Characterization of Plant Growth-Promoting Traits and Inoculation Effects on Triticum durum of Actinomycetes Isolates under Salt Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Actinomycetes Strains
2.2. Estimation of PGP Traits under Salt Stress
2.2.1. Hydrocyanic Acid and Ammonia Production
2.2.2. Phosphate Solubilization Ability
2.2.3. Production of Indole-3-Acetic Acid
2.3. Estimation of 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Activity
2.4. Greenhouse Experiment on Triticum durum
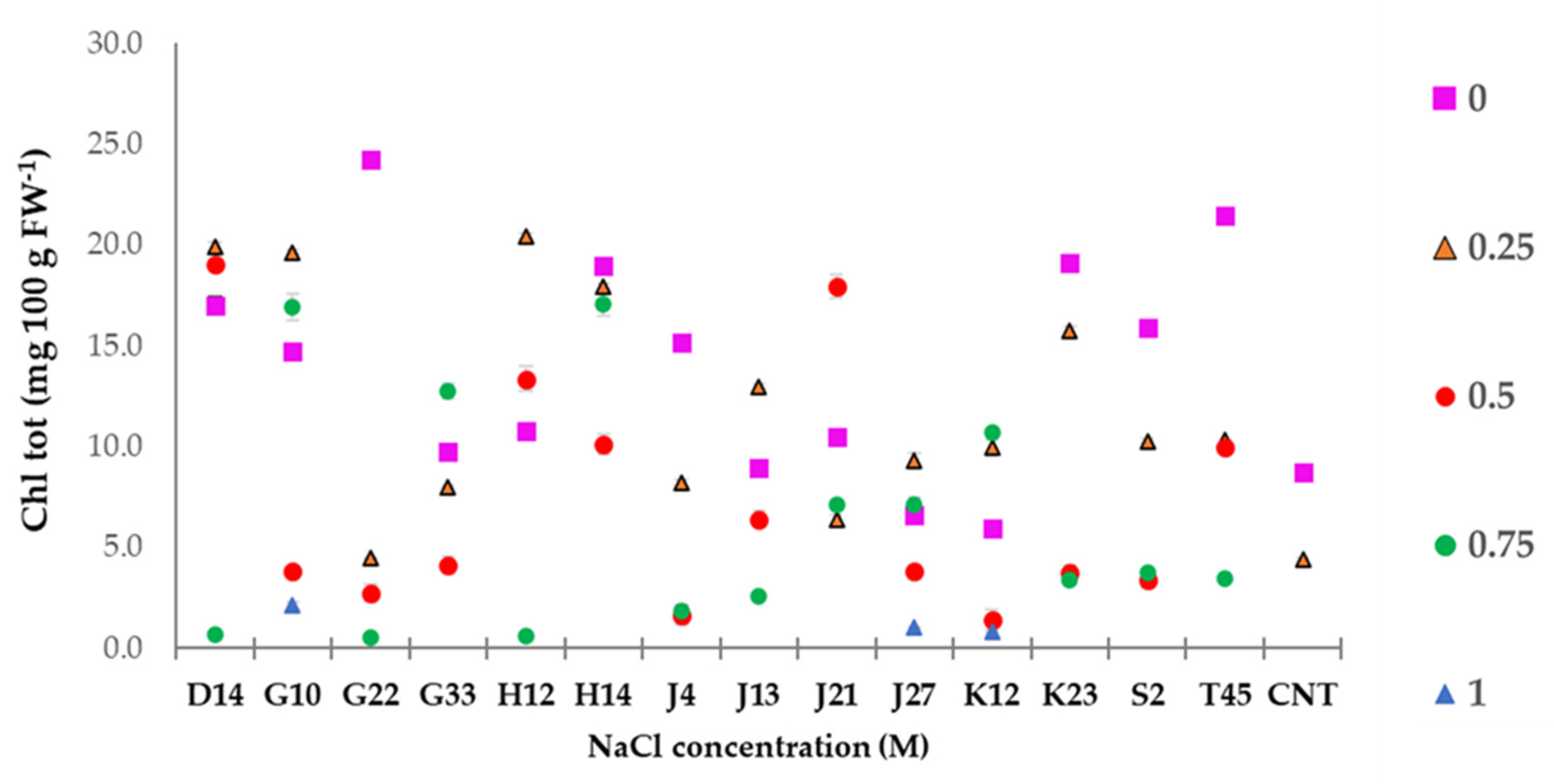
2.4.1. Chlorophyll Measurement
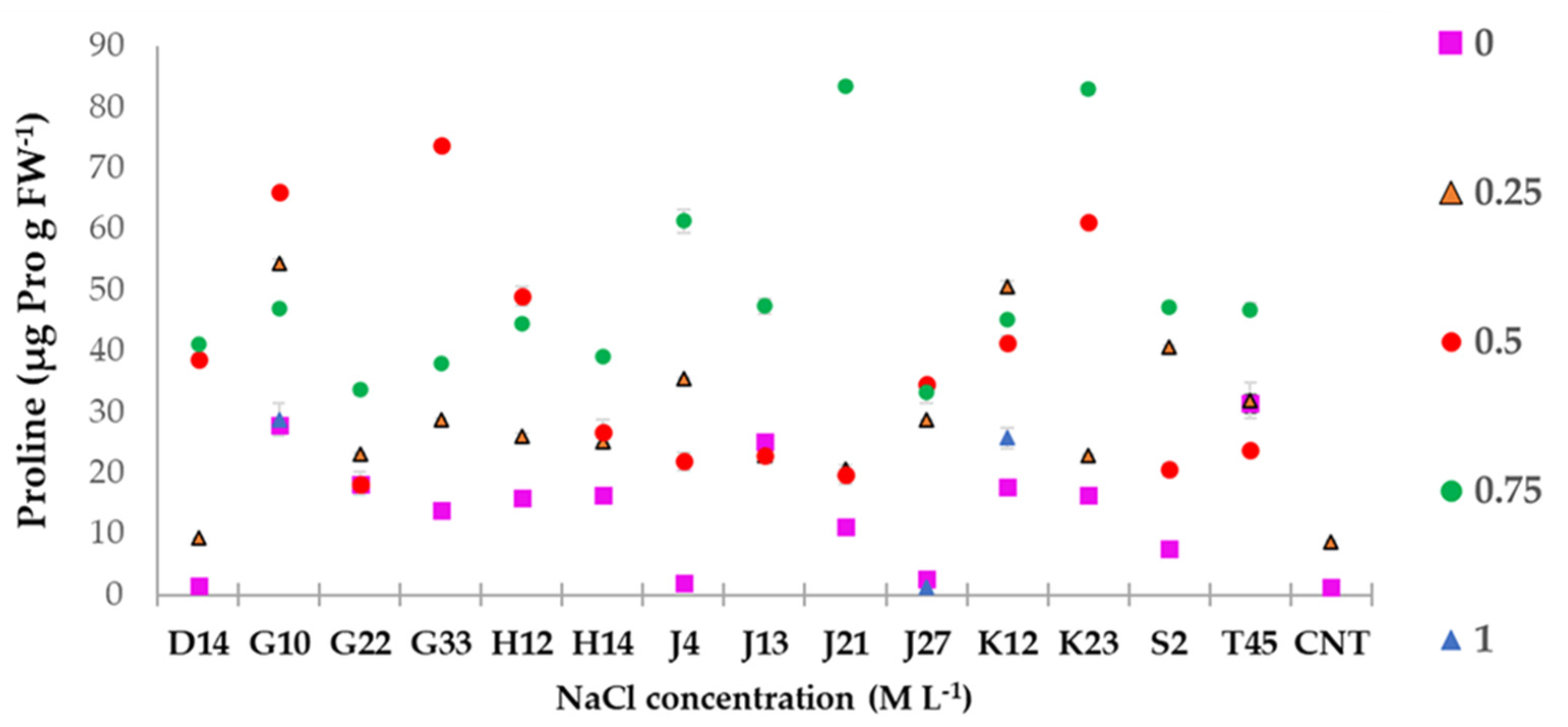
2.4.2. Proline Determination
2.5. Statistical Analysis
3. Results
3.1. Actinomycetes Strains
- Nocardiopsis aegyptica (MG597543)—H14;
- Nocardiopsis aegyptica (MG597572)—S2;
- Nocardiopsis alba (MG597576)—J21;
- Nocardiopsis dassonvillei subsp. dassonvillei (MG597514)—D14;
- Nocardiopsis dassonvillei subsp. dassonvillei (MG597502)—T45.
