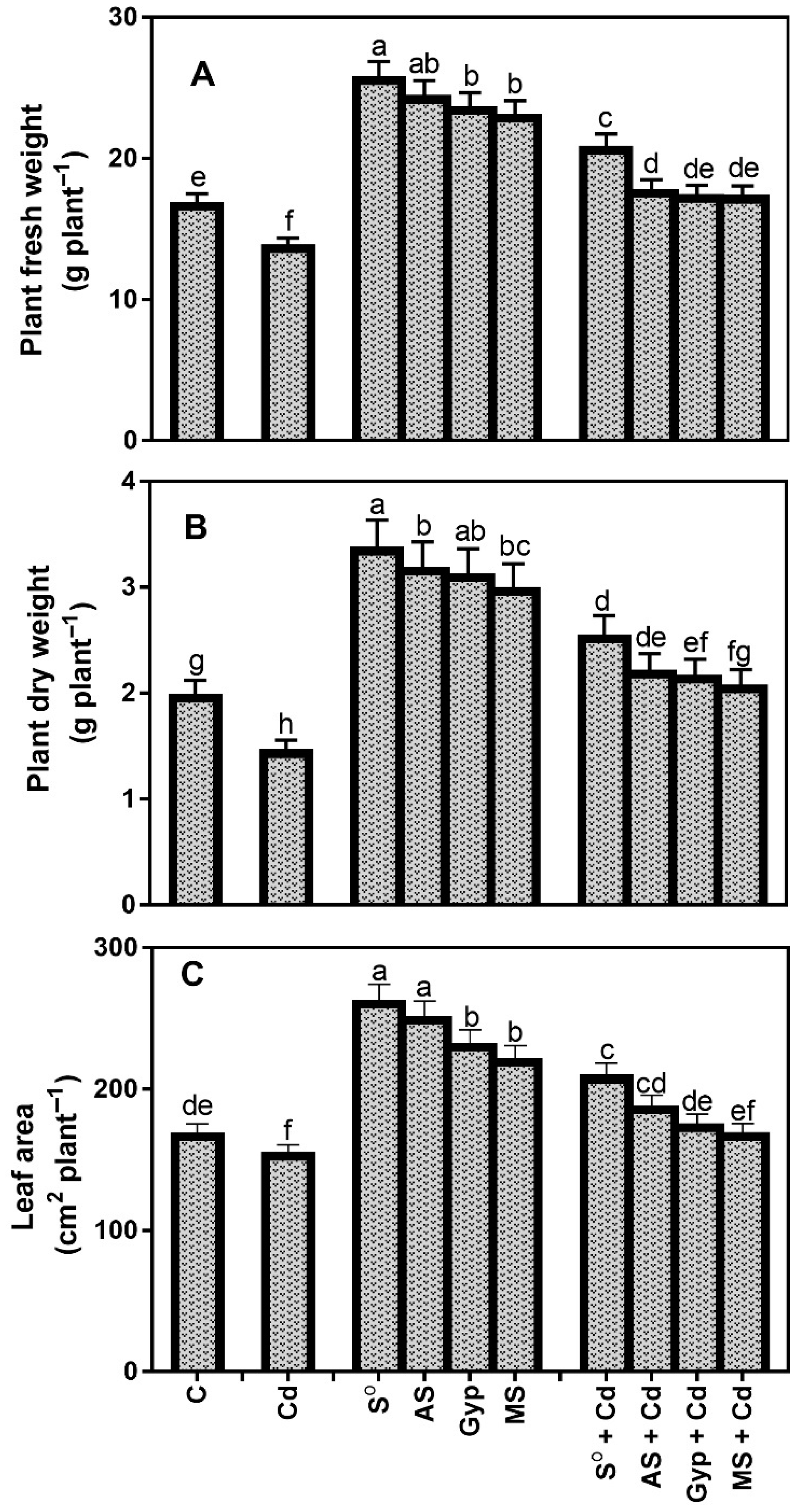
3.4. Effects of Different S Sources on Plant Growth under Cd Stress
3.5. Effect of Different S Sources in Preventing Adverse Effects of Cd on Photosynthesis
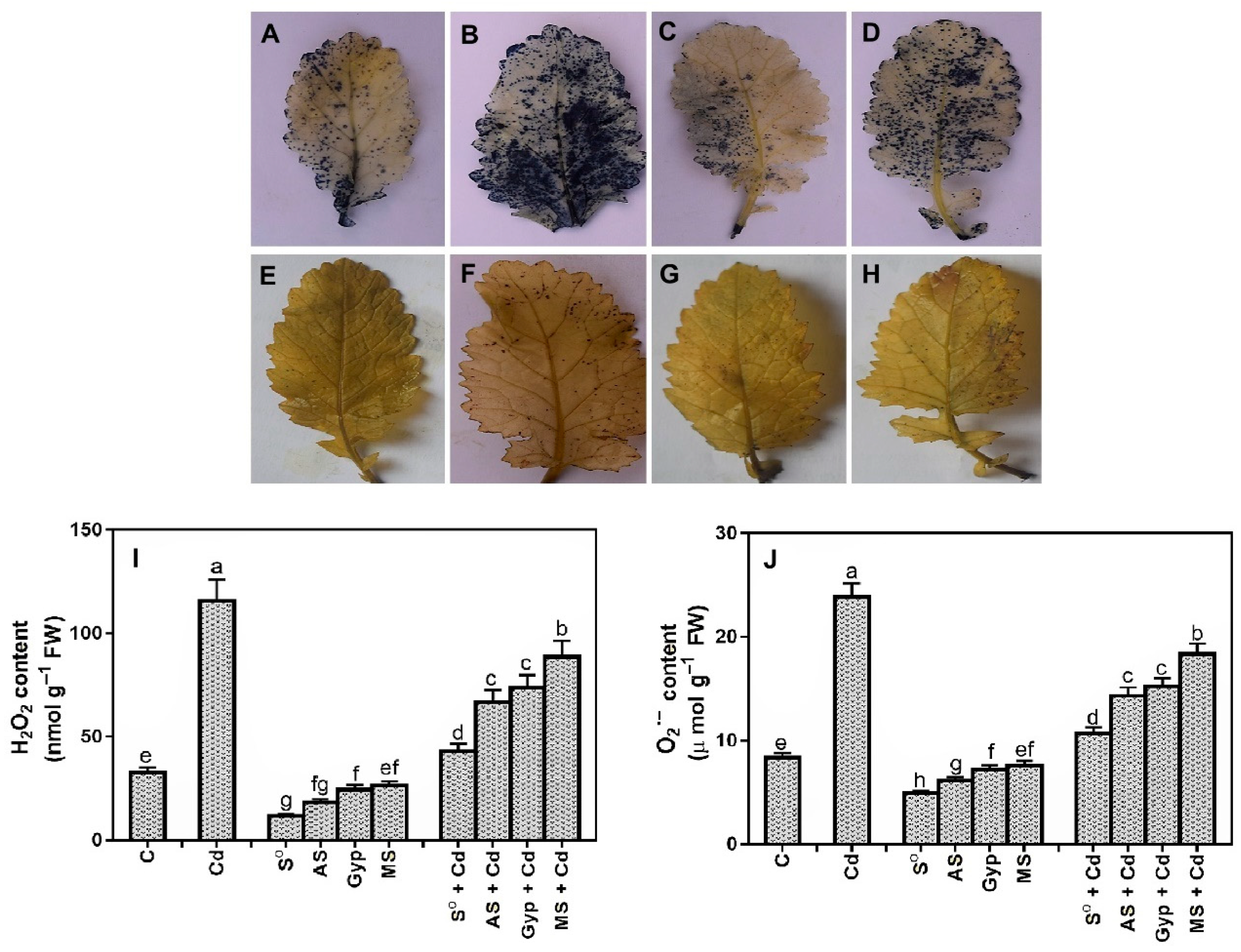
3.6. Effects of Different S Sources on Oxidative Stress and Antioxidants
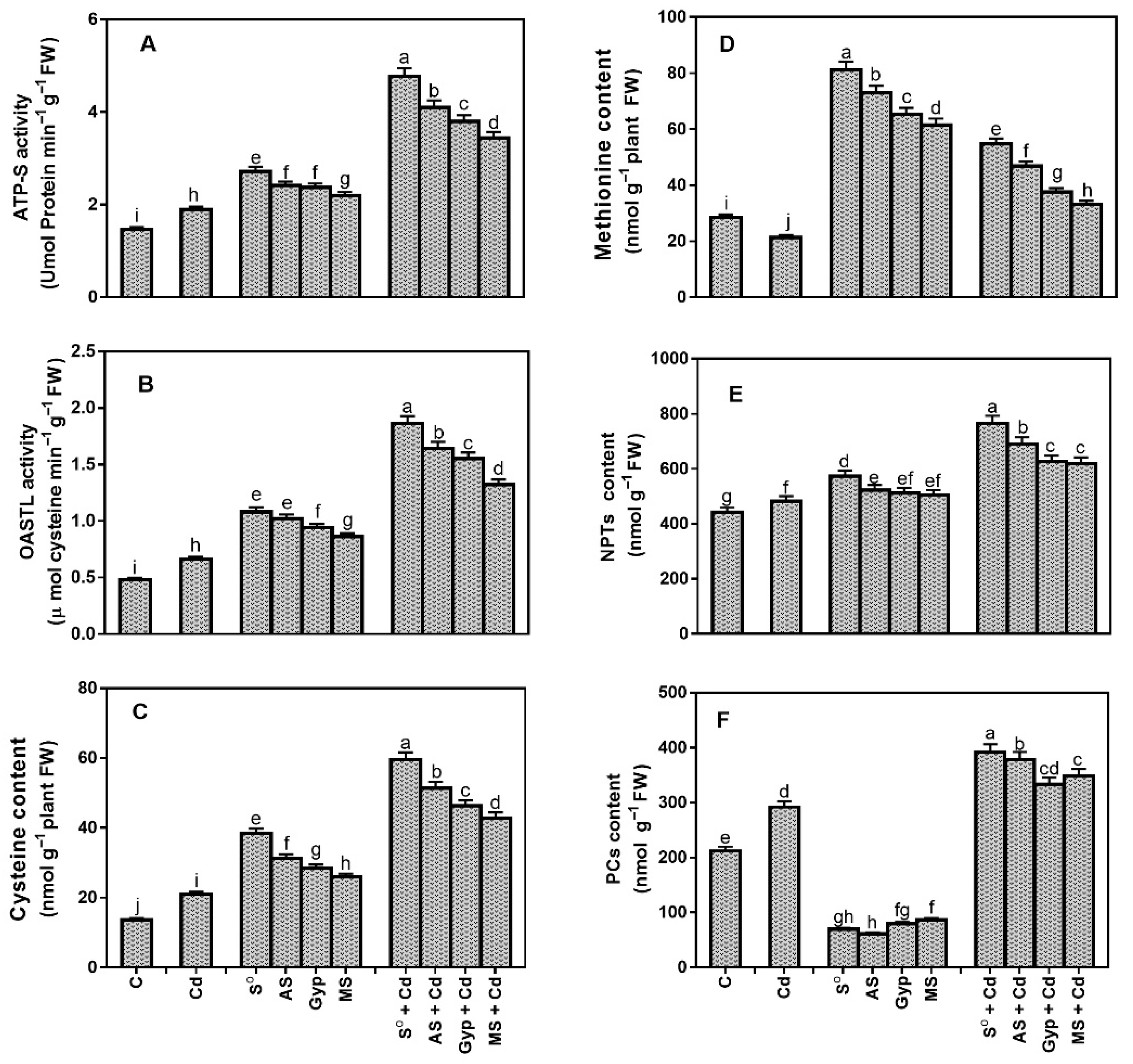
3.7. Effect of Different Sources of S on Variations in S Assimilation under Cd Stress
3.8. Influence of Different S Sources on Cell Viability and Stomatal Studies under Cd Stress
4. Discussion
4.1. S-Assimilation Plays a Central Role in Enhancing Defense against Cd Stress
4.2. Cd Accumulation, Translocation, and Role of Sulfur
4.3. Sulfur Increases Plant Growth by Mitigating Cd-Induced Toxicity
4.4. Sulfur Prevents Negative Effects of Cd on Photosynthesis
4.5. Sulfur Is Involved in the Reversal of Cd-Induced Oxidative Burst
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 1104692020. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric Oxide Pre-Treatment Advances Seed Germination and Alleviates Copper-Induced Photosynthetic Inhibition in Indian Mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Coakley, S.; Cahill, G.; Enright, A.M.; O’Rourke, B.; Petti, C. Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend? Sustainability 2019, 11, 5018. [Google Scholar] [CrossRef]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium Accumulation in Plants: Structure–Function Relations and Tissue-Specific Operation of Transporters in the Spotlight. Plants 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A. Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica Juncea L. Plants 2019, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, D.M. Toxicity and subcellular distribution of cadmium in wheat as affected by dissolved organic acids. J. Environ. Sci. 2012, 24, 903–911. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Photosynthesis mediated decrease in cadmium translocation protect shoot growth of Oryza sativa seedlings up on ammonium phosphate–sulfur fertilization. Environ. Sci. Pollut. Res. 2014, 21, 986–997. [Google Scholar] [CrossRef]
