Pteris vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion during Field-Scale Phytoextraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil, Fern, and Porewater Sample Collection
2.3. Fern and Soil Sample Analyses
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Temporal Evolution of Fern Biomass and Arsenic Uptake
3.2. Effects of Soil Fertilization and Inoculation with F. mosseae on Fern Arsenic Uptake
3.3. Soil Arsenic Depletion Compared to Fern Arsenic Accumulation
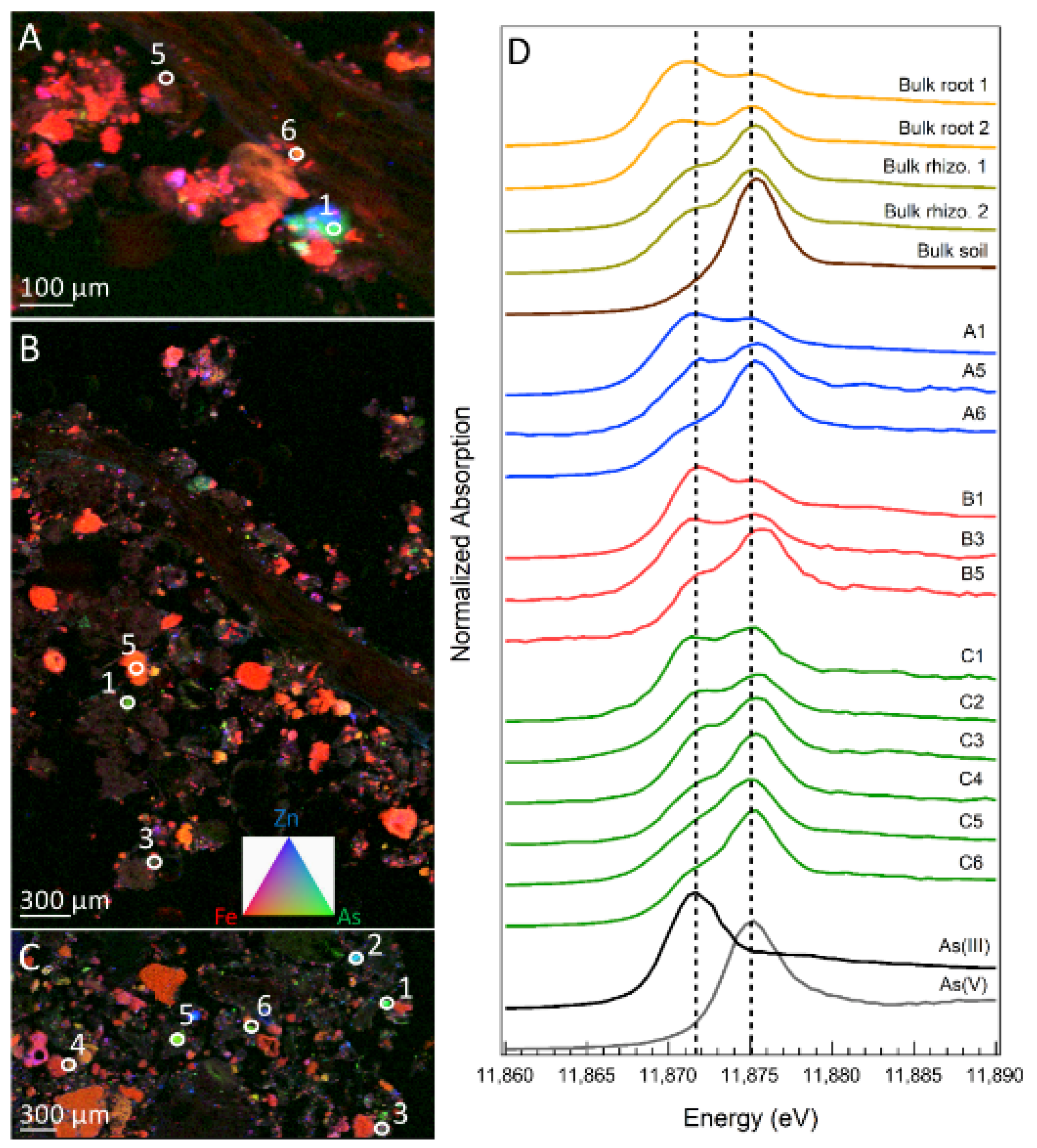
3.4. Arsenic Speciation in Soil and Fern Roots
4. Discussion
4.1. Fern Arsenic Uptake Varies with Soil Arsenic Content
4.2. Soil Treatment Did Not Affect Fern Arsenic Accumulation
4.3. Fern Arsenic Accumulation Is Less than Soil Arsenic Depletion
4.4. Leaching Could Explain Discrepancy in Soil–Plant Mass Balance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Occurrence of arsenic contamination in Canada: Sources, behavior and distribution. Sci. Total Environ. 2006, 366, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Crecelius, E.A.; Johnson, C.J.; Hofer, G.C. Contamination of soils near a copper smelter by arsenic, antimony and lead. Water Air Soil Pollut. 1974, 3, 337–342. [Google Scholar]
- Colbourn, P.; Alloway, B.J.; Thornton, I. Arsenic and heavy metals in soils associated with regional geochemical anomalies in South-West England. Sci. Total Environ. 1975, 4, 359–363. [Google Scholar] [CrossRef]
- Moreno-Jimenez, E.; Esteban, E.; Penalosa, J.M. The fate of arsenic in soil-plant systems. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; Volume 225, pp. 1–37. [Google Scholar]
- Teixeira, M.C.; Santos, A.C.; Fernandes, C.S.; Ng, J.C. Arsenic contamination assessment in Brazil – Past, present and future concerns: A historical and critical review. Sci. Total Environ. 2020, 730, 138–217. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2011; pp. 1–548. [Google Scholar]
- Mandal, B.K.; Suzuki, K.T. Arsenic around the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic-a review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef]
- Mitchell, V.L. Health risks associated with chronic exposures to arsenic in the environment. Rev. Miner. Geochem. 2014, 79, 435–449. [Google Scholar] [CrossRef]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J.; Lombi, E. Phytoremediation of metals, metalloids, and radionuclides. Adv. Agron. 2002, 75, 1–56. [Google Scholar]
- Robinson, B.H.; Anderson, C.W.N.; Dickinson, N.M. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Niazi, N.K.; Singh, B.; Van Zwieten, L.; Kachenko, A.G. Phytoremediation of an arsenic-contaminated site using Pteris vittata L. and Pityrogramma calomelanos var. austroamericana: A long-term study. Environ. Sci. Pollut. Res. 2012, 19, 3506–3515. [Google Scholar] [CrossRef]