- Streptomyces albidoflavus (MG597552)—H12;
- Streptomyces ambofaciens (MG597599)—J27;
- Streptomyces anulatus (MG597579)—J13;
- Streptomyces iakyrus (MG597593)—G10;
- Streptomyces thinghirensis (MG597560)—K23;
- Streptomyces thinghirensis (MG597590)—J4;
- Streptomyces xantholiticus (MG597545)—K12;
- Streptomyces xantholiticus (MG597582)—G22;
- Streptomyces xantholiticus (MG597585)—G33;
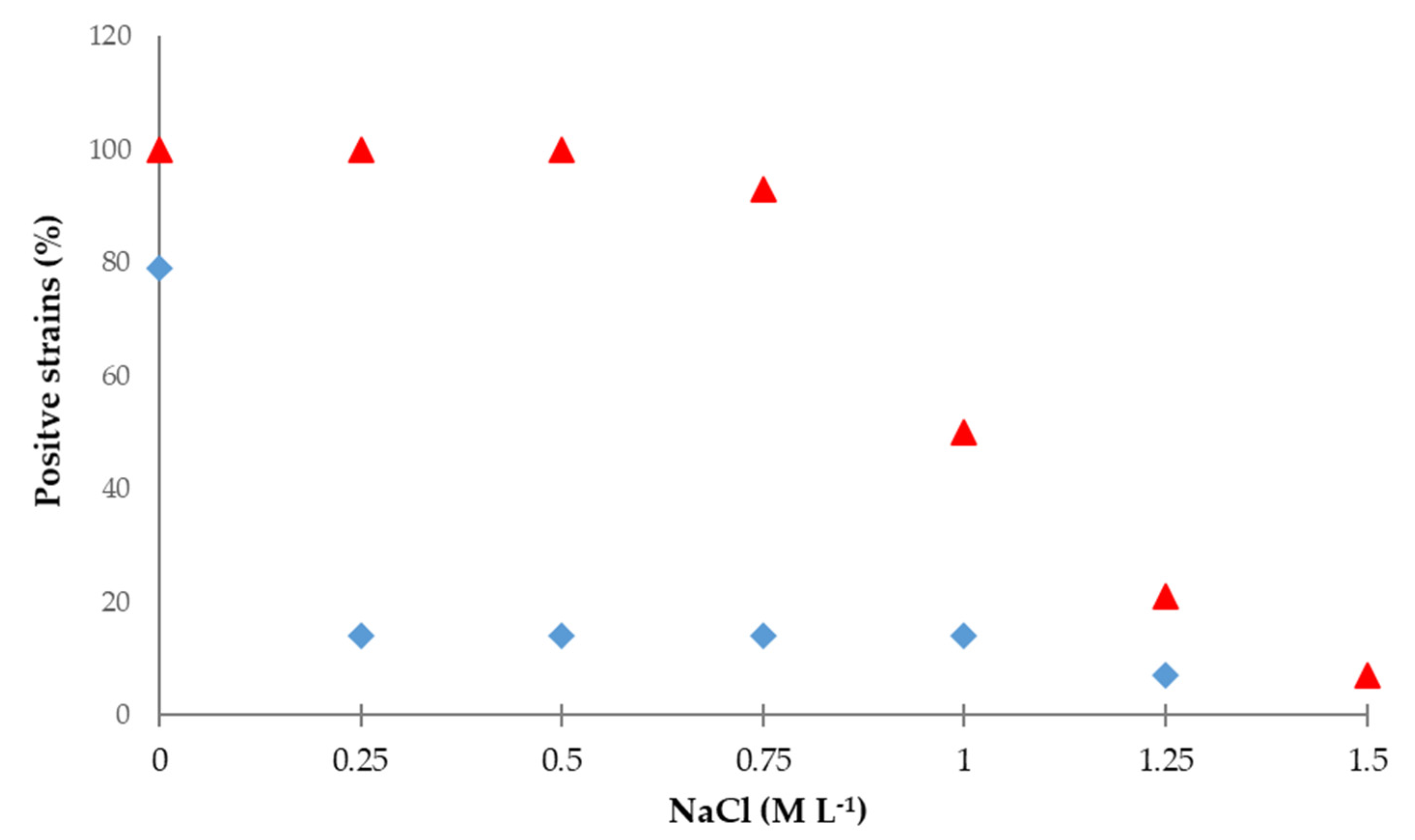
3.2. Hydrocyanic Acid and Ammonia Production under Salt Stress
3.3. Phosphate Solubilization under Salt Stress
3.4. Indole Acetic Acid Production under Salt Stress
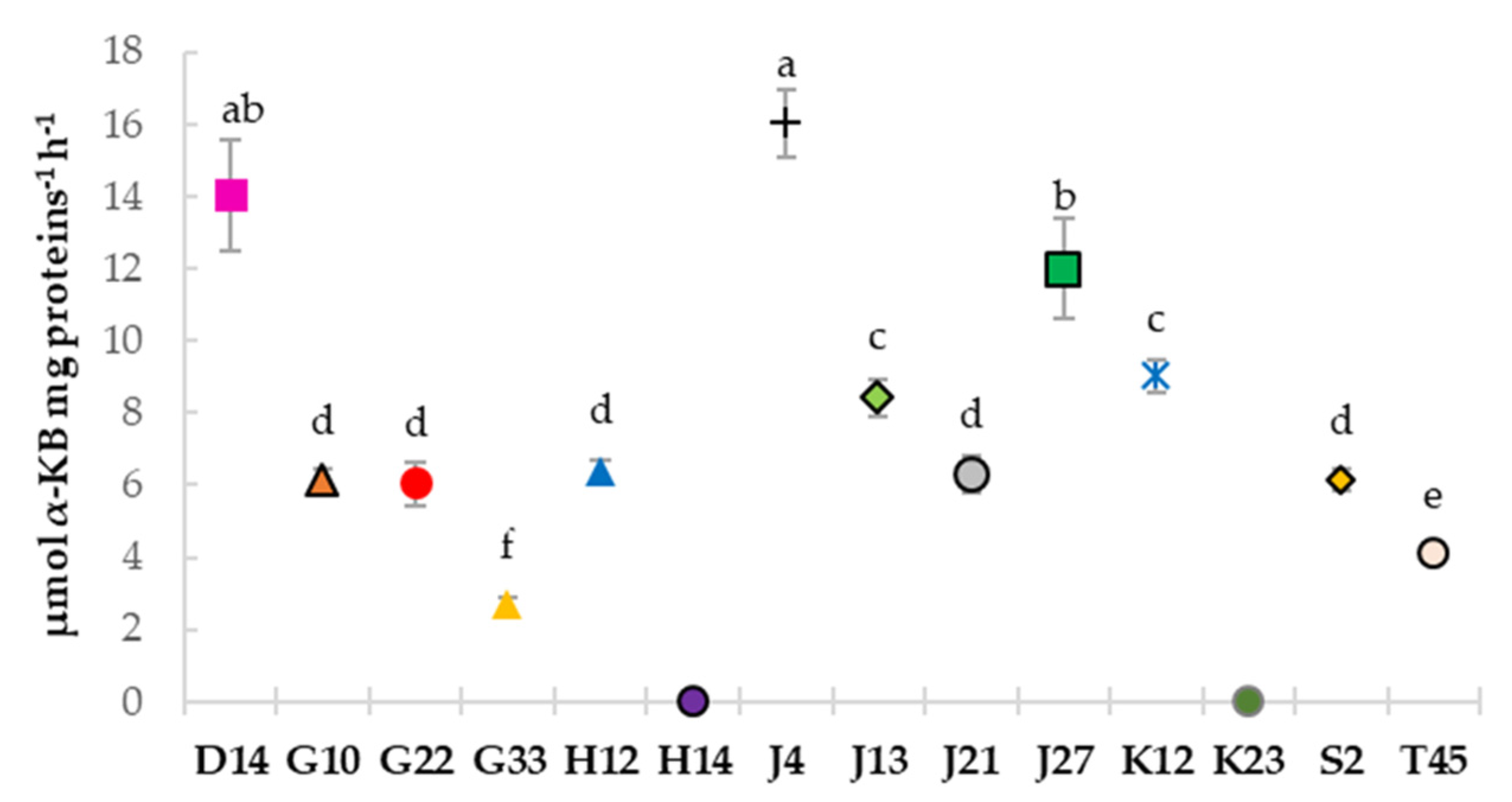
3.5. Estimation of 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Activity
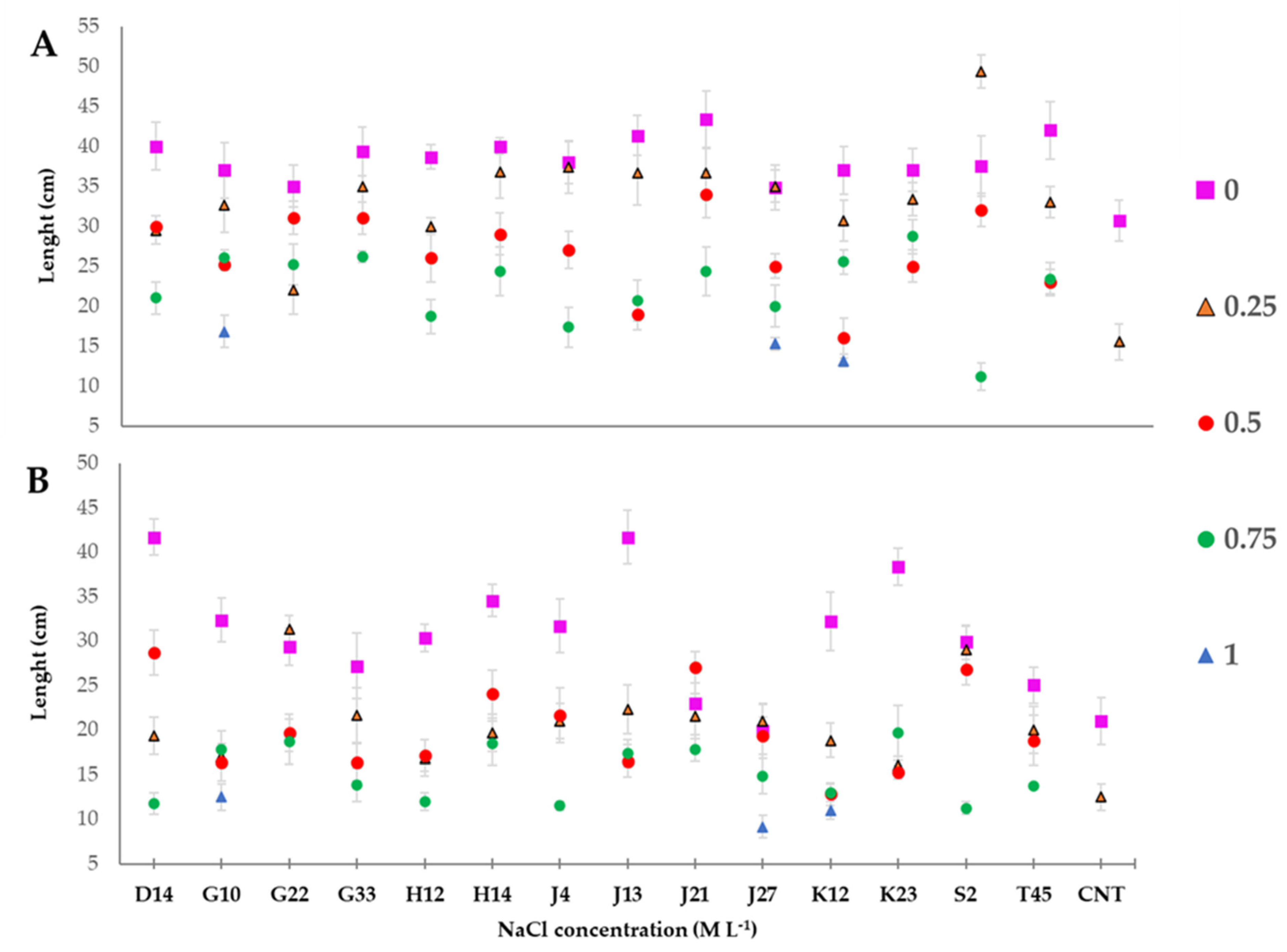
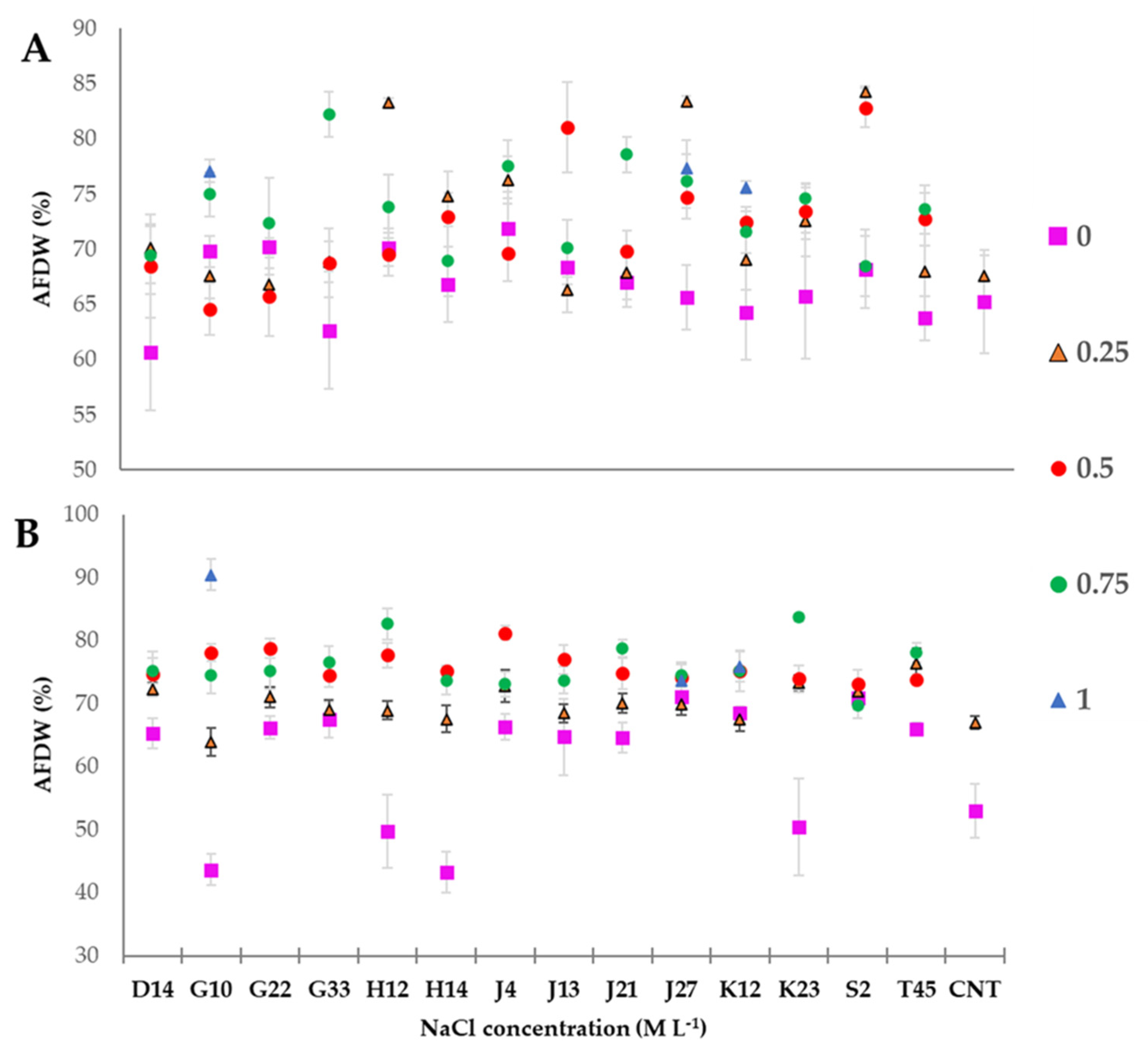