- Astolfi, S.; Zuchi, S.; Passera, C. Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J. Plant Physiol. 2004, 161, 795–802. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Mobin, M.; Anjum, N.A.; Khan, N.A. Modulation and significance of nitrogen and sulfur metabolism in cadmium challenged plants. Plant Growth Regul. 2016, 78, 1–11. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.F.; Li, J.; Xiong, J.; Zhou, L.N.; He, S.L.; Liu, H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Hu, K.D.; Bai, G.S.; Li, W.J.; Yan, H.; Hu, L.Y.; Li, Y.H.; Zhang, H. Sulfur dioxide promotes germination and plays an antioxidant role in cadmium-stressed wheat seeds. Plant Growth Regul. 2015, 75, 271–280. [Google Scholar] [CrossRef]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Tropical Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Liang, T.; Ding, H.; Wang, G.; Kang, J.; Pang, H.; Lv, J. Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulfur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2016, 124, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Umar, S.; Ahmad, A.; Iqbal, M.; Khan, N.A. Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul. 2008, 54, 271–279. [Google Scholar] [CrossRef]
- Adhikari, S.; Ghosh, S.; Azahar, I.; Adhikari, A.; Shaw, A.K.; Konar, S.; Hossain, Z. Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ. Exp. Bot. 2018, 153, 143–162. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 657, 1460–1467. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: An evaluation of the role of antioxidant machinery. Plant Signal. Behav. 2011, 6, 293–300. [Google Scholar] [CrossRef]
- Masood, A.; Khan, M.I.R.; Fatma, M.; Asgher, M.; Per, T.S.; Khan, N.A. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Chesnin, L.; Yien, C.H. Turbidimetric determination of available sulphates. Soil Sci. Soc. Am. Proc. 1950, 15, 149–151. [Google Scholar] [CrossRef]
- Usuda, H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Masuda, Y.; Yamanka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B.; Biosciences, J. Histochemical detection of superoxide 780 and H2O2 accumulation in Brassica juncea seedlings. Biol. Protoc. 2014, 4, e1108. [Google Scholar]