- Kertulis-Tartar, G.M.; Ma, L.Q.; Tu, C.; Chirenje, T. Phytoremediation of an arsenic-contaminated site using Pteris vittata L.: A two-year study. Int. J. Phytoremediat. 2006, 8, 311–322. [Google Scholar] [CrossRef]
- Lessl, J.T.; Ma, L.Q. Sparingly-soluble phosphate rock induced significant plant growth and arsenic uptake by Pteris vittata from three contaminated soils. Environ. Sci. Technol. 2013, 47, 5311–5318. [Google Scholar] [CrossRef]
- Liao, X.-Y.; Chen, T.-B.; Xiao, X.-Y.; Xie, H.; Yan, X.-L.; Zhai, L.-M.; Wu, B. Selecting appropriate forms of nitrogen fertilizer to enhance soil arsenic removal by Pteris vittata: A new approach in phytoremediation. Int. J. Phytoremediat. 2007, 9, 269–280. [Google Scholar] [CrossRef]
- da Silva, E.B.; Lessel, J.T.; Wilkie, A.C.; Liu, X.; Liu, Y.; Ma, L.Q. Arsenic removal by As-hyperaccumulator Pteris vittata from two contaminated soils: A 5-year study. Chemosphere 2018, 206, 736–741. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Yan, Y.; Yang, J.; Wan, X.; Guo, J.; Guo, J.; Chen, T.; Lei, M. Phytoaccumulation of As by Pteris vittata supplied with fertilizers under different soil moisture regimes—A field case. Ecol. Eng. 2019, 138, 274–280. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q. Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci. Total Environ. 2006, 359, 17–25. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Shiralipour, A. Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator, Pteris Vittata L. Environ. Pollut. 2003, 126, 157–167. [Google Scholar] [CrossRef]
- Hua, C.-Y.; Chen, J.-X.; Cao, Y.; Li, H.-B.; Chen, Y.; Ma, L.Q. Pteris vittata coupled with phosphate rock effectively reduced As and Cd uptake by water spinach from contaminated soil. Chemosphere 2020, 247, 1–7. [Google Scholar] [CrossRef]
- Chen, T.; Fan, Z.; Lei, M.; Huang, Z.; Wei, C. Effect of phosphorus on arsenic accumulation in As-hyperaccumulator Pteris vittata L. and its implication. Chin. Sci. Bull. 2002, 47, 1876–1879. [Google Scholar] [CrossRef]
- Tu, S.; Ma, L.Q. Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyperaccumulator Pteris vittata L. under hydroponic conditions. Environ. Exp. Bot. 2003, 50, 243–251. [Google Scholar] [CrossRef]
- Caille, N.; Swanwick, S.; Zhao, F.J.; McGrath, S.P. Arsenic hyperaccumulation by Pteris vittata from arsenic contaminated soils and the effect of liming and phosphate fertilisation. Environ. Pollut. 2004, 132, 113–120. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Tu, C. Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.). Environ. Pollut. 2004, 128, 317–325. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Kalyvas, G.; Ioannou, D.; Massas, I. Desorption of arsenic from calcareous mine affected soils by phosphate fertilizers application in relation to soil properties and As partitioning. Soil Syst. 2019, 3, 54. [Google Scholar] [CrossRef]