3.6. Greenhouse Experiment on T. durum
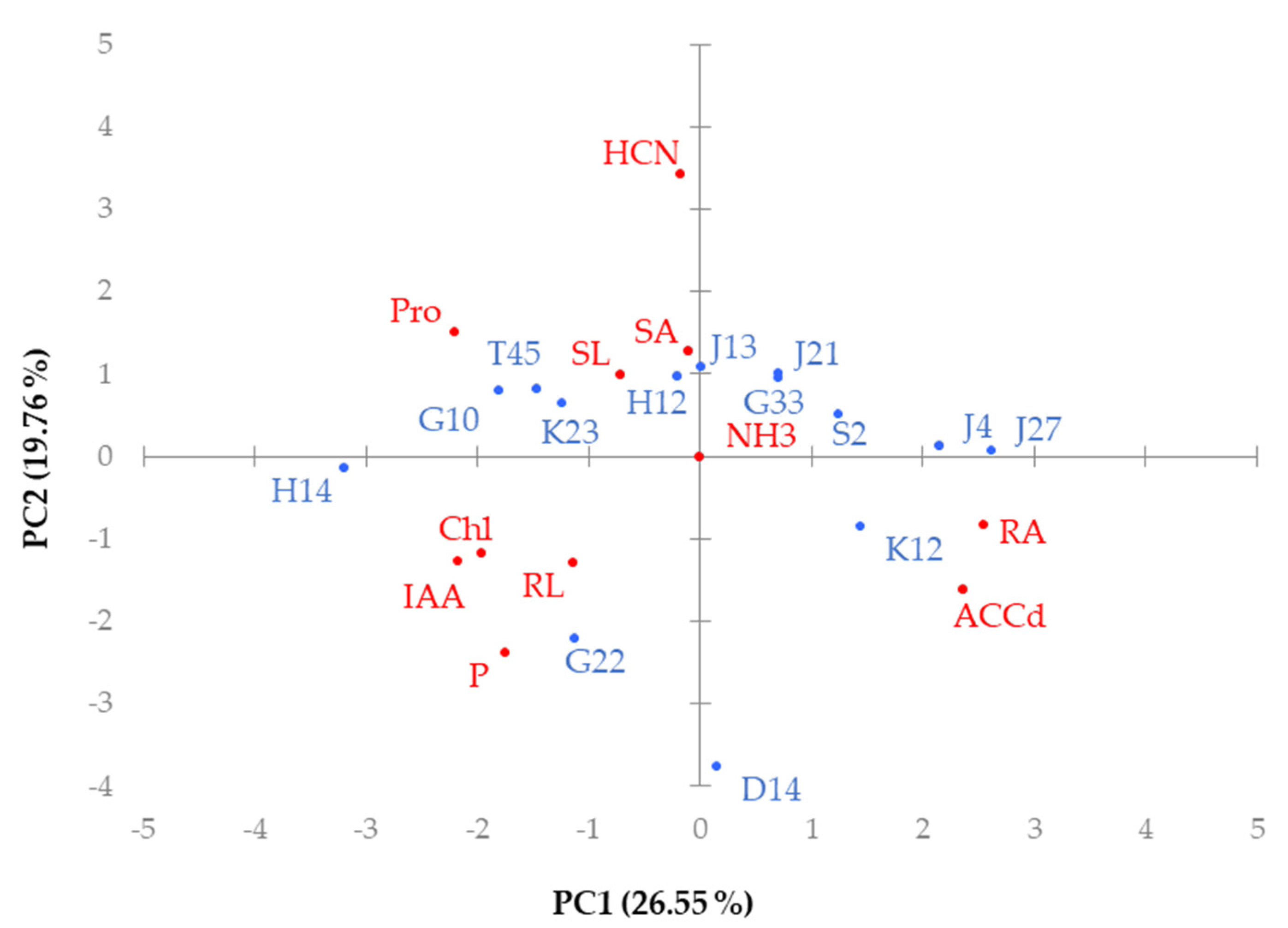
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fatima, T.; Arora, N.K. Plant Growth-Promoting Rhizospheric Microbes for Remediation of Saline Soils. In Phyto and Rhizo Remediation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–146. [Google Scholar]
- Bharti, N.; Yadav, D.; Barnawal, D.; Maji, D.; Kalra, A. Exiguobacterium Oxidotolerans, a Halotolerant Plant Growth Promoting Rhizobacteria, Improves Yield and Content of Secondary Metabolites in Bacopa Monnieri (L.) Pennell under Primary and Secondary Salt Stress. World J. Microbiol. Biotechnol. 2013, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Glick, B.R. Halotolerant Plant Growth–Promoting Bacteria: Prospects for Alleviating Salinity Stress in Plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Mahmoud, O.M.B.; Hidri, R.; Talbi-Zribi, O.; Taamalli, W.; Abdelly, C.; Djébali, N. Auxin and Proline Producing Rhizobacteria Mitigate Salt-Induced Growth Inhibition of Barley Plants by Enhancing Water and Nutrient Status. S. Afr. J. Bot. 2020, 128, 209–217. [Google Scholar] [CrossRef]
- Silini, A.; Cherif-Silini, H.; Yahiaoui, B. Growing Varieties Durum Wheat (Triticum Durum) in Response to the Effect of Osmolytes and Inoculation by Azotobacter Chroococcum under Salt Stress. Afr. J. Microbiol. Res. 2016, 10, 387–399. [Google Scholar]
- FAO. 2015 Global Soil Partnership—World Soil Charter. Available online: http://www.fao.org/3/mn442e/mn442e.pdf (accessed on 13 August 2020).