- Sullivan, C.Y.; Ross, W.M. Selecting the Drought and Heat Resistance in Grain Sorghum. In Stress Physiology in Crop Plants; Mussel, H., Staples, R.C., Eds.; John Wiley & Sons: New York, NY, USA, 1979; pp. 263–281. [Google Scholar]
- Dhindsa, R.H.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Pinto, M.C.D.; Tommasi, F.; Gara, L.D. Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco bright yellow 2. Plant Physiol. Biochem. 2000, 38, 541–550. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–211. [Google Scholar] [CrossRef]
- Lou, L.; Kang, J.; Pang, H.; Li, Q.; Du, X.; Wu, W.; Lv, J. Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. Int. J. Mol. Sci. 2017, 18, 1628. [Google Scholar] [CrossRef]
- Lappartient, A.G.; Touraine, B. Demand-driven control of root ATP sulfurylase activity and SO42-uptake in intact canola (the role of phloem-translocated glutathione). Plant Physiol. 1996, 111, 147–157. [Google Scholar] [CrossRef]
- Riemenschneider, A.; Riedel, K.; Hoefgen, R.; Papenbrock, J.; Hesse, H. Impact of reduced O-acetylserine (thiol) lyase isoform contents on potato plant metabolism. Plant Physiol. 2005, 137, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front. Plant Sci. 2016, 7, 1933. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, N.A.; Khan, M.I.R.; Fatma, M.; Masood, A. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol. Environ. Saf. 2014, 106, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Flávio, H.S.R.; Ricardo, A.A.; Francisco, A.M. The Proper Supply of S Increases Amino Acid Synthesis and Antioxidant Enzyme Activity in Tanzania Guinea Grass Used for Cd Phytoextraction. Water Air Soil Pollut. 2017, 228, 394–411. [Google Scholar]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol. Plant. 2020, 168, 490–510. [Google Scholar] [PubMed]
- Hussain, S.J.; Masood, A.; Anjum, N.A.; Khan, N.A. Sulfur-mediated control of salinity impact on photosynthesis and growth in mungbean cultivars screened for salt tolerance involves glutathione and proline metabolism, and glucose sensitivity. Acta Physiol. Plant. 2019, 41, 129. [Google Scholar] [CrossRef]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef]
- Wangeline, A.L.; Burkhead, J.L.; Hale, K.L.; Lindblom, S.D.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Overexpression of ATP Sulfurylase in Indian Mustard—Effects on Tolerance and Accumulation of Twelve Metals. J. Environ. Qual. 2004, 33, 54–60. [Google Scholar]
