Accessing Legacy Phosphorus in Soils
Abstract
1. Introduction
2. Occurrence and Bioaccessibility of Different Chemical Forms of Soil Legacy P
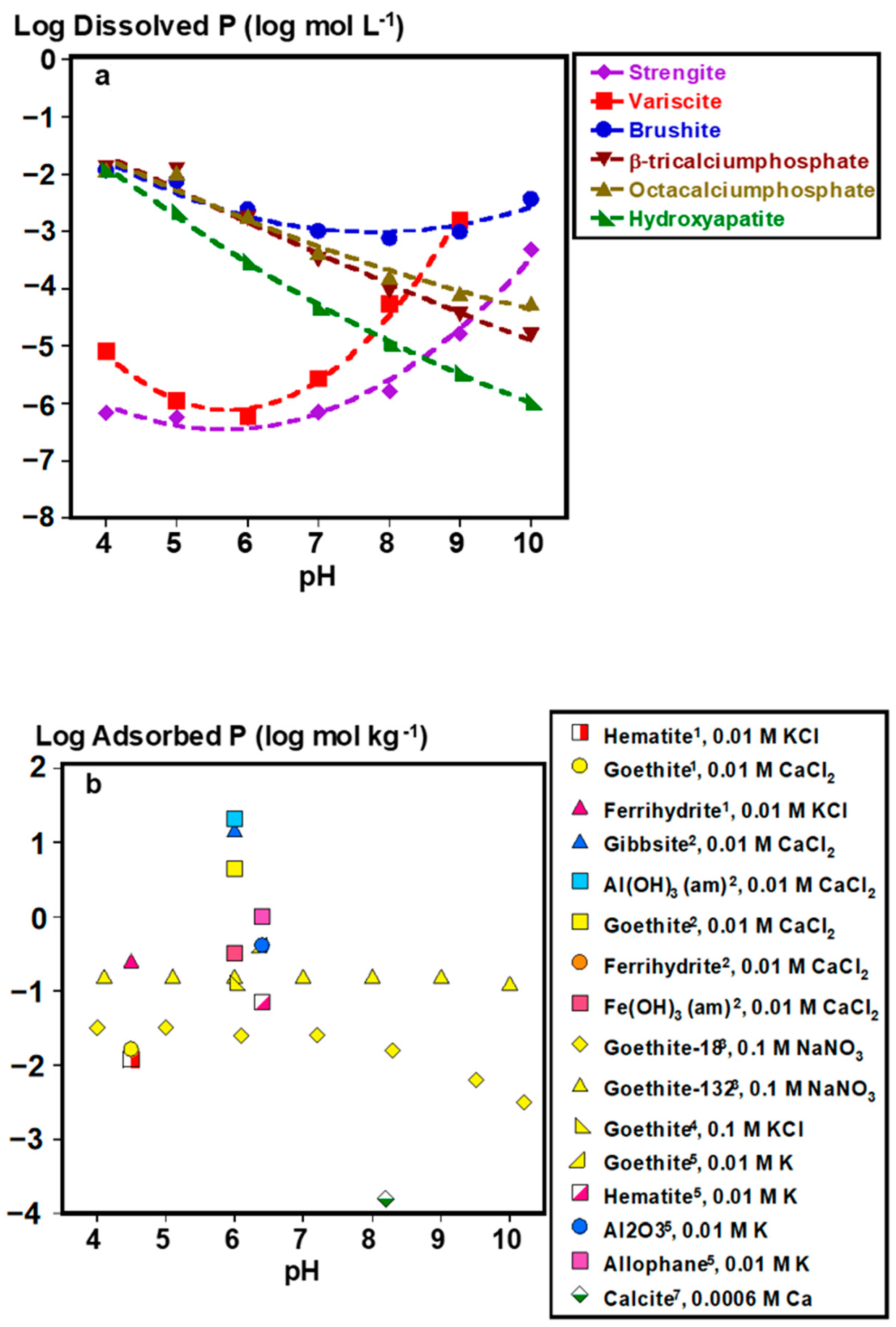
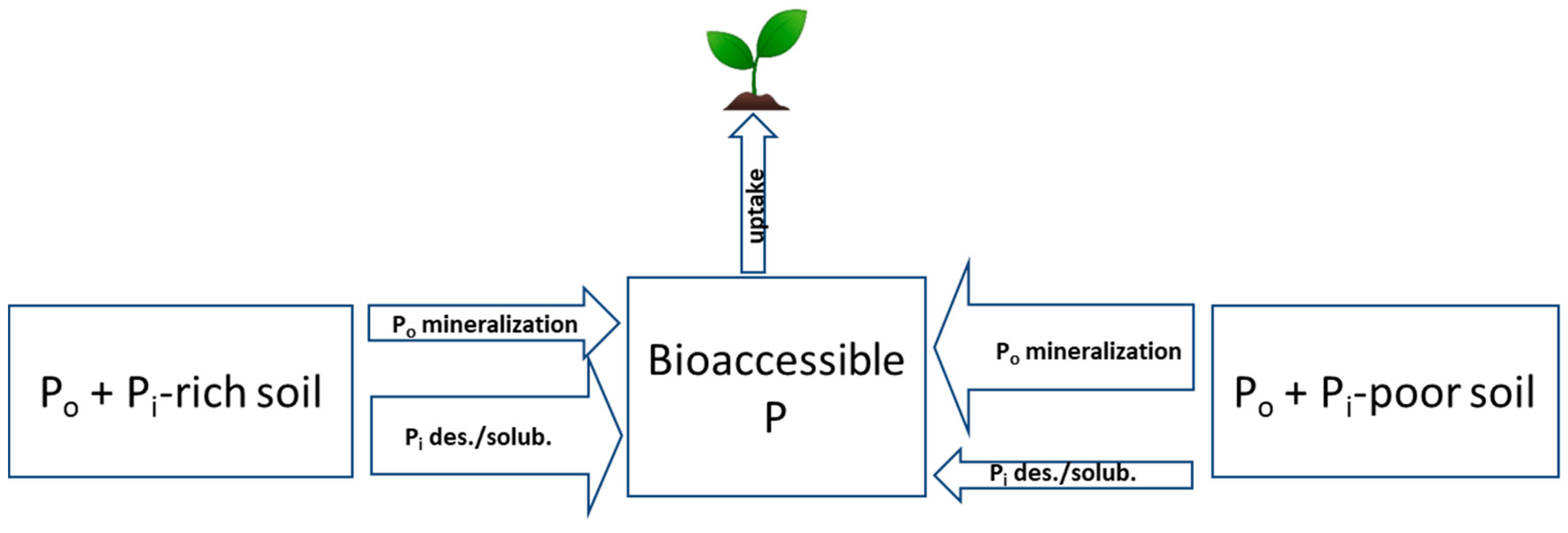
2.1. Inorganic P Forms and Bioaccessibility
2.2. Organic P Forms and Bioaccessibility
3. Legacy P Transformations with Mineral and Organic Fertilizer Applications
3.1. Soil Inorganic Legacy P Transformations
3.2. Soil Organic P Transformations
4. Approaches for Accessing Native Soil P and Associated Challenges
4.1. Plant-Based Strategies
4.2. Phosphate-Solubilizing Microorganisms
4.3. Immobilized Organic P Hydrolyzing Enzymes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fraústo da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Stewart, W.M.; Hammond, L.L.; Van Kauwenbergh, S.J. Phosphorus as a Natural Resource. In Phosphorus: Agriculture and the Environment; Pierzynski, G.M., McDowell, R.W., Sims, J.T., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; pp. 1–22. [Google Scholar]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for a Food Secure Future. Ann. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Pierzynski, G.M.; McDowell, R.W.; Sims, J.T. Chemistry, Cycling, and Potential Movement of Inorganic Phosphorus in Soils. In Phosphorus: Agriculture and the Environment; Sims, J.T., Sharpley, A.N., Westermann, T.D., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; pp. 51–86. [Google Scholar]