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M.M. Isolation and Identification of Salt-Tolerant Plant-Growth-Promoting Rhizobacteria and Their Application for Rice Cultivation under Salt Stress. Can. J. Microbiol. 2020, 66, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.Z. Bacteria Able to Control Foot and Root Rot and to Promote Growth of Cucumber in Salinated Soils. Biol. Fertil. Soils 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress: A Review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by Arbuscular Mycorrhizae of the Integrated Physiological Response to Salinity in Plants: New Challenges in Physiological and Molecular Studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential Biochemical Indicators of Salinity Tolerance in Plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Baniaghil, N.; Arzanesh, M.H.; Ghorbanli, M.; Shahbazi, M. The Effect of Plant Growth Promoting Rhizobacteria on Growth Parameters, Antioxidant Enzymes and Microelements of Canola under Salt Stress. J. Appl. Environ. Biol. Sci. 2013, 3, 17–27. [Google Scholar]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere Bacteria Help Plants Tolerate Abiotic Stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, X.; Smith, D.L. Enhanced Soybean Plant Growth Resulting from Coinoculation of Bacillus Strains with Bradyrhizobium Japonicum. Crop Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Davranov, K.; Wirth, S.; Hashem, A.; Abd_Allah, E.F. Impact of Soil Salinity on the Plant-Growth–Promoting and Biological Control Abilities of Root Associated Bacteria. Saudi J. Biol. Sci. 2017, 24, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of Endophytic and Rhizospheric Plant Growth Promoting Rhizobacteria in Oryza Sativa Shows Higher Accumulation of Osmoprotectant against Saline Stress. Acta Physiol. Plant. 2011, 33, 797–802. [Google Scholar] [CrossRef]
- Goswami, M.; Suresh, D. Plant Growth-Promoting Rhizobacteria—Alleviators of Abiotic Stresses in Soil: A Review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Nadeem, S.M.; Khan, M.Y.; Binyamin, R.; Waqas, M.R. Role of Halotolerant Microbes in Plant Growth Promotion under Salt Stress Conditions. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 209–253. [Google Scholar]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular Response to Hyperosmotic Stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef]
- Jha, B.; Singh, V.K.; Weiss, A.; Hartmann, A.; Schmid, M. Zhihengliuella Somnathii Sp. Nov., a Halotolerant Actinobacterium from the Rhizosphere of a Halophyte Salicornia Brachiata. Int. J. Syst. Evol. Microbiol. 2015, 65, 3137–3142. [Google Scholar] [CrossRef]
- Jha, B.; Gontia, I.; Hartmann, A. The Roots of the Halophyte Salicornia Brachiata Are a Source of New Halotolerant Diazotrophic Bacteria with Plant Growth-Promoting Potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Mahmood, A.; Kataoka, R.; Turgay, O.C.; Yaprak, A.E. Halophytic Microbiome in Ameliorating the Stress. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 171–194. [Google Scholar]
- Kaushal, M.; Wani, S.P. Rhizobacterial-Plant Interactions: Strategies Ensuring Plant Growth Promotion under Drought and Salinity Stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Ahmed, A.-H. Plant Growth Promoting Bacteria as an Alternative Strategy for Salt Tolerance in Plants: A Review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.C.M.; Galindo, F.S.; Gazola, R.P.D.; Dupas, E.; Rosa, P.A.L.; Mortinho, E.S. Corn Yield and Phosphorus Use Efficiency Response to Phosphorus Rates Associated With Plant Growth Promoting Bacteria. Front. Environ. Sci. 2020, 8, 40. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking Bacterial Community Composition to Soil Salinity along Environmental Gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef] [PubMed]
- ZHANG, W.; Chong, W.; Rui, X.; WANG, L. Effects of Salinity on the Soil Microbial Community and Soil Fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Smati, M.; Kitouni, M. Diversity of Actinobacteria in the Marshes of Ezzemoul and Djendli in Northeastern Algeria. Eur. J. Ecol. 2019, 5, 41–53. [Google Scholar] [CrossRef][Green Version]
- Djebaili, R.; Pellegrini, M.; Smati, M.; Gallo, M.D.; Kitouni, M. Actinomycete Strains Isolated from Saline Soils: Plant-Growth-Promoting Traits and Inoculation Effects on Solanum Lycopersicum. Sustainability 2020, 12, 4617. [Google Scholar] [CrossRef]
- Pochon, J.; Tardieux, P. Techniques d’analyse En Microbiologie Du Sol; Editions de la Tourelle: Paris, France, 1962; Volume 11. [Google Scholar]
- Donate-Correa, J.; León-Barrios, M.; Pérez-Galdona, R. Screening for Plant Growth-Promoting Rhizobacteria in Chamaecytisus Proliferus (Tagasaste), a Forage Tree-Shrub Legume Endemic to the Canary Islands. Plant Soil 2005, 266, 261–272. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Sherman, N. Biochemical Activities of Microorganisms. In Microbiology—A Laboratory Manual; The Benjamin/Cummings Publishing Co., Inc.: Menlo Park, CA, USA, 1996. [Google Scholar]
- Pikovskaya, R.I. Mobilization of Phosphorus in Soil in Connection with Vital Activity of Some Microbial Species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; (Soil Science Society of America); American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Khiangte, L.; Lalfakzuala, R. In Vitro Production of Growth Regulator (IAA) and Phosphatase by Phosphate Solubilizing Bacteria. Sci. Technol. J 2011, 5, 32–35. [Google Scholar] [CrossRef]