- Codling, E.E.; Dao, T.H. Short-term effect of lime, phosphorus, and iron amendments on water-extractable lead and arsenic in orchard soils. Commun. Soil Sci. Plant Anal. 2007, 38, 903–919. [Google Scholar] [CrossRef]
- Peryea, F.J. Phosphate-induced release of arsenic from soils contaminated with lead arsenate. Soil Sci. Soc. Am. J. 1991, 55, 1301–1306. [Google Scholar] [CrossRef]
- Davenport, J.R.; Peryea, F.J. Phosphate fertilizers influence leaching of lead and arsenic in a soil contaminated with lead arsenate. Water. Air. Soil Pollut. 1991, 57–58, 101–110. [Google Scholar] [CrossRef]
- Trotta, A.; Falaschi, P.; Cornara, L.; Minganti, V.; Fusconi, A.; Drava, G.; Berta, G. Arbuscular mycorrhizae increase the arsenic translocation factor in the As hyperaccumulating fern Pteris vittata L. Chemosphere 2006, 65, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.G.; Chen, B.D.; Christie, P.; Li, X.L. Influence of the arbuscular mycorrhizal fungus Glomus mosseae on uptake of arsenate by the As hyperaccumulator fern Pteris vittata L. Mycorrhiza 2005, 15, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Tlustoš, P.; Komárek, M.; Száková, J. Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: Laboratory versus field scale measures of efficiency. Geoderma 2008, 144, 446–454. [Google Scholar] [CrossRef]
- Audet, P. Examining the ecological paradox of the mycorrhizal metal hyperaccumulators. Arch. Agron. Soil Sci. 2012, 59, 549–558. [Google Scholar] [CrossRef]
- Marschner, H.; Romheld, V. Strategies of plants for acquisition of iron. Plant Soil 1994, 165, 261–274. [Google Scholar] [CrossRef]
- Lombi, E.; Sletten, R.S.; Wenzel, W.W. Sequentially extracted arsenic from different size fractions of contaminated soils. Water Air Soil Pollut. 2000, 124, 319–332. [Google Scholar] [CrossRef]
- Smith, E.; Smith, J.; Naidu, R. Distribution and nature of arsenic along former railway corridors of South Australia. Sci. Total Environ. 2006, 363, 175–182. [Google Scholar] [CrossRef]
- Gonzaga, M.I.; Santos, J.A.G.; Ma, L.Q. Arsenic chemistry in the rhizosphere of Pteris vittata L. and Nephrolepis exaltata L. Environ. Pollut. 2006, 143, 254–260. [Google Scholar] [CrossRef]
- Tu, S.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant Soil 2004, 258, 9–19. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W.; Zhang, H.; Nurmi, J.; Stipek, K.; Fischerova, Z.; Schweiger, P.; Köllensperger, G.; Ma, L.Q.; Stingeder, G. Rhizosphere characteristics of the arsenic hyperaccumulator Pteris vittata L. and monitoring of phytoremoval efficiency. Environ. Sci. Technol. 2003, 37, 5008–5014. [Google Scholar] [CrossRef] [PubMed]
- Reichard, P.U.; Kretzschmar, R.; Kraemer, S.M. Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochim. Cosmochim. Acta 2007, 71, 5635–5650. [Google Scholar] [CrossRef]