- Fox, R.L.; Kamprath, E.J. Phosphate Sorption Isotherms for Evaluating the Phosphate Requirements of Soils. Soil Sci. Soc. Am. J. 1970, 34, 902–907. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil Fertilizer Phosphorus Use—Reconciling Changing Concepts of Soil Phosphorus Behavior with Agronomic Information; Food And Agriculture Organization of The United Nations (FAO): Rome, Italy, 2008. [Google Scholar]
- Dhillon, J.; Torres, G.; Driver, E.; Figueiredo, B.; Raun, W.R. World Phosphorus Use Efficiency in Cereal Crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. BioScience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Asal, S.; Toor, G.S. Residential catchments to coastal waters: Forms, fluxes, and mechanisms of phosphorus transport. Sci. Total Environ. 2020, 142767. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Sharpley, A.N.; Withers, P.J.A.; Scott, J.T.; Haggard, B.E.; Neal, C. Phosphorus Mitigation to Control River Eutrophication: Murky Waters, Inconvenient Truths, and “Postnormal” Science. J. Environ. Qual. 2013, 42, 295–304. [Google Scholar] [CrossRef]
- USEPA. National Lakes Assessment 2012 Results. Available online: https://www.epa.gov/national-aquatic-resource-surveys/national-lakes-assessment-2012-results (accessed on 15 June 2020).
- CDC. Harmful Algal Bloom (HAB)-Associated Illness. Available online: https://www.cdc.gov/habs/general.html#:~:text=Fresh%20water%20%E2%80%94In%202014%2C%20a,or%20other%20fresh%20water%20bodies (accessed on 15 June 2020).