- Xiang, C.; Oliver, D.J. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998, 10, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, X.; Gao, K. Non-protein thiols and glutathione S-transferase alleviate Cd stress and reduce root-to-shoot translocation of Cd in rice. J. Plant Nutr. Soil Sci. 2013, 176, 626–633. [Google Scholar] [CrossRef]
- Yamaguchi, C.; Khamsalath, S.; Takimoto, Y.; Suyama, A.; Mori, Y.; Ohkama-Ohtsu, N.; Maruyama-Nakashita, A. SLIM1 Transcription Factor Promotes Sulfate Uptake and Distribution to Shoot, Along with Phytochelatin Accumulation, Under Cadmium Stress in Arabidopsis thaliana. Plants 2020, 9, 163. [Google Scholar] [CrossRef]
- Yang, Z.H.; Stöven, K.; Haneklaus, S.; Singh, B.R.; Schnug, E. Elemental sulfur oxidation by Thiobacillus spp. and aerobic heterotrophic sulfur-oxidizing bacteria. Pedosphere 2010, 20, 71–79. [Google Scholar] [CrossRef]
- Germida, J.J.; Janzen, H.H. Factors affecting the oxidation of elemental sulfur in soils. Fertil. Res. 1993, 35, 101–114. [Google Scholar] [CrossRef]
- Gao, M.X.; Hu, Z.Y.; Wang, G.D.; Xia, X. Effect of elemental sulfur supply on cadmium uptake into rice seedlings when cultivated in low and excess cadmium soils. Commun. Soil Sci. Plant Anal. 2010, 41, 990–1003. [Google Scholar] [CrossRef]
- Yamaguchi, C.; Takimoto, Y.; Ohkama-Ohtsu, N.; Hokura, A.; Shinano, T.; Nakamura, T.; Suyama, A.; Maruyama-Nakashita, A. Effects of cadmium treatment on the uptake and translocation of sulfate in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Lancilli, C.; Giacomini, B.; Lucchini, G.; Davidian, J.C.; Cocucci, M.; Sacchi, G.A.; Nocito, F.F. Cadmium exposure and sulfate limitation reveal differences in the transcriptional control of three sulfate transporter (Sultr1;2) genes in Brassica juncea. BMC Plant Biol. 2014, 14, 132. [Google Scholar] [CrossRef]
- Ferri, A.; Lancilli, C.; Maghrebi, M.; Lucchini, G.; Sacchi, G.A.; Nocito, F.F. The sulfate supply maximizing Arabidopsis shoot growth is higher under long-than short-term exposure to cadmium. Front. Plant Sci. 2017, 8, 854. [Google Scholar] [CrossRef]