- Furrer, G.; Stumm, W.; Zinder, B. The coordination chemistry of weathering: II. Dissolution of Fe(III) oxides. Geochim. Cosmochim. Acta 1986, 50, 1861–1869. [Google Scholar] [CrossRef]
- Dobran, S.; Zagury, G.J. Arsenic speciation and mobilization in CCA-contaminated soils: Influence of organic matter content. Sci. Total Environ. 2006, 364, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chou, M.L.; Jean, J.S.; Yang, H.J.; Kim, P.J. Arsenic-enrichment enhanced root exudates and altered rhizosphere microbial communities and activities in hyperaccumulator Pteris vittata. J. Hazard. Mater. 2017, 325, 279–287. [Google Scholar] [CrossRef]
- Madrid, F.; Liphadzi, M.S.; Kirkham, M.B. Heavy metal displacement in chelate-irrigated soil during phytoremediation. J. Hydrol. 2003, 272, 107–119. [Google Scholar] [CrossRef]
- Martin del Campo, M.V.; Esteller, M.V.; Morell, I.; Expósito, J.L.; Bandenay, G.L.; Díaz-Delgado, C. A lysimeter study under field conditions of nitrogen and phosphorus leaching in a turf grass crop amended with peat and hydrogel. Sci. Total Environ. 2019, 648, 530–541. [Google Scholar] [CrossRef]
- Meredith, L.K.; Boye, K.; Savage, K.; Vargas, R. Formation and fluxes of soil trace gases. Soil Syst. 2020, 4, 22. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Mlakar, T.L.; Horvat, M.; Vuk, T.; Stergaršek, A.; Kotnik, J.; Tratnik, J.; Fajon, V. Mercury species, mass flows and processes in a cement plant. Fuel 2010, 89, 1936–1945. [Google Scholar] [CrossRef]
- Shi, J.; Thompson, V.S.; Yancey, N.A.; Stavila, V.; Simmons, B.A.; Singh, S. Impact of mixed feedstocks and feedstock densification on ionic liquid pretreatment efficiency. Biofuels 2013, 4, 63–72. [Google Scholar] [CrossRef]
- Burns, K.; Villeneuve, J.-P. Chlorinated hydrocarbons in the open Mediterranean ecosystem and implications for mass balance calculations. Mar. Chem. 1987, 20, 337–359. [Google Scholar] [CrossRef]
- Tetra Tech EM. Final Current Conditions Report, University of California, Berkeley Richmond Field Station, Richmond; Tetra Tech EM, Inc.: San Francisco, CA, USA, 2008; pp. 1–193. [Google Scholar]
- Department of Toxic Substances Control, California Environmental Protection Agency, State of California. State of California Department of Toxic Substances Control Site Investigation and Remediation Order, Docket No. I/SE-RAO 0607-004; State of California: Sacramento, CA, USA, 2006; pp. 1–47.
- Duvergé, D.J. Establishing Background Arsenic in Soil of the Urbanized San Francisco Bay Region; San Francisco State University: San Francisco, CA, USA, 2011. [Google Scholar]
- Human Health Risk Assessment (HHRA) Note Number 3, DTSC-Modified Screening Levels (DTSC-SLs); California Department of Toxic Substance Control (Cal DTSC): Sacramento, CA, USA, 2020; pp. 1–46.
- Poppe, L.J.; Paskevich, V.F.; Hathaway, J.C.; Blackwood, D.S. A Laboratory Manual for X-ray Powder Diffraction; United States Geological Survey: Reston, VA, USA, 2002.