- Schlesinger, W.H.; Bernhardt, E.S. Chapter 12—The Global Cycles of Nitrogen and Phosphorus. In Biogeochemistry, 3rd ed.; Schlesinger, W.H., Bernhardt, E.S., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 445–467. [Google Scholar]
- Kamprath, E.J. Changes in phosphate availability of ultisols with long-term cropping. Commun. Soil Sci. Plant Anal. 1999, 30, 909–919. [Google Scholar] [CrossRef]
- Roberts, T.; Johnston, A. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R. Phosphorus in Agriculture: A Review of Results from 175 Years of Research at Rothamsted, UK. J. Environ. Qual. 2019, 48, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Kizewski, F.; Liu, Y.-T.; Morris, A.; Hesterberg, D. Spectroscopic Approaches for Phosphorus Speciation in Soils and Other Environmental Systems. J. Environ. Qual. 2011, 40, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Hou, E.; Tan, X.; Heenan, M.; Wen, D. A global dataset of plant available and unavailable phosphorus in natural soils derived by Hedley method. Sci. Data 2018, 5, 180166. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Pierzynski, G.M. Chemistry of Phosphorus in Soils. In Chemical Processes in Soils; Tabatabai, M.A., Sparks, D.L., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; pp. 151–192. [Google Scholar]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Yang, J.; Abdi, D.; Cade-Menun, B.J. Investigation of Soil Legacy Phosphorus Transformation in Long-Term Agricultural Fields Using Sequential Fractionation, P K-edge XANES and Solution P NMR Spectroscopy. Environ. Sci. Technol. 2015, 49, 168–176. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; He, Z.; Zhang, H.; Endale, D.M.; Schomberg, H.H.; Liu, C.W. Stratification of Phosphorus Forms from Long-Term Conservation Tillage and Poultry Litter Application. Soil Sci. Soc. Am. J. 2015, 79, 504–516. [Google Scholar] [CrossRef]
- Vincent, A.G.; Vestergren, J.; Gröbner, G.; Persson, P.; Schleucher, J.; Giesler, R. Soil organic phosphorus transformations in a boreal forest chronosequence. Plant Soil 2013, 367, 149–162. [Google Scholar] [CrossRef]
- Turner, B.L.; Cheesman, A.W.; Godage, H.Y.; Riley, A.M.; Potter, B.V.L. Determination of neo- and d-chiro-Inositol Hexakisphosphate in Soils by Solution 31P NMR Spectroscopy. Environ. Sci. Technol. 2012, 46, 4994–5002. [Google Scholar] [CrossRef]
- Ahlgren, J.; Djodjic, F.; Börjesson, G.; Mattsson, L. Identification and quantification of organic phosphorus forms in soils from fertility experiments. Soil Use Manag. 2013, 29, 24–35. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Carter, M.R.; James, D.C.; Liu, C.W. Phosphorus Forms and Chemistry in the Soil Profile under Long-Term Conservation Tillage: A Phosphorus-31 Nuclear Magnetic Resonance Study. J. Environ. Qual. 2010, 39, 1647–1656. [Google Scholar] [CrossRef]
- Dou, Z.; Ramberg, C.F.; Toth, J.D.; Wang, Y.; Sharpley, A.N.; Boyd, S.E.; Chen, C.R.; Williams, D.; Xu, Z.H. Phosphorus Speciation and Sorption-Desorption Characteristics in Heavily Manured Soils. Soil Sci. Soc. Am. J. 2009, 73, 93–101. [Google Scholar] [CrossRef]
- Koch, M.; Kruse, J.; Eichler-Löbermann, B.; Zimmer, D.; Willbold, S.; Leinweber, P.; Siebers, N. Phosphorus stocks and speciation in soil profiles of a long-term fertilizer experiment: Evidence from sequential fractionation, P K-edge XANES, and 31P NMR spectroscopy. Geoderma 2018, 316, 115–126. [Google Scholar] [CrossRef]
- Weyers, E.; Strawn, D.G.; Peak, D.; Moore, A.D.; Baker, L.L.; Cade-Menun, B. Phosphorus Speciation in Calcareous Soils Following Annual Dairy Manure Amendments. Soil Sci. Soc. Am. J. 2016, 80, 1531–1542. [Google Scholar] [CrossRef]
- Oh, Y.-M.; Hesterberg, D.L.; Nelson, P.V.; Niedziela, C.E. Desorption Characteristics of Three Mineral Oxides and a Non-crystalline Aluminosilicate for Supplying Phosphate in Soilless Root Media. Commun. Soil Sci. Plant Anal. 2016, 47, 753–760. [Google Scholar] [CrossRef]
- Parfitt, R.L. The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by ryegrass. Plant Soil 1979, 53, 55–65. [Google Scholar] [CrossRef]
- Gypser, S.; Hirsch, F.; Schleicher, A.M.; Freese, D. Impact of crystalline and amorphous iron- and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. In Clays and Clay Minerals; Ingerson, E., Ed.; Pergamon: Oxford, UK, 2013; pp. 317–327. [Google Scholar]
- Nair, V.D. Soil phosphorus saturation ratio for risk assessment in land use systems. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef]
- Beauchemin, S.; Simard, R.R. Soil phosphorus saturation degree: Review of some indices and their suitability for P management in Québec, Canada. Can. J. Soil Sci. 1999, 79, 615–625. [Google Scholar] [CrossRef]
- Wang, X.M.; Liu, F.; Tan, W.F.; Li, W.; Feng, X.H.; Sparks, D.L. Characteristics of Phosphate Adsorption-Desorption Onto Ferrihydrite: Comparison With Well-Crystalline Fe (Hydr)Oxides. Soil Sci. 2013, 178, 1–11. [Google Scholar] [CrossRef]