- Kaya, M.; Zeliha, K.; Erdal, I. Effects of elemental sulfur and sulfur containing waste on nutrient concentrations and growth of bean and corn plants grown on a calcareous soil. Afr. J. Biotechnol. 2009, 8, 4481–4489. [Google Scholar]
- Cui, Y.; Wang, Q. Physiological responses of maize to elemental sulphur and cadmium stress. Plant Soil Environ. 2006, 52, 523. [Google Scholar] [CrossRef]
- Ervio, R.A.I.M.O. Acid-induced leaching of elements from cultivated soils. Ann. Agric. Fenn. 1991, 30, 331–344. [Google Scholar]
- Tichý, R.; Fajtl, J.; Kužel, S.; Kolář, L. Use of elemental sulphur to enhance a cadmium solubilization and its vegetative removal from contaminated soil. Nutr. Cycl. Agroecosyst. 1996, 46, 249–255. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, R.; Singh, M.P.; Singh, V.P. Impact of sulfur fertilization on different forms and balance of soil sulfur and the nutrition of wheat in wheat-soybean cropping sequence in tarai soil. J. Plant Nutr. 2014, 37, 618–632. [Google Scholar] [CrossRef]
- Bashir, H.; Ahmad, J.; Bagheri, R.; Nauman, M.; Qureshi, M.I. Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot. 2013, 94, 19–32. [Google Scholar] [CrossRef]
- Nazar, R.; Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 2014, 152, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Ibrahim, M.M.; Bagheri, R.; Ahmad, J.; Arif, I.A.; Baig, M.A.; Qureshi, M.I. Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB Plants 2015, 7, plv001. [Google Scholar] [CrossRef]
- Singh, M.; Kushwaha, B.K.; Singh, S.; Kumar, V.; Singh, V.P.; Prasad, S.M. Sulphur alters chromium (VI) toxicity in Solanum melongena seedlings: Role of sulphur assimilation and sulphur-containing antioxidants. Plant. Physiol. Biochem. 2017, 112, 183–192. [Google Scholar] [CrossRef]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a first line defence against cadmium toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef] [PubMed]




| Soil Parameter | Unit of Measure | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|---|
| Texture | Sandy loam | Sandy loam | Sandy loam | |
| pH | 7.83 | 7.64 | 7.78 | |
| Electrical conductivity | (ds m−1) | 0.48 | 0.51 | 0.43 |
| S | (mg kg−1 soil) | 31.56 | 29.85 | 26.17 |