- Chen, T.; Wei, C.; Huang, Z.; Huang, Q.; Quanguo, L.; Fan, Z. Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Brewer, R.; Peard, J.; Heskett, M. A critical review of discrete soil sample data reliability: Part 1—Field study results. Soil Sediment Contam. 2017, 26, 1–22. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.-J.; Meharg, A.A.; Raab, A.; Feldmann, J.; McGrath, S.P. Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol. 2002, 130, 1552–1561. [Google Scholar] [CrossRef]
- Matzen, S.L. Effects of Soil Biogeochemical and Physical Characteristics on Arsenic Hyperaccumulation in Pteris vittata L.; University of California: Berkeley, CA, USA, 2020. [Google Scholar]
- Arai, Y.; Dahle, J.T. Redox-ligand complexation controlled chemical fate of ceria nanoparticles in an agricultural soil. J. Agric. Food Chem. 2018, 66, 6646–6653. [Google Scholar] [CrossRef]
- Sarret, G.; Willems, G.; Isaure, M.-P.; Marcus, M.A.; Fakra, S.C.; Frérot, H.; Pairis, S.; Geoffroy, N.; Manceau, A.; Saumitou-Laprade, P. Zinc distribution and speciation in Arabidopsis halleri × Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol. 2009, 184, 581–595. [Google Scholar] [CrossRef]
- McIntosh, J.L. Bray and Morgan soil extractants modified for testing acid soils from different parent materials 1. Agron. J. 1969, 61, 259–265. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Santos, J.A.G.; Ma, L.Q. Phytoextraction by arsenic hyperaccumulator Pteris vittata L. from six arsenic-contaminated soils: Repeated harvests and arsenic redistribution. Environ. Pollut. 2008, 154, 212–218. [Google Scholar] [CrossRef]
- Marcus, M.A.; MacDowell, A.A.; Celestre, R.; Manceau, A.; Miller, T.; Padmore, H.A.; Sublett, R.E. Beamline 10.3.2 at ALS: A hard X-ray microprobe for environmental and materials sciences. J. Synchrotron Radiat. 2004, 11, 239–247. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Niazi, N.K.; Singh, B.; Van Zwieten, L.; Kachenko, A.G. Phytoremediation potential of Pityrogramma calomelanos var. austroamericana and Pteris vittata L. grown at a highly variable arsenic contaminated site. Int. J. Phytoremediat. 2011, 13, 912–932. [Google Scholar] [CrossRef]
- Lei, M.; Wan, X.; Guo, G.; Yang, J.; Chen, T. Phytoextraction of arsenic-contaminated soil with Pteris vittata in Henan Province, China: Comprehensive evaluation of remediation efficiency correcting for atmospheric depositions. Environ. Sci. Pollut. Res. 2018, 25, 124–131. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Ent, A.; Van, D.; Baker, A.J.M. Hyperaccumulators of metal and metalloid trace elements: Facts and Fiction. Plant Soil 2013, 319–334. [Google Scholar] [CrossRef]
- Ciurli, A.; Lenzi, L.; Alpi, A.; Pardossi, A. Arsenic uptake and translocation by plants in pot and field experiments. Int. J. Phytoremediat. 2014, 16, 804–823. [Google Scholar] [CrossRef]
- Tu, C.; Ma, L. Effects of arsenic concentrations and forms on arsenic uptake by the hyperaccumulator ladder brake. J. Environ. Qual. 2002, 31, 641–647. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q.; Cao, X.; Rathinasabapathi, B. Effects of heavy metals on growth and arsenic accumulation in the arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2004, 132, 289–296. [Google Scholar] [CrossRef]