- Strauss, R.; Brümmer, G.W.; Barrow, N.J. Effects of crystallinity of goethite: II. Rates of sorption and desorption of phosphate. Eur. J. Soil Sci. 1997, 48, 101–114. [Google Scholar] [CrossRef]
- Yan, Y.; Koopal, L.K.; Liu, F.; Huang, Q.; Feng, X. Desorption of myo-inositol hexakisphosphate and phosphate from goethite by different reagents. J. Plant Nutr. Soil Sci. 2015, 178, 878–887. [Google Scholar] [CrossRef]
- Krumina, L.; Kenney, J.P.L.; Loring, J.S.; Persson, P. Desorption mechanisms of phosphate from ferrihydrite and goethite surfaces. Chem. Geol. 2016, 427, 54–64. [Google Scholar] [CrossRef]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Geochim. Cosmochim. Acta 2011, 75, 2911–2923. [Google Scholar]
- Hesterberg, D. Macroscale Chemical Properties and X-Ray Absorption Spectroscopy of Soil Phosphorus. In Developments in Soil Science; Singh, B., Gräfe, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 313–356. [Google Scholar]
- Gu, C.; Dam, T.; Hart, S.C.; Turner, B.L.; Chadwick, O.A.; Berhe, A.A.; Hu, Y.; Zhu, M. Quantifying Uncertainties in Sequential Chemical Extraction of Soil Phosphorus Using XANES Spectroscopy. Environ. Sci. Technol. 2020, 54, 2257–2267. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- McIntosh, J.L. Bray and Morgan Soil Extractants Modified for Testing Acid Soils from Different Parent Materials1. Agron. J. 1969, 61, 259–265. [Google Scholar] [CrossRef]
- Hochmuth, G.J.; Mylavarapu, R.S.; Hanlon, E.A. Developing a Soil Test Extractant: The Correlation and Calibration Processes; University of Florida: Gainesville, FL, USA, 2017. [Google Scholar]
- Khatiwada, R.; Hettiarachchi, G.M.; Mengel, D.B.; Fei, M. Speciation of Phosphorus in a Fertilized, Reduced-Till Soil System: In-Field Treatment Incubation Study. Soil Sci. Soc. Am. J. 2012, 76, 2006–2018. [Google Scholar] [CrossRef]
- Sims, J.T.; Edwards, A.C.; Schoumans, O.F.; Simard, R.R. Integrating Soil Phosphorus Testing into Environmentally Based Agricultural Management Practices. J. Environ. Qual. 2000, 29, 60–71. [Google Scholar] [CrossRef]
- Mallarino, A.P.; Atia, A.M. Correlation of a Resin Membrane Soil Phosphorus Test with Corn Yield and Routine Soil Tests. Soil Sci. Soc. Am. J. 2005, 69, 266–272. [Google Scholar] [CrossRef]
- Sims, J.T. Soil Test Phosphorus: Principles and Methods In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, 2nd ed.; Kovar, J., Pierzynski, G., Eds.; Southern Cooperative Series Bulletin 408; Southern Extension and Research Activity (SERA): Blacksburg, VA, USA, 2009; Volume 408. [Google Scholar]
- Turner, B.L.; Engelbrecht, B.M.J. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 2011, 103, 297–315. [Google Scholar] [CrossRef]
- Murphy, P.N.C.; Bell, A.; Turner, B.L. Phosphorus speciation in temperate basaltic grassland soils by solution 31P NMR spectroscopy. Eur. J. Soil Sci. 2009, 60, 638–651. [Google Scholar] [CrossRef]
- Hou, E.; Wen, D.; Kuang, Y.; Cong, J.; Chen, C.; He, X.; Heenan, M.; Lu, H.; Zhang, Y. Soil pH predominantly controls the forms of organic phosphorus in topsoils under natural broadleaved forests along a 2500km latitudinal gradient. Geoderma 2018, 315, 65–74. [Google Scholar] [CrossRef]
- Turner, B.L.; Blackwell, M.S.A. Isolating the influence of pH on the amounts and forms of soil organic phosphorus. Eur. J. Soil Sci. 2013, 64, 249–259. [Google Scholar] [CrossRef]
- Li, R.; Zhao, J.; Sun, C.; Lu, W.; Guo, C.; Xiao, K. Biochemical properties, molecular characterizations, functions, and application perspectives of phytases. Front. Agric. China 2010, 4, 195–209. [Google Scholar] [CrossRef]
- Vohra, A.; Satyanarayana, T. Phytases: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Crit. Rev. Biotechnol. 2003, 23, 29–60. [Google Scholar] [CrossRef]
- McKelvie, I.D.; Hart, B.T.; Cardwell, T.J.; Cattrall, R.W. Use of immobilized 3-phytase and flow injection for the determination of phosphorus species in natural waters. Anal. Chim. Acta 1995, 316, 277–289. [Google Scholar] [CrossRef]
- Ullah, A.H.J.; Gibson, D.M. Extracellular Phytase (E. C. 3.1.3.8) from Aspergillus Ficuum NRRL 3135: Purification and Characterization. Prep. Biochem. 1987, 17, 63–91. [Google Scholar]
- Konietzny, U.; Greiner, R. Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int. J. Food Sci. Technol. 2002, 37, 791–812. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphatase activity in temperate pasture soils: Potential regulation of labile organic phosphorus turnover by phosphodiesterase activity. Sci. Total Environ. 2005, 344, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wan, B.; Liu, F.; Tan, W.; Liu, M.; Feng, X. Adsorption-Desorption of Myo-Inositol Hexakisphosphate on Hematite. Soil Sci. 2014, 179, 476–485. [Google Scholar] [CrossRef]