- Leaungvutiviroj, C.; Ruangphisarn, P.; Hansanimitkul, P.; Shinkawa, H.; Sasaki, K. Development of a New Biofertilizer with a High Capacity for N2 Fixation, Phosphate and Potassium Solubilization and Auxin Production. Biosci. Biotechnol. Biochem. 2010, 74, 1098–1101. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ponmurugan, P.; Gopi, C. In Vitro Production of Growth Regulators and Phosphatase Activity by Phosphate Solubilizing Bacteria. Afr. J. Biotechnol. 2006, 5, 348–350. [Google Scholar]
- Wahyudi, A.T.; Priyanto, J.A.; Afrista, R.; Kurniati, D.; Astuti, R.I.; Akhdiya, A. Plant Growth Promoting Activity of Actinomycetes Isolated from Soybean Rhizosphere. Online J. Biol. Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation of Indoleacetic Acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef]
- Brígido, C.; Duan, J.; Glick, B.R. Methods to Study 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase in Plant Growth-Promoting Bacteria. In Handbook for Azospirillum; Springer: Berlin/Heidelberg, Germany, 2015; pp. 287–305. [Google Scholar]
- Dworkin, M.; Foster, J.W. Experiments with Some Microorganisms Which Utilize Ethane and Hydrogen. J. Bacteriol. 1958, 75, 592. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ayadi, M.; Cavez, D.; Miled, N.; Chaumont, F.; Masmoudi, K. Identification and Characterization of Two Plasma Membrane Aquaporins in Durum Wheat (Triticum Turgidum L. Subsp. Durum) and Their Role in Abiotic Stress Tolerance. Plant Physiol. Biochem. 2011, 49, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Naghavi, M.R.; Alizadeh, H.; Poostini, K.; Abd Mishani, C. Comparison of Protein Changes in the Leaves of Two Bread Wheat Cultivars with Different Sensitivity under Salt Stress. Annu. Res. Rev. Biol. 2014, 4, 1784–1797. [Google Scholar] [CrossRef]
- Ouerghi, Z.; Rémy, R.; Ouelhazi, L.; Ayadi, A.; Brulfert, J. Two-Dimensional Electrophoresis of Soluble Leaf Proteins, Isolated from Two Wheat Species (Triticum Durum and Triticum Aestivum) Differing in Sensitivity towards NaCl. Electrophor. Int. J. 2000, 21, 2487–2491. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Naidu, B.P.; Cameron, D.F.; Konduri, S.V. Improving Drought Tolerance of Cotton by Glycinebetaine Application and Selection. In Proceedings of the 9th Australian Agronomy Conference, Wagga Wagga, Australia, 20–23 July 1998. [Google Scholar]
- Bhise, K.K.; Dandge, P.B. Mitigation of Salinity Stress in Plants Using Plant Growth Promoting Bacteria. Symbiosis 2019, 79, 191–204. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Lv, J.; Gao, T.; Song, Y. The Expression of Alfalfa MsPP2CA1 Gene Confers ABA Sensitivity and Abiotic Stress Tolerance on Arabidopsis Thaliana. Plant Physiol. Biochem. 2019, 143, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hmaeid, N.; Wali, M.; Mahmoud, O.M.-B.; Pueyo, J.J.; Ghnaya, T.; Abdelly, C. Efficient Rhizobacteria Promote Growth and Alleviate NaCl-Induced Stress in the Plant Species Sulla Carnosa. Appl. Soil Ecol. 2019, 133, 104–113. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant Growth and Development under Salinity Stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–32. [Google Scholar]
- Yildirim, E.; Turan, M.; Ekinci, M.; Dursun, A.; Cakmakci, R. Plant Growth Promoting Rhizobacteria Ameliorate Deleterious Effect of Salt Stress on Lettuce. Sci. Res. Essays 2011, 6, 4389–4396. [Google Scholar]
- Tolba, S.T.; Ibrahim, M.; Amer, E.A.; Ahmed, D.A. First Insights into Salt Tolerance Improvement of Stevia by Plant Growth-Promoting Streptomyces Species. Arch. Microbiol. 2019, 201, 1295–1306. [Google Scholar] [CrossRef]
- Dhillon, J.; Torres, G.; Driver, E.; Figueiredo, B.; Raun, W.R. World Phosphorus Use Efficiency in Cereal Crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bavaresco, J.; Sánchez-Rodríguez, A.R.; Barrón, V.; Torrent, J.; Bayer, C. Diffusion and Uptake of Phosphorus, and Root Development of Corn Seedlings, in Three Contrasting Subtropical Soils under Conventional Tillage or No-Tillage. Biol. Fertil. Soils 2016, 52, 203–210. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, T.; Chi, X.; Wang, M.; Chen, N.; Chen, M.; Pan, L.; Qi, P. Isolation and Characterization of Halotolerant Phosphate Solubilizing Bacteria Naturally Colonizing the Peanut Rhizosphere in Salt-Affected Soil. Geomicrobiol. J. 2020, 37, 110–118. [Google Scholar] [CrossRef]
- Lollato, R.P.; Figueiredo, B.M.; Dhillon, J.S.; Arnall, D.B.; Raun, W.R. Wheat Grain Yield and Grain-Nitrogen Relationships as Affected by N, P, and K Fertilization: A Synthesis of Long-Term Experiments. Field Crop. Res. 2019, 236, 42–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, C.L.; Xiang, J.; Long, Y.; Wang, X.; Zou, J.; Luo, Z.; Ding, G.; Cai, H.; Graham, N.S. QTL Meta-Analysis of Root Traits in Brassica Napus under Contrasting Phosphorus Supply in Two Growth Systems. Sci. Rep. 2016, 6, 33113. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of Inoculation with Plant Growth-Promoting Bacteria (PGPB) on Amelioration of Saline Stress in Maize (Zea Mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate Solubilizing Bacteria with Glucose Dehydrogenase Gene for Phosphorus Uptake and Beneficial Effects on Wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant Growth Promoting Activity of an Auxin and Siderophore Producing Isolate of Streptomyces under Saline Soil Conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- Gupta, N.; Sahoo, D. Evaluation of in Vitro Solubilization Potential of Phosphate Solubilising Streptomyces Isolated from Phyllosphere of Heritiera Fomes (Mangrove). Afr. J. Microbiol. Res. 2010, 4, 136–142. [Google Scholar]
- Kim, K.Y.; Jordan, D.; Krishnan, H.B. Rahnella Aquatilis, a Bacterium Isolated from Soybean Rhizosphere, Can Solubilize Hydroxyapatite. FEMS Microbiol. Lett. 1997, 153, 273–277. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of Potassium-and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses, Volume 1; Springer: Berlin/Heidelberg, Germany, 2014; pp. 73–96. [Google Scholar]