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Effects of nitrogen and phosphorus levels, and frond-harvesting on absorption, translocation and accumulation of arsenic by Chinese brake fern (Pteris vittata L.). Int. J. Phytoremediat. 2009, 11, 313–328. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Traina, S.J.; Logan, T.J.; Ryan, J.A. In situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Mkhabela, M.S.; Warman, P.R. The influence of municipal solid waste compost on yield, soil phosphorus availability and uptake by two vegetable crops grown in a Pugwash sandy loam soil in Nova Scotia. Agric. Ecosyst. Environ. 2005, 106, 57–67. [Google Scholar] [CrossRef]
- Giusquiani, P.L.; Marucchini, C.; Businelli, M. Chemical properties of soils amended with compost of urban waste. Plant Soil 1988, 109, 73–78. [Google Scholar] [CrossRef]
- Leung, H.M.; Leung, A.O.W.; Ye, Z.H.; Cheung, K.C.; Yung, K.K.L. Mixed arbuscular mycorrhizal (AM) fungal application to improve growth and arsenic accumulation of Pteris vittata (As hyperaccumulator) grown in As-contaminated soil. Chemosphere 2013, 92, 1367–1374. [Google Scholar] [CrossRef]
- Leung, H.M.; Wu, F.Y.; Cheung, K.C.; Ye, Z.H.; Wong, M.H. Synergistic effects of arbuscular mycorrhizal fungi and phosphate rock on heavy metal uptake and accumulation by an arsenic hyperaccumulator. J. Hazard. Mater. 2010, 181, 497–507. [Google Scholar] [CrossRef]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 184, 1029. [Google Scholar] [CrossRef]
- Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Arsenic hyperaccumulation by different fern species. New Phytol. 2002, 156, 27–31. [Google Scholar] [CrossRef]
- Liao, X.-Y.; Chen, T.-B.; Lei, M.; Huang, Z.-C.; Xiao, X.-Y.; An, Z.-Z. Root distributions and elemental accumulations of Chinese brake (Pteris vittata L.) from As-contaminated soils. Plant Soil 2004, 261, 109–116. [Google Scholar] [CrossRef]
- Wilde, E.W.; Brigmon, R.L.; Dunn, D.L.; Heitkamp, M.A.; Dagnan, D.C. Phytoextraction of lead from firing range soil by Vetiver Grass. Chemosphere 2005, 61, 1451–1457. [Google Scholar] [CrossRef]
- Hadley, P.W.; Petrisor, I.G. Incremental sampling: Challenges and opportunities for environmental forensics. Environ. Forensics 2013, 14, 109–120. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Tu, C.; Ma, L.Q. Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci. Total Environ. 2002, 300, 167–177. [Google Scholar] [CrossRef]
- Lin, Z.; Puls, R.W. Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environ. Geol. 2000, 39, 753–759. [Google Scholar] [CrossRef]
- Roberts, L.C.; Hug, S.J.; Voegelin, A.; Dittmar, J.; Kretzschmar, R.; Wehrli, B.; Saha, G.C.; Badruzzaman, A.B.M.; Ali, M.A. Arsenic dynamics in porewater of an intermittently irrigated paddy field in Bangladesh. Environ. Sci. Technol. 2011, 45, 971–976. [Google Scholar] [CrossRef]
- Liu, X.; Fu, J.-W.; Guan, D.-X.; Cao, Y.; Luo, J.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2016, 50, 9070–9077. [Google Scholar] [CrossRef]