- Saeki, K.; Kunito, T.; Sakai, M. Effects of pH, ionic strength, and solutes on DNA adsorption by andosols. Biol. Fertil. Soils 2010, 46, 531–535. [Google Scholar] [CrossRef]
- Deiss, L.; de Moraes, A.; Maire, V. Environmental drivers of soil phosphorus composition in natural ecosystems. Biogeosciences 2018, 15, 4575–4592. [Google Scholar] [CrossRef]
- Siebers, N.; Sumann, M.; Kaiser, K.; Amelung, W. Climatic Effects on Phosphorus Fractions of Native and Cultivated North American Grassland Soils. Soil Sci. Soc. Am. J. 2017, 81, 299–309. [Google Scholar] [CrossRef]
- Dalai, R.C. Soil Organic Phosphorus. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1977; Volume 29, pp. 83–117. [Google Scholar]
- Moata, M.R.S.; Doolette, A.L.; Smernik, R.J.; McNeill, A.M.; Macdonald, L.M. Organic phosphorus speciation in Australian Red Chromosols: Stoichiometric control. Soil Res. 2016, 54, 11–19. [Google Scholar] [CrossRef]
- Spohn, M. Phosphorus and carbon in soil particle size fractions: A synthesis. Biogeochemistry 2020, 147, 225–242. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Allison, V.J.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Turner, B.L. Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol. Biochem. 2007, 39, 1770–1781. [Google Scholar] [CrossRef]
- Yadav, R.S.; Tarafdar, J.C. Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol. Biochem. 2003, 35, 745–751. [Google Scholar] [CrossRef]
- George, T.S.; Simpson, R.J.; Gregory, P.J.; Richardson, A.E. Differential interaction of Aspergillus niger and Peniophora lycii phytases with soil particles affects the hydrolysis of inositol phosphates. Soil Biol. Biochem. 2007, 39, 793–803. [Google Scholar] [CrossRef]
- Renella, G.; Szukics, U.; Landi, L.; Nannipieri, P. Quantitative assessment of hydrolase production and persistence in soil. Biol. Fertil. Soils 2007, 44, 321–329. [Google Scholar] [CrossRef]
- Skujiņš, J.; Burns, R.G. Extracellular Enzymes in Soil. CRC Crit. Rev. Microbiol. 1976, 4, 383–421. [Google Scholar]
- Gerke, J. Phytate (Inositol Hexakisphosphate) in Soil and Phosphate Acquisition from Inositol Phosphates by Higher Plants. A Review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef]
- Doolette, A.L.; Smernik, R.J.; Dougherty, W.J. Rapid decomposition of phytate applied to a calcareous soil demonstrated by a solution 31P NMR study. Eur. J. Soil Sci. 2010, 61, 563–575. [Google Scholar] [CrossRef]
- Leytem, A.B.; Smith, D.R.; Applegate, T.J.; Thacker, P.A. The Influence of Manure Phytic Acid on Phosphorus Solubility in Calcareous Soils. Soil Sci. Soc. Am. J. 2006, 70, 1629–1638. [Google Scholar] [CrossRef]
- Annaheim, K.E.; Rufener, C.B.; Frossard, E.; Bünemann, E.K. Hydrolysis of organic phosphorus in soil water suspensions after addition of phosphatase enzymes. Biol. Fertil. Soils 2013, 49, 1203–1213. [Google Scholar] [CrossRef][Green Version]
- Turner, B.L.; McKelvie, I.D.; Haygarth, P.M. Characterisation of water-extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol. Biochem. 2002, 34, 27–35. [Google Scholar] [CrossRef]
- Toor, G.S.; Condron, L.M.; Cade-Menun, B.J.; Di, H.J.; Cameron, K.C. Preferential phosphorus leaching from an irrigated grassland soil. Eur. J. Soil Sci. 2005, 56, 155–168. [Google Scholar] [CrossRef]
- Toor, G.S.; Condron, L.M.; Di, H.J.; Cameron, K.C.; Cade-Menun, B.J. Characterization of organic phosphorus in leachate from a grassland soil. Soil Biol. Biochem. 2003, 35, 1317–1323. [Google Scholar] [CrossRef]
- Darch, T.; Blackwell, M.S.A.; Hawkins, J.M.B.; Haygarth, P.M.; Chadwick, D. A Meta-Analysis of Organic and Inorganic Phosphorus in Organic Fertilizers, Soils, and Water: Implications for Water Quality. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2172–2202. [Google Scholar] [CrossRef]
- Jarosch, K.A.; Doolette, A.L.; Smernik, R.J.; Tamburini, F.; Frossard, E.; Bünemann, E.K. Characterisation of soil organic phosphorus in NaOH-EDTA extracts: A comparison of 31P NMR spectroscopy and enzyme addition assays. Soil Biol. Biochem. 2015, 91, 298–309. [Google Scholar] [CrossRef]
- McLaren, T.I.; Smernik, R.J.; McLaughlin, M.J.; McBeath, T.M.; Kirby, J.K.; Simpson, R.J.; Guppy, C.N.; Doolette, A.L.; Richardson, A.E. Complex Forms of Soil Organic Phosphorus–A Major Component of Soil Phosphorus. Environ. Sci. Technol. 2015, 49, 13238–13245. [Google Scholar] [CrossRef] [PubMed]
- Pierzynski, J.; Hettiarachchi, G.M. Reactions of Phosphorus Fertilizers with and without a Fertilizer Enhancer in Three Acidic Soils with High Phosphorus-Fixing Capacity. Soil Sci. Soc. Am. J. 2018, 82, 1124–1139. [Google Scholar] [CrossRef]
- Luo, L.; Ma, Y.; Sanders, R.L.; Xu, C.; Li, J.; Myneni, S.C.B. Phosphorus speciation and transformation in long-term fertilized soil: Evidence from chemical fractionation and P K-edge XANES spectroscopy. Nutr. Cycl. Agroecosystems 2017, 107, 215–226. [Google Scholar] [CrossRef]