- Raval, V.H.; Saraf, M. Biosynthesis and Purification of Indole-3-Acetic Acid by Halotolerant Rhizobacteria Isolated from Little Runn of Kachchh. Biocatal. Agric. Biotechnol. 2020, 23, 101435. [Google Scholar]
- Arora, N.K.; Tewari, S.; Singh, R. Multifaceted Plant-Associated Microbes and Their Mechanisms Diminish the Concept of Direct and indirect PGPRs. In Plant Microbe Symbiosis: Fundamentals and Advances; Springer: Berlin/Heidelberg, Germany, 2013; pp. 411–449. [Google Scholar]
- Egamberdieva, D.; Kucharova, Z. Selection for Root Colonising Bacteria Stimulating Wheat Growth in Saline Soils. Biol. Fertil. Soils 2009, 45, 563–571. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.; Sharma, P.; Prasad, S.R. Role of Indole Acetic Acid (IAA) Producing Rhizobacteria and Its Effect on Plant Growth of Mustard Crop under Salt Stress Condition. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2439–2445. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- AzcON, R.; Barea, J.M. Synthesis of Auxins, Gibberellins and Cytokinins ByAzotobacter Vinelandii AndAzotobacter Beijerinckii Related to Effects Produced on Tomato Plants. Plant Soil 1975, 43, 609–619. [Google Scholar] [CrossRef]
- Li, H.Q.; Jiang, X.W. Inoculation with Plant Growth-Promoting Bacteria (PGPB) Improves Salt Tolerance of Maize Seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
- Werner, J.E.; Finkelstein, R.R. Arabidopsis Mutants with Reduced Response to NaCl and Osmotic Stress. Physiol. Plant. 1995, 93, 659–666. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial Amelioration of Crop Salinity Stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Boër, B.; Ȫzturk, M.; Clüsener-Godt, M.; Gul, B.; Breckle, S.-W. Sabkha Ecosystems: Volume V: The Americas; Springer: Berlin/Heidelberg, Germany, 2016; Volume 48. [Google Scholar]
- Liu, W.; Li, R.-J.; Han, T.-T.; Cai, W.; Fu, Z.-W.; Lu, Y.-T. Salt Stress Reduces Root Meristem Size by Nitric Oxide-Mediated Modulation of Auxin Accumulation and Signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, R.; Alikhani, H.A.; Towfighi, H.; Khavazi, K.; Pourbabaee, A.A. Isolated Bacteria from Saline–Sodic Soils Alter the Response of Wheat under High Adsorbed Sodium and Salt Stress. Int. J. Environ. Sci. Technol. 2017, 14, 143–150. [Google Scholar] [CrossRef]
- Anwar, S.; Ali, B.; Sajid, I. Screening of Rhizospheric Actinomycetes for Various In-Vitro and in-Vivo Plant Growth Promoting (PGP) Traits and for Agroactive Compounds. Front. Microbiol. 2016, 7, 1334. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Gomaa, E.Z. Effect of Plant Growth Promoting Bacillus Subtilis and Pseudomonas Fluorescens on Growth and Pigment Composition of Radish Plants (Raphanus Sativus) under NaCl Stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Plant Growth Promotion by Microbes Ben Lugtenberg—Academia.Edu. Available online: https://www.academia.edu/15172071/Plant_Growth_Promotion_by_Microbes (accessed on 23 August 2020).
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Plant Growth-Promoting Characteristics of Salt Tolerant Enterobacter Cloacae Strain KBPD and Its Efficacy in Amelioration of Salt Stress in Vigna radiata L. J. Plant Growth Regul. 2017, 36, 215–226. [Google Scholar] [CrossRef]
- Del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Ethylene in Plant Biology, 2nd ed.; Academic Press: New York, NY, USA, 1992; Volume 414. [Google Scholar]
- Glick, B.R.; Penrose, D.M.; Li, J. A Model for the Lowering of Plant Ethylene Concentrations by Plant Growth-Promoting Bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chauhan, P.S. ACC Deaminase-Producing Rhizosphere Competent Bacillus Spp. Mitigate Salt Stress and Promote Zea Mays Growth by Modulating Ethylene Metabolism. 3 Biotech 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Arshad, M.; Frankenberger, W.T. Plant Growth Promoting Rhizobacteria: Applications and Perspectives in Agriculture. Adv. Agron. 2004, 81, 98–169. [Google Scholar]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC Deaminase Producing PGPR from Rice Rhizosphere and Evaluating Their Plant Growth Promoting Activity under Salt Stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Mohamed, I.; Eid, K.E.; Abbas, M.H.; Salem, A.A.; Ahmed, N.; Ali, M.; Shah, G.M.; Fang, C. Use of Plant Growth Promoting Rhizobacteria (PGPR) and Mycorrhizae to Improve the Growth and Nutrient Utilization of Common Bean in a Soil Infected with White Rot Fungi. Ecotoxicol. Environ. Saf. 2019, 171, 539–548. [Google Scholar] [CrossRef]
- Nagargade, M.; Tyagi, V.; Singh, M.K. Plant Growth-Promoting Rhizobacteria: A Biological Approach toward the Production of Sustainable Agriculture. In Role of Rhizospheric Microbes in Soil; Springer: Berlin/Heidelberg, Germany, 2018; pp. 205–223. [Google Scholar]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M. Effects of Inoculation with Plant Growth Promoting Rhizobacteria on Photosynthesis, Antioxidant Status and Yield of Runner Bean. Rom. Biotechnol. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Orhan, F. Alleviation of Salt Stress by Halotolerant and Halophilic Plant Growth-Promoting Bacteria in Wheat (Triticum Aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved Salinity Tolerance of Arachishypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Munshi, A.D.; Kumar, R.; Pandey, R.N.; Arora, A.; Bhat, J.S.; Sureja, A.K. Effect of Salt Stress on Cucumber: Na+–K+ Ratio, Osmolyte Concentration, Phenols and Chlorophyll Content. Acta Physiol. Plant. 2010, 32, 103–114. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of Foliar Salicylic Acid Applications on Growth, Chlorophyll, and Mineral Content of Cucumber Grown under Salt Stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Allam, N.G.; Kinany, R.; El-Refai, E.; Ali, W.Y. Potential Use of Beneficial Salt Tolerant Bacteria for Improving Wheat Productivity Grown in Salinized Soil. J. Microbiol. Res. 2018, 8, 43–53. [Google Scholar]