- Hutton, C.; Bryce, D.W.; Russeau, W.; Glass, H.J.; Jenkin, L.E.T.; Corns, W.T.; Stockwell, P.B. Aqueous and solid-phase speciation of arsenic in Cornish soils. Mineral. Mag. 2005, 69, 577–589. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ. Sci. Technol. 1991, 25, 1414–1419. [Google Scholar] [CrossRef]
- Vinther, F.P.; Hansen, E.M.; Eriksen, J. Leaching of soil organic carbon and nitrogen in sandy soils after cultivating grass-clover swards. Biol. Fertil. Soils 2006, 43, 12–19. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Lilienfein, J. Contribution of root vs. leaf litter to dissolved organic carbon leaching through soil. Soil Sci. Soc. Am. J. 2007, 71, 1555–1563. [Google Scholar] [CrossRef]
- Tiberg, C.; Bendz, D.; Theorin, G.; Kleja, D.B. Evaluating solubility of Zn, Pb, Cu and Cd in pyrite cinder using leaching tests and geochemical modelling. Appl. Geochem. 2017, 85, 106–117. [Google Scholar] [CrossRef]
- Duan, G.-L.; Zhu, Y.-G.; Tong, Y.-P.; Cai, C.; Kneer, R. Characterization of arsenate reductase in the extract of roots and fronds of Chinese brake fern, an arsenic hyperaccumulator. Plant Physiol. 2005, 138, 461–469. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.Q.; Rathinasabapathi, B.; Cai, Y.; Liu, Y.G.; Zeng, G.M. Mechanisms of efficient arsenite uptake by arsenic hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2011, 45, 9719–9725. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-L.; Liao, X.-Y.; Chen, T. Leaching potential of arsenic from Pteris vittata L. under field conditions. Sci. Total Environ. 2009, 408, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, J.W.; Han, Y.H.; Rathinasabapathi, B.; Ma, L.Q. High As exposure induced substantial arsenite efflux in As-hyperaccumulator Pteris vittata. Chemosphere 2016, 144, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hoefer, C.; Puschenreiter, M.; Wenzel, W.; Oburger, E.; Hann, S.; Robinson, B.; Kretzschmar, R.; Santner, J. Arsenic redox transformations and cycling in the rhizosphere of Pteris vittata and Pteris quadriaurita. Environ. Exp. Bot. 2020, 177, 1–12. [Google Scholar] [CrossRef]
- Masue-Slowey, Y.; Ying, S.C.; Kocar, B.D.; Pallud, C.E.; Fendorf, S. Dependence of arsenic fate and transport on biogeochemical heterogeneity arising from the physical structure of soils and sediments. J. Environ. Qual. 2013, 42, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tu, S.; Wang, G.; Liao, X.; Yan, X. Effectiveness of applying arsenate reducing bacteria to enhance arsenic removal from polluted soils by Pteris vittata L. Int. J. Phytoremediation 2012, 14, 89–99. [Google Scholar] [CrossRef]


| Soil Characteristic | Unit | Value | Range |
|---|---|---|---|
| pH 1 before liming | 5.5 ± 0.02 | n/a | |
| pH after liming | 6.1 ± 0.22 | n/a | |
| Total concentrations 2 | |||
| As | (mg/kg) | 78.3 ± 4.47 | 23.5–118.6 |
| P | (mg/kg) | 290.3 ± 20.2 | 98.0–735.5 |
| Fe | % | 3.4 ± 0.12 | 1.9–4.6 |
| Pb | (mg/kg) | 143.9 ± 5.23 | 95.2–223.0 |
| Cu | (mg/kg) | 550.6 ± 11.75 | 404.5–772.0 |
| Zn | (mg/kg) | 401.5 ± 9.96 | 293.6–562.3 |
| Modified Morgan extractable concentrations 3 | |||
| P | (mg/kg) | 2.7 ± 0.04 | n/a |
| K | (mg/kg) | 129.0 ± 0.58 | n/a |
| Ca | (mg/kg) | 2,479.1 ± 0.16 | n/a |
| Mg | (mg/kg) | 341.2 ± 2.96 | n/a |
| Zn | (mg/kg) | 38.1 ± 0.05 | n/a |
| B | (mg/kg) | 0.3 ± 0 | n/a |