| N | (mg kg−1 soil) | 72.51 | 76.94 | 78.68 |
| P | (mg kg−1 soil) | 8.32 | 9.79 | 8.11 |
| K | (mg kg−1 soil) | 115.64 | 133.81 | 138.65 |
| Cd | (mg kg−1 soil) | nd | nd | nd |
| Cultivar | Cd Level | Plant Fresh Biomass | Plant Dry Biomass | Leaf Area | Chlorophyll Content | Net Photosynthesis | Stomatal Conductance | Cd Root Content | Cd Leaf Content | Tolerance Index |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg Cd kg−1 Soil) | (g plant−1) | (cm2 plant−1) | (µmol CO2 m−2 s−1) | (mmol H2O m−2 s−1) | (µg g−1 DW) | |||||
| Giriraj | 0 (control) | 20.39 ± 0.51 a | 2.04 ± 0.10 a | 140.35 ± 7.06 a | 28.69 ± 1.44 a | 20.96 ± 1.05 a | 229.17 ± 11.53 a | nd | nd | |
| 100 | 18.59 ± 0.47 c,d | 1.73 ± 0.09 b,c | 122.45 ± 6.16 b | 26.53 ± 1.34 b,c | 17.41 ± 0.88 d,e | 205.17 ± 10.33 c,d | 125.66 ± 6.32 f | 28.96 ± 1.46 i | ||
| 200 | 15.36 ± 0.39 f | 1.61 ± 0.08 c | 105.51 ± 5.31 c,d | 23.85 ± 1.20 d | 13.24 ± 0.67 h | 179.46 ± 9.03 d | 191.14 ± 9.62 d,e | 113.35 ± 5.71 e | 0.789 ± 0.041 a | |
| RH-0749 | 0 (control) | 20.06 ± 0.50 a | 2.01 ± 0.10 a | 128.71 ± 6.45 a,b | 28.26 ± 1.42 a | 20.35 ± 1.02 a | 227.62 ± 11.46 a,b | nd | nd | |
| 100 | 18.33 ± 0.46 d,e | 1.55 ± 0.08 c,d | 116.29 ± 5.85 b,c | 26.14 ± 1.32 b,c | 17.06 ± 0.86 d,e | 198.38 ± 9.98 c,d | 151.35 ± 0.62 e,f | 40.14 ± 2.02 h | ||
| 200 | 14.92 ± 0.38 f | 0.95 ± 0.05 f | 87.14 ± 4.39 e | 23.19 ± 1.17 d,e | 12.45 ± 0.63 h,i | 174.39 ± 8.78 d,e | 215.98 ± 10.87 c | 129.46 ± 6.52 d | 0.472 ± 0.026 b | |
| Pusa Agrini | 0 (control) | 19.88 ± 0.50 a,b | 1.97 ± 0.10 a,b | 117.62 ± 5.92 b,c | 28.13 ± 1.42 a,b | 19.69 ± 0.99 a,b | 224.31 ± 11.29 b,c | nd | nd | |
| 100 | 18.09 ± 0.46 d,e | 1.42 ± 0.07 d | 92.34 ± 4.65 d,e | 25.87 ± 1.30b c | 16.31 ± 0.82 e,f | 195.48 ± 9.84 c,d | 175.22 ± 8.82 d,e | 61.95 ± 3.12 g | ||
| 200 | 13.65 ± 0.34 g | 0.81 ± 0.04 g | 60.32 ± 3.04 g,h | 22.82 ± 1.15 e | 12.07 ± 0.61 h,i | 172.63 ± 8.69 d,e | 269.27 ± 13.55 b | 135.88 ± 6.84 c | 0.411 ± 0.035 c | |
| RH-406 | 0 (control) | 19.64 ± 0.49 b,c | 1.94 ± 0.10 a,b | 106.76 ± 5.37 c,d | 28.02 ± 1.41 a,b | 19.11 ± 0.96 b,c | 223.44 ± 11.25 b,c | nd | nd | |
| 100 | 17.83 ± 0.45 e | 1.36 ± 0.07 d,e | 84.81 ± 4.27 e,f | 25.44 ± 1.28 c,d | 15.94 ± 0.80 f,g | 193.56 ± 9.74 c,d | 188.65 ± 9.50 d | 76.54 ± 3.85 f,g | ||