- Kar, G.; Peak, D.; Schoenau, J.J. Spatial Distribution and Chemical Speciation of Soil Phosphorus in a Band Application. Soil Sci. Soc. Am. J. 2012, 76, 2297–2306. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Cade-Menun, B.J.; Hu, Y.; Li, J.; Peng, C.; Ma, Y. Molecular speciation and transformation of soil legacy phosphorus with and without long-term phosphorus fertilization: Insights from bulk and microprobe spectroscopy. Sci. Rep. 2017, 7, 15354. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, B.; Akinremi, O.O.; Hu, Y.; Jürgensen, A. XANES Speciation of Phosphorus in Organically Amended and Fertilized Vertisol and Mollisol. Soil Sci. Soc. Am. J. 2008, 72, 1256–1262. [Google Scholar] [CrossRef]
- Gamble, A.V.; Northrup, P.A.; Sparks, D.L. Elucidation of soil phosphorus speciation in mid-Atlantic soils using synchrotron-based microspectroscopic techniques. J. Environ. Qual. 2020, 49, 184–193. [Google Scholar] [CrossRef]
- Abdala, D.B.; Moore, P.A.; Rodrigues, M.; Herrera, W.F.; Pavinato, P.S. Long-term effects of alum-treated litter, untreated litter and NH4NO3 application on phosphorus speciation, distribution and reactivity in soils using K-edge XANES and chemical fractionation. J. Environ. Manag. 2018, 213, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Cade-Menun, B.J. Characterizing phosphorus forms in cropland soils with solution 31P-NMR: Past studies and future research needs. Chem. Biol. Technol. Agric. 2017, 4, 19. [Google Scholar] [CrossRef]
- Annaheim, K.E.; Doolette, A.L.; Smernik, R.J.; Mayer, J.; Oberson, A.; Frossard, E.; Bünemann, E.K. Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions. Geoderma 2015, 257-258, 67–77. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Doody, D.G.; Liu, C.W.; Watson, C.J. Long-term Changes in Grassland Soil Phosphorus with Fertilizer Application and Withdrawal. J. Environ. Qual. 2017, 46, 537–545. [Google Scholar] [CrossRef]
- Recena, R.; Torrent, J.; del Campillo, M.C.; Delgado, A. Accuracy of Olsen P to assess plant P uptake in relation to soil properties and P forms. Agron. Sustain. Dev. 2015, 35, 1571–1579. [Google Scholar] [CrossRef]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2012, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Finnegan, P.M.; Laliberté, E.; Pearse, S.J.; Ryan, M.H.; Shane, M.W.; Veneklaas, E.J. Phosphorus Nutrition of Proteaceae in Severely Phosphorus-Impoverished Soils: Are There Lessons To Be Learned for Future Crops? Plant Physiol. 2011, 156, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.C.; Ribrioux, S.P.C.P.; Fitter, A.H.; Leyser, H.M.O. Phosphate Availability Regulates Root System Architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tang, C.; Armstrong, R.; Sale, P. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 2012, 358, 91–104. [Google Scholar] [CrossRef]
- Rose, T.J.; Impa, S.M.; Rose, M.T.; Pariasca-Tanaka, J.; Mori, A.; Heuer, S.; Johnson-Beebout, S.E.; Wissuwa, M. Enhancing phosphorus and zinc acquisition efficiency in rice: A critical review of root traits and their potential utility in rice breeding. Ann. Bot. 2013, 112, 331–345. [Google Scholar] [CrossRef]
- Lynch, J.; van Beem, J.J. Growth and Architecture of Seedling Roots of Common Bean Genotypes. Crop Sci. 1993, 33, cropsci1993.0011183X003300060028x. [Google Scholar] [CrossRef]
- Wang, Y.L.; Almvik, M.; Clarke, N.; Eich-Greatorex, S.; Øgaard, A.F.; Krogstad, T.; Lambers, H.; Clarke, J.L. Contrasting responses of root morphology and root-exuded organic acids to low phosphorus availability in three important food crops with divergent root traits. AoB Plants 2015, 7. [Google Scholar] [CrossRef]
- Henry, A.; Chaves, N.F.; Kleinman, P.J.A.; Lynch, J.P. Will nutrient-efficient genotypes mine the soil? Effects of genetic differences in root architecture in common bean (Phaseolus vulgaris L.) on soil phosphorus depletion in a low-input agro-ecosystem in Central America. Field Crops Res. 2010, 115, 67–78. [Google Scholar] [CrossRef]
- da Silva, A.; Bruno, I.P.; Franzini, V.I.; Marcante, N.C.; Benitiz, L.; Muraoka, T. Phosphorus uptake efficiency, root morphology and architecture in Brazilian wheat cultivars. J. Radioanal. Nucl. Chem. 2016, 307, 1055–1063. [Google Scholar] [CrossRef]
- Salvi, S. An evo-devo perspective on root genetic variation in cereals. J. Exp. Bot. 2017, 68, 351–354. [Google Scholar] [CrossRef][Green Version]