- Upadhyay, S.K.; Singh, D.P. Effect of Salt-Tolerant Plant Growth-Promoting Rhizobacteria on Wheat Plants and Soil Health in a Saline Environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Sadeghi, A.; Safaie, N. Streptomyces Alleviate Drought Stress in Tomato Plants and Modulate the Expression of Transcription Factors ERF1 and WRKY70 Genes. Sci. Hortic. 2020, 265, 109206. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt Stress Encourages Proline Accumulation by Regulating Proline Biosynthesis and Degradation in Jerusalem Artichoke Plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef] [PubMed]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of Four Olive Cultivars during Salt Stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Xiong, Y.-W.; Gong, Y.; Li, X.-W.; Chen, P.; Ju, X.-Y.; Zhang, C.-M.; Yuan, B.; Lv, Z.-P.; Xing, K.; Qin, S. Enhancement of Growth and Salt Tolerance of Tomato Seedlings by a Natural Halotolerant Actinobacterium Glutamicibacter Halophytocola KLBMP 5180 Isolated from a Coastal Halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]

| Strain | 0 M | 0.25 M | 0.5 M | 0.75 M | 1 M | 1.25 M | 1.5 M |
|---|---|---|---|---|---|---|---|
| D14 | 24.8 aA | 12.1 cB | 9.1 cCD | 9.1 eEF | 12.7 cB | 17.3 bA | 10.4 dCD |
| G10 | 17.2 aF | 10.4 cCD | 12.8 bB | 10.1 cCDE | 10.7 cD | 7.1 dG | 10.5 cCD |
| G22 | 23.3 aB | 14.5 bA | 14.4 bA | 11.3 cBC | 10.6 cdDE | 12.1 cB | 9.1 dEF |
| G33 | 10.8 cL | 9.0 dEF | 12.4 dEF | 9.7 cdDE | 9.1 dF | 9.9 cdDE | 14.4 aA |
| H12 | 18.8 aE | 9.9 dDE | 13.3 dDE | 13.2 bA | 11.3 cCD | 9.9 dDE | 9.2 dEF |
| H14 | 22.2 aC | 10.7 cCD | 13.0 cCD | 8.5 dF | 6.6 eG | 6.3 eG | 11.4 cC |
| J4 | 12.1 bcK | 11.4 cBC | 7.9 cBC | 12.9 bA | 16.5 aA | 8.4 eF | 10.1 dDE |
| J13 | 9.9 deM | 15.0 aA | 11.9 aA | 9.5 deEF | 9.3 eEF | 10.8 cdBCD | 12.8 bB |
| J21 | 19.3 aD | 4.6 fG | 8.5 fG | 6.0 eG | 9.1 cF | 11.5 bBC | 8.0 dFG |
| J27 | 12.6 aJ | 4.8 dG | 10.0 dG | 10.7 bCD | 11.1 bCD | 11.0 bBCD | 7.3 cGH |
| K12 | 10.8 cL | 13.8 aA | 12.3 bB | 10.0 cDE | 12.3 bBC | 11.2 bcBC | 10.0 cDE |
| K23 | 14.8 aG | 8.4 eF | 9.9 cDC | 12.1 bAB | 10.5 cDE | 10.0 cdDE | 9.4 deDE |
| S2 | 12.9 aI | 8.0 dF | 6.8 eE | 12.4 aAB | 7.3 deG | 9.3 cEF | 10.5 bCD |
| T45 | 13.9 aH | 11.5 bcBC | 12.2 bB | 11.3 bcBC | 13.3 aB | 10.7 cCD | 6.5 dH |
| Strain | 0 M | 0.25 M | 0.5 M | 0.75 M | 1 M | 1.25 M | 1.5 M |
|---|---|---|---|---|---|---|---|
| D14 | 10.7 aD | 4.8 bH | - | - | - | - | - |
| G10 | 12.2 bcC | 11.3 cCD | 13.8 bC | 25.9 aA | 6.1 dE | 6.4 dD | 6.8 d |
| G22 | 12.4 dC | 19.8 bA | 35.6 aA | 18.6 bB | 15.3 cB | 10.7 dB | - |
| G33 | 7.2 F | - | - | - | - | - | - |
| H12 | - | 4.8 cH | 5.1 cF | 8.2 bE | 10.2 aD | - | - |
| H14 | 21.4 aA | 6.9 dFG | 9.7 cE | 12.8 bD | - | - | - |
| J4 | - | 9.8 bcDE | 8.7 cE | 10.2 bE | 12.8 aC | - | - |
| J13 | 9.5 deE | 8.3 cEF | 11.6 dD | 13.9 cCD | 17.6 bA | 25.4 aA | - |
| J21 | - | 6.2 GH | - | - | - | - | - |
| J27 | 9.8 cE | 10.2 cCD | 6.4 dF | 15.2 bC | 18.1 aA | - | - |
| K12 | 7.6 cF | 11.7 bC | 15.2 aBC | 8.2 cE | 14.7 aB | - | - |
| K23 | - | - | - | - | - | - | - |
| S2 | - | - | - | - | - | - | - |
| T45 | 14.8 abB | 13.9 abB | 15.8 aB | 6.0 cF | 12.9 bC | 8.2 cC | - |
| NaCl (M L−1) | Survival Rate (%) | |
|---|---|---|
| PGPB | CNT | |
| 0 | 100 | 100 |
| 0.25 | 100 | 100 |
| 0.5 | 100 | - |
| 0.75 | 100 | - |
| 1 | 21 | - |
| 1.25 | - | - |
| 1.5 | - | - |
| 0 M | 0.25 M | 0.5 M | 0.75 M | 1 M | |
|---|---|---|---|---|---|
| D14 | 1.9 a | 1.9 a | 1.9 a | 2.1 a | - |
| G10 | 1.9 bc | 2.0 ab | 2.2 a | 1.6 c | 0.3 d |
| G22 | 1.9 b | 2.4 a | 2.2 a | 0.5 c | - |
| G33 | 2.3 a | 1.9 b | 2.2 ab | 2.1 ab | - |
| H12 | 1.8 b | 2.0 b | 1.8 b | 7.7 a | - |
| H14 | 1.9 a | 2.1 a | 2.1 a | 2.2 a | - |
| J4 | 2.0 c | 2.2 c | 3.5 b | 4.1 a | - |
| J13 | 2.3 a | 1.0 b | 2.2 a | 2.0 a | - |
| J21 | 2.1 a | 2.1 a | 1.8 a | 1.5 a | - |
| J27 | 5.5 a | 1.8 b | 1.6 b | 2.3 b | 1.5 b |
| K12 | 2.2 a | 1.9 ab | 0.2 c | 2.1 a | 1.7 b |
| K23 | 2.0 b | 1.9 b | 2.2 b | 3.2 a | - |
| S2 | 2.0 a | 2.0 a | 2.2 a | 1.8 a | - |
| T45 | 1.3 b | 2.3 a | 2.3 a | 2.1 a | - |
| CNT | 2.5 b | 2.8 a | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djebaili, R.; Pellegrini, M.; Rossi, M.; Forni, C.; Smati, M.; Del Gallo, M.; Kitouni, M. Characterization of Plant Growth-Promoting Traits and Inoculation Effects on Triticum durum of Actinomycetes Isolates under Salt Stress Conditions. Soil Syst. 2021, 5, 26. https://doi.org/10.3390/soilsystems5020026
Djebaili R, Pellegrini M, Rossi M, Forni C, Smati M, Del Gallo M, Kitouni M. Characterization of Plant Growth-Promoting Traits and Inoculation Effects on Triticum durum of Actinomycetes Isolates under Salt Stress Conditions. Soil Systems. 2021; 5(2):26. https://doi.org/10.3390/soilsystems5020026
Chicago/Turabian StyleDjebaili, Rihab, Marika Pellegrini, Massimiliano Rossi, Cinzia Forni, Maria Smati, Maddalena Del Gallo, and Mahmoud Kitouni. 2021. "Characterization of Plant Growth-Promoting Traits and Inoculation Effects on Triticum durum of Actinomycetes Isolates under Salt Stress Conditions" Soil Systems 5, no. 2: 26. https://doi.org/10.3390/soilsystems5020026
APA StyleDjebaili, R., Pellegrini, M., Rossi, M., Forni, C., Smati, M., Del Gallo, M., & Kitouni, M. (2021). Characterization of Plant Growth-Promoting Traits and Inoculation Effects on Triticum durum of Actinomycetes Isolates under Salt Stress Conditions. Soil Systems, 5(2), 26. https://doi.org/10.3390/soilsystems5020026