| 200 | 12.67 ± 0.32 g | 0.74 ± 0.047 g | 52.74 ± 2.65 h | 20.46 ± 1.03 f | 11.35 ± 0.57 i,j | 166.13 ± 8.36 e | 295.84 ± 14.89 b | 142.69 ± 7.18 b | 0.381 ± 0.026 d | |
| Pusa tarak | 0 (control) | 19.25 ± 0.48 b,c | 1.80 ± 0.09 b | 99.21 ± 4.99 d,e | 27.91 ± 1.40 a,b | 18.65 ± 0.94 c,d | 219.27 ± 11.04 b,c | nd | nd | |
| 100 | 17.68 ± 0.44 e | 1.26 ± 0.06 e | 70.59 ± 3.55 f,g | 25.06 ± 1.26 c,d | 14.06 ± 0.71 g,h | 192.81 ± 9.70 c,d | 228.35 ± 11.49 c | 89.75 ± 4.52 f | ||
| 200 | 11.06 ± 0.28 h | 0.65 ± 0.03 h | 45.38 ± 2.28 h | 18.37 ± 0.92 g | 11.06 ± 0.56 i,j | 152.79 ± 79.20 f | 364.48 ± 18.35 a | 158.23 ± 7.96 a | 0.361 ± 0.023 d | |
| S Source | S Level | Plant Fresh Biomass | Plant Dry Biomass | Chlorophyll Content | Net Photosynthesis | Stomatal Conductance | Intercellular CO2 | Maximal PSII Efficiency |
|---|---|---|---|---|---|---|---|---|
| (mg S kg−1 Soil) | (g plant−1) | (µmol CO2 m−2 s−1) | (mmol H2O m−2 s−1) | (µmol CO2 mol−1) | ||||
| Control | 0 | 19.69 ± 0.83 h | 1.81 ± 0.08 e | 26.48 ± 1.56 g | 19.23 ± 1.13 f | 235.62 ± 11.11 e | 241.38 ± 10.15 f | 0.578 ± 0.03 i |
| S0 | 100 | 23.19 ± 1.07 d | 2.24 ± 0.11 d | 33.41 ± 1.97 d | 23.67 ± 1.39 c | 347.92 ± 15.19 c | 303.35 ± 12.56 c,d | 0.683 ± 0.03 e |
| 200 | 26.19 ± 1.16 a | 3.76 ± 0.16 a | 39.45 ± 2.32 a | 26.98 ± 1.56 a | 430.49 ± 19.46 a | 394.66 ± 17.35 a | 0.814 ± 0.04 a | |
| AS | 100 | 22.45 ± 0.99 e | 2.19 ± 0.10 d | 32.59 ± 1.92 d,e | 22.45 ± 1.32 d | 317.35 ± 12.80 c,d | 296.17 ± 11.90 d,e | 0.664 ± 0.03 f |
| 200 | 25.64 ± 1.14 b | 3.19 ± 0.14 b | 37.62 ± 2.22 b | 25.17 ± 1.48 b | 410.39 ± 18.28 a,b | 371.76 ± 16.59 a,b | 0.785 ± 0.03 b | |
| Gyp | 100 | 22.91 ± 1.01 f | 2.06 ± 0.10 d,e | 31.87 ± 1.88 e,f | 22.38 ± 1.30 d | 308.37 ± 13.45 c,d | 291.64 ± 11.28 d,e | 0.646 ± 0.03 g |
| 200 | 25.31 ± 1.12 b,c | 3.02 ± 0.13 b,c | 36.44 ± 2.15 b,c | 25.03 ± 1.43 b | 396.45 ± 11.57 b,c | 330.51 ± 13.54 b | 0.769 ± 0.03 c | |
| MS | 100 | 21.56 ± 1.01 g | 2.03 ± 0.09 d,e | 29.33 ± 1.73 f | 21.54 ± 1.26 e | 291.49 ± 11.98 d,e | 269.46 ± 10.57 e | 0.621 ± 0.03 h |
| 200 | 24.76 ± 1.09 c | 2.88 ± 0.10 cd | 35.45 ± 2.09 c,d | 24.62 ± 1.41 b,c | 389.44 ± 17.04 b,c | 318.62 ± 13.05 b,c | 0.713 ± 0.03 d | |