- Rich, S.M.; Watt, M. Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver. J. Exp. Bot. 2013, 64, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; Moosavi, S.; Cunningham, S.C.; Kirkegaard, J.A.; Rebetzke, G.J.; Richards, R.A. A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 2013, 112, 447–455. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; George, T.S.; Gregory, P.J.; Bengough, A.G.; Hallett, P.D.; McKenzie, B.M. Matching roots to their environment. Ann. Bot. 2013, 112, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Erel, R.; Bérard, A.; Capowiez, L.; Doussan, C.; Arnal, D.; Souche, G.; Gavaland, A.; Fritz, C.; Visser, E.J.W.; Salvi, S.; et al. Soil type determines how root and rhizosphere traits relate to phosphorus acquisition in field-grown maize genotypes. Plant Soil 2017, 412, 115–132. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Wang, X.; Li, H.; Rengel, Z.; Shen, J. Competition between Zea mays genotypes with different root morphological and physiological traits is dependent on phosphorus forms and supply patterns. Plant Soil 2019, 434, 125–137. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Oburger, E.; Leitner, D.; Jones, D.L.; Zygalakis, K.C.; Schnepf, A.; Roose, T. Adsorption and desorption dynamics of citric acid anions in soil. Eur. J. Soil Sci. 2011, 62, 733–742. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Touhami, D.; McDowell, R.W.; Condron, L.M. Role of Organic Anions and Phosphatase Enzymes in Phosphorus Acquisition in the Rhizospheres of Legumes and Grasses Grown in a Low Phosphorus Pasture Soil. Plants 2020, 9, 1185. [Google Scholar] [CrossRef]
- Pearse, S.J.; Veneklaas, E.J.; Cawthray, G.R.; Bolland, M.D.A.; Lambers, H. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 2006, 288, 127–139. [Google Scholar] [CrossRef]
- Wang, Y.; Krogstad, T.; Clarke, J.L.; Hallama, M.; Øgaard, A.F.; Eich-Greatorex, S.; Kandeler, E.; Clarke, N. Rhizosphere Organic Anions Play a Minor Role in Improving Crop Species’ Ability to Take Up Residual Phosphorus (P) in Agricultural Soils Low in P Availability. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Gao, Y.; Wu, X.; Ma, H.; Zheng, C.; Wang, X.; Zhang, H.; Li, Z.; Yang, H. The relative contributions of pH, organic anions, and phosphatase to rhizosphere soil phosphorus mobilization and crop phosphorus uptake in maize/alfalfa polyculture. Plant Soil 2020, 447, 117–133. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Phenes for Enhanced Soil Exploration and Phosphorus Acquisition: Tools for Future Crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Hasan, M.M.; Teixeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell. Mol. Biol. Lett. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.; Zhang, L.; Lu, W.; Li, X.; Xiao, K. TaPht1;4, a high-affinity phosphate transporter gene in wheat (Triticum aestivum), plays an important role in plant phosphate acquisition under phosphorus deprivation. Funct. Plant Biol. 2013, 40, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gu, M.; Cao, Y.; Huang, X.; Zhang, X.; Ai, P.; Zhao, J.; Fan, X.; Xu, G. A Constitutive Expressed Phosphate Transporter, OsPht1;1, Modulates Phosphate Uptake and Translocation in Phosphate-Replete Rice. Plant Physiol. 2012, 159, 1571–1581. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Huang, T.-K.; Tseng, C.-Y.; Lai, Y.-S.; Lin, S.-I.; Lin, W.-Y.; Chen, J.-W.; Chiou, T.-J. PHO2-Dependent Degradation of PHO1 Modulates Phosphate Homeostasis in Arabidopsis. Plant Cell 2012, 24, 2168–2183. [Google Scholar] [CrossRef]
- Ye, Y.; Yuan, J.; Chang, X.; Yang, M.; Zhang, L.; Lu, K.; Lian, X. The Phosphate Transporter Gene OsPht1;4 Is Involved in Phosphate Homeostasis in Rice. PLoS ONE 2015, 10, e0126186. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.-N.; Zhou, H.-M.; Wang, D.-F.; Jiang, T.-T.; Sun, Y.-F.; Cao, Y.; Pei, W.-X.; Sun, S.-B.; Xu, G.-H. Overexpression of rice phosphate transporter gene OsPT6 enhances phosphate uptake and accumulation in transgenic rice plants. Plant Soil 2014, 384, 259–270. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, W.; Shi, J.; Zhang, L.; Xiang, D.; Yang, C.; Li, C.; Wu, Z.; Liu, Y.; Yu, Y.; et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 14953–14958. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Shimizu, A.; Kato, K.; Komatsu, A.; Motomura, K.; Ikehashi, H. Genetic analysis of root elongation induced by phosphorus deficiency in rice (Oryza sativa L.): Fine QTL mapping and multivariate analysis of related traits. Theor. Appl. Genet. 2008, 117, 987–996. [Google Scholar] [CrossRef]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant Soil 2005, 270, 299–310. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, X.; Mei, M.; Yan, X.; Liao, H. QTL analysis of root traits as related to phosphorus efficiency in soybean. Ann. Bot. 2010, 106, 223–234. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar] [CrossRef]
- Li, Q.; Sapkota, M.; van der Knaap, E. Perspectives of CRISPR/Cas-mediated cis-engineering in horticulture: Unlocking the neglected potential for crop improvement. Hortic. Res. 2020, 7, 36. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Regmi, K.; Hirschi, K.D. Moving On Up: H(+)-PPase Mediated Crop Improvement. Trends Biotechnol. 2016, 34, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Rouached, H.; Stefanovic, A.; Secco, D.; Bulak Arpat, A.; Gout, E.; Bligny, R.; Poirier, Y. Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis. Plant J. 2011, 65, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.; Sun, S.; Zhao, J.; Fan, X.; Xin, W.; Guo, Q.; Yu, L.; Shen, Q.; Wu, P.; Miller, A.J.; et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef]