| Treatment | Cd Treatment | Cd Content | S Content | Cd TF | ||
|---|---|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | |||
| (µg g−1 DW) | (mg g−1 DW) | |||||
| Control | −Cd | nd | nd | 4.07 ± 0.07 e | 4.29 ± 0.08 g | nd |
| Cd | +Cd | 166.96 ± 9.33 a | 138.59 ± 4.18 a | 3.12 ± 0.06 f | 3.32 ± 0.06 h | 0.830 a |
| S0 | −Cd | nd | nd | 6.77 ± 0.12 a | 7.98 ± 0.16 a | nd |
| +Cd | 89.69 ± 1.05 e | 52.94 ± 3.22 d | 5.30 ± 0.10 c,d | 5.88 ± 0.11 d | 0.590 d | |
| AS | −Cd | nd | nd | 6.35 ± 0.12 b | 7.25 ± 0.13 b | nd |
| +Cd | 95.59 ± 4.04 d | 71.49 ± 1.32 cd | 5.06 ± 0.09 d | 5.47 ± 0.10 e | 0.747 b | |
| Gyp | −Cd | nd | nd | 5.85 ± 0.11 c | 6.92 ± 0.13 b,c | nd |
| +Cd | 109.56 ± 4.62 c | 75.62 ± 1.62 c | 4.88 ± 0.09 d | 5.09 ± 0.09 f | 0.694 c | |
| MS | −Cd | nd | nd | 5.27 ± 0.12 c,d | 6.37 ± 0.12 c | nd |
| +Cd | 126.84 ± 5.62 b | 81.24 ± 1.85 b | 4.64 ± 0.08 d,e | 4.75 ± 0.09 f,g | 0.642 c,d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, I.R.; Rather, B.A.; Masood, A.; Majid, A.; Sehar, Z.; Anjum, N.A.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Soil Sulfur Sources Differentially Enhance Cadmium Tolerance in Indian Mustard (Brassica juncea L.). Soil Syst. 2021, 5, 29. https://doi.org/10.3390/soilsystems5020029
Mir IR, Rather BA, Masood A, Majid A, Sehar Z, Anjum NA, Sofo A, D’Ippolito I, Khan NA. Soil Sulfur Sources Differentially Enhance Cadmium Tolerance in Indian Mustard (Brassica juncea L.). Soil Systems. 2021; 5(2):29. https://doi.org/10.3390/soilsystems5020029
Chicago/Turabian StyleMir, Iqbal R., Bilal A. Rather, Asim Masood, Arif Majid, Zebus Sehar, Naser A. Anjum, Adriano Sofo, Ilaria D’Ippolito, and Nafees A. Khan. 2021. "Soil Sulfur Sources Differentially Enhance Cadmium Tolerance in Indian Mustard (Brassica juncea L.)" Soil Systems 5, no. 2: 29. https://doi.org/10.3390/soilsystems5020029
APA StyleMir, I. R., Rather, B. A., Masood, A., Majid, A., Sehar, Z., Anjum, N. A., Sofo, A., D’Ippolito, I., & Khan, N. A. (2021). Soil Sulfur Sources Differentially Enhance Cadmium Tolerance in Indian Mustard (Brassica juncea L.). Soil Systems, 5(2), 29. https://doi.org/10.3390/soilsystems5020029