- Kafle, A.; Cope, K.R.; Raths, R.; Krishna Yakha, J.; Subramanian, S.; Bücking, H.; Garcia, K. Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems. Agronomy 2019, 9, 127. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Ann. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Eissenstat, D.M.; Graham, J.H.; Williams, K.; Hodge, N.C. Growth Depression in Mycorrhizal Citrus at High-Phosphorus Supply (Analysis of Carbon Costs). Plant Physiol. 1993, 101, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Krey, T.; Baum, C.; Ruppel, S.; Seydel, M.; Eichler-Löbermann, B. Organic and Inorganic P Sources Interacting with Applied Rhizosphere Bacteria and Their Effects on Growth and P Supply of Maize. Commun. Soil Sci. Plant Anal. 2013, 44, 3205–3215. [Google Scholar] [CrossRef]
- Mercl, F.; Tejnecký, V.; Ságová-Marečková, M.; Dietel, K.; Kopecký, J.; Břendová, K.; Kulhánek, M.; Košnář, Z.; Száková, J.; Tlustoš, P. Co-application of wood ash and Paenibacillus mucilaginosus to soil: The effect on maize nutritional status, root exudation and composition of soil solution. Plant Soil 2018, 428, 105–122. [Google Scholar] [CrossRef]
- Güneş, A.; Ataoğlu, N.; Turan, M.; Eşitken, A.; Ketterings, Q.M. Effects of phosphate-solubilizing microorganisms on strawberry yield and nutrient concentrations. J. Plant Nutr. Soil Sci. 2009, 172, 385–392. [Google Scholar]
- Mosimann, C.; Oberhänsli, T.; Ziegler, D.; Nassal, D.; Kandeler, E.; Boller, T.; Mäder, P.; Thonar, C. Tracing of Two Pseudomonas Strains in the Root and Rhizoplane of Maize, as Related to Their Plant Growth-Promoting Effect in Contrasting Soils. Front. Microbiol. 2017, 7, 2150. [Google Scholar] [CrossRef]
- Varga, T.; Hixson, K.K.; Ahkami, A.H.; Sher, A.W.; Barnes, M.E.; Chu, R.K.; Battu, A.K.; Nicora, C.D.; Winkler, T.E.; Reno, L.R.; et al. Endophyte-Promoted Phosphorus Solubilization in Populus. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Jorquera, M.; Gianfreda, L.; Rao, M.; Greiner, R.; Garrido, E.; de la Luz Mora, M. Activity stabilization of Aspergillus niger and Escherichia coli phytases immobilized on allophanic synthetic compounds and montmorillonite nanoclays. Bioresour. Technol. 2011, 102, 9360–9367. [Google Scholar] [CrossRef] [PubMed]
- Calabi-Floody, M.; Velásquez, G.; Gianfreda, L.; Saggar, S.; Bolan, N.; Rumpel, C.; Mora, M.L. Improving bioavailability of phosphorous from cattle dung by using phosphatase immobilized on natural clay and nanoclay. Chemosphere 2012, 89, 648–655. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Jorquera, M.A.; Gianfreda, L.; Greiner, R.; de la Luz Mora, M. A novel phosphorus biofertilization strategy using cattle manure treated with phytase–nanoclay complexes. Biol. Fertil. Soils 2014, 50, 583–592. [Google Scholar] [CrossRef]
- Jones, J.L.; Yingling, Y.G.; Reaney, I.M.; Westerhoff, P. Materials matter in phosphorus sustainability. MRS Bull. 2020, 45, 7–10. [Google Scholar] [CrossRef]


| Inorganic P Fertilizers | |||
|---|---|---|---|
| Superphosphates | Diammonium phosphate (DAP) (18-46-0) | Monoammonium phosphate (MAP) (11-(51-55)-0) | Other nitrogen–phosphate grades |
| metric tons of material | |||
| 651,162 | 2,236,864 | 2,458,331 | 1,723,509 |
| Organic P Fertilizers | |||
| Compost | Dried manure | Sewage sludge | Other organic materials |
| metric tons of material | |||
| 101,062 | 86,900 | 192,101 | 196,800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doydora, S.; Gatiboni, L.; Grieger, K.; Hesterberg, D.; Jones, J.L.; McLamore, E.S.; Peters, R.; Sozzani, R.; Van den Broeck, L.; Duckworth, O.W. Accessing Legacy Phosphorus in Soils. Soil Syst. 2020, 4, 74. https://doi.org/10.3390/soilsystems4040074
Doydora S, Gatiboni L, Grieger K, Hesterberg D, Jones JL, McLamore ES, Peters R, Sozzani R, Van den Broeck L, Duckworth OW. Accessing Legacy Phosphorus in Soils. Soil Systems. 2020; 4(4):74. https://doi.org/10.3390/soilsystems4040074
Chicago/Turabian StyleDoydora, Sarah, Luciano Gatiboni, Khara Grieger, Dean Hesterberg, Jacob L. Jones, Eric S. McLamore, Rachel Peters, Rosangela Sozzani, Lisa Van den Broeck, and Owen W. Duckworth. 2020. "Accessing Legacy Phosphorus in Soils" Soil Systems 4, no. 4: 74. https://doi.org/10.3390/soilsystems4040074
APA StyleDoydora, S., Gatiboni, L., Grieger, K., Hesterberg, D., Jones, J. L., McLamore, E. S., Peters, R., Sozzani, R., Van den Broeck, L., & Duckworth, O. W. (2020). Accessing Legacy Phosphorus in Soils. Soil Systems, 4(4), 74. https://doi.org/10.3390/soilsystems4040074