| Mn | (mg/kg) | 44.7 ± 0.11 | n/a |
| Cu | (mg/kg) | 18.6 ± 0.13 | n/a |
| Fe | (mg/kg) | 24.4 ± 0.3 | n/a |
| Pb | (mg/kg) | 4.4 ± 0.02 | n/a |
| Al | (mg/kg) | 15.2 ± 0.15 | n/a |
| Na | (mg/kg) | 32 ± 0.4 | n/a |
| S | (mg/kg) | 46.3 ± 0.01 | n/a |
| CEC 3 | meq/100g | 23.7 ± 0.22 | n/a |
| Organic matter 3 | % | 8.6 ± 0.17 | n/a |
| Bulk density 4 | g/cm3 | 1.1 ± 0.01 | n/a |
| Sand content 4 | % | 53.6 ± 0.16 | n/a |
| Silt content 4 | % | 29.8 ± 0.29 | n/a |
| Clay content 4 | % | 16.6 ± 0.45 | n/a |
| Texture 4 | Sandy loam | ||
| 0–20 cm | 0–10 cm | 10–20 cm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plot | Treatment | Fern As Accumulation per kg Soil (per 1 Depth) | Initial Soil As | % of Initial Soil As Accumulated in Fern after 58 Weeks | Soil As Depletion after 58 Weeks | % of Depleted Soil As Accumulated in Fern after 58 Weeks | Soil As Depletion after 58 Weeks | Soil As Depletion after 58 Weeks | ||
| mg As/kg Soil | mg/kg | % | mg/kg | % | mg/kg | % decr | mg/kg | % decr | ||
| 16 | Control | 8.1 | 118.6 ± 4.0 | 3.4 | 1.4 | 281.4 | −2.4 | −2.0 | 5.3 | 4.4 |
| 11 | P—high | 5.3 | 108.7 ± 3.6 | 2.4 | 31.0 | 8.5 | 15.3 | 14.1 | 46.6 | 42.9 |
| 17 | Compost | 11.2 | 108.6 ± 5.0 | 5.2 | −2.5 | NA | −2.3 | −2.2 | −2.7 | −2.5 |
| 21 | Fungi | 29.3 | 108.4 ± 1.7 | 13.5 | 14.0 | 104.6 | 20.6 | 19.0 | 7.4 | 6.9 |
| 18 | P—high | 7.4 | 107.1 ± 3.5 | 3.4 | 11.9 | 31.1 | 10.0 | 9.3 | 13.8 | 12.8 |
| 9 | Control | 9.0 | 104.4 ± 4.9 | 4.3 | 10.0 | 45.3 | 8.1 | 7.7 | 11.9 | 11.4 |
| 3 | P—low | 4.7 | 99.6 ± 2.3 | 2.4 | 24.0 | 9.8 | 7.0 | 7.0 | 41.1 | 41.2 |
| 8 | Fungi | 6.7 | 98.1 ± 1.3 | 3.4 | 6.2 | 54.6 | 3.8 | 3.8 | 8.6 | 8.7 |
| 25 | P—low | 8.1 | 97.8 ± 4.7 | 4.1 | 15.0 | 26.9 | 16.9 | 17.2 | 13.2 | 13.5 |
| 5 | P—low | 4.5 | 97.7 ± 0.9 | 2.3 | 30.3 | 7.4 | 17.9 | 18.4 | 42.6 | 43.6 |
| 6 | Compost | 5.7 | 97.0 ± 1.9 | 2.9 | 21.2 | 13.5 | 9.4 | 9.7 | 33.0 | 34.1 |
| 2 | Compost | 3.9 | 95.6 ± 0.6 | 2.1 | 12.4 | 15.9 | 8.5 | 8.9 | 16.2 | 17.0 |
| 24 | Nitrogen | 9.5 | 94.2 ± 6.1 | 5.0 | 13.0 | 36.6 | 14.2 | 15.1 | 11.7 | 12.4 |
| 14 | P—low | 5.3 | 93.2 ± 3.3 | 2.9 | 11.0 | 24.2 | 9.0 | 9.7 | 13.0 | 13.9 |
| 36 | Control | 3.3 | 92.7 ± 6.1 | 1.8 | 12.0 | 13.9 | 14.7 | 15.8 | 9.4 | 10.1 |
| 23 | Control | 8.4 | 91.3 ± 7.6 | 4.6 | 13.4 | 31.5 | 12.1 | 13.2 | 14.7 | 16.1 |
| 7 | P—high | 6.0 | 90.0 ± 2.7 | 3.3 | 11.7 | 25.6 | 4.7 | 5.3 | 18.7 | 20.8 |
| 10 | Nitrogen | 4.2 | 87.9 ± 3.1 | 2.4 | 16.3 | 12.9 | 3.8 | 4.4 | 28.9 | 32.8 |
| 35 | Compost | 7.7 | 85.6 ± 2.4 | 4.5 | 5.8 | 66.4 | 4.2 | 4.9 | 7.4 | 8.7 |
| 22 | P—high | 6.2 | 82.4 ± 4.4 | 3.7 | 4.4 | 70.2 | 3.2 | 3.8 | 5.6 | 6.8 |
| 15 | Compost | 4.8 | 81.1 ± 1.0 | 3.0 | 10.5 | 22.8 | 5.8 | 7.1 | 15.2 | 18.8 |
| 19 | Nitrogen | 6.8 | 81.0 ± 0.9 | 4.2 | 6.8 | 50.0 | 4.5 | 5.5 | 9.2 | 11.3 |
| 1 | Nitrogen | 2.2 | 80.6 ± 2.6 | 1.4 | 14.7 | 7.5 | 11.5 | 14.3 | 18.0 | 22.3 |
| 4 | Fungi | 2.9 | 76.3 ± 2.1 | 1.9 | 13.9 | 10.3 | 3.6 | 4.8 | 24.2 | 31.7 |
| 33 | P—low | 10.7 | 65.6 ± 2.0 | 8.2 | 9.6 | 55.5 | 10.8 | 16.4 | 8.5 | 13.0 |
| 34 | Nitrogen | 2.7 | 61.2 ± 2.8 | 2.2 | 6.6 | 20.6 | 10.9 | 17.8 | 2.4 | 4.0 |
| 20 | Fungi | 6.6 | 61.0 ± 0.5 | 5.4 | 2.9 | 114.6 | 2.7 | 4.5 | 3.0 | 4.9 |
| 12 | Control | 3.4 | 59.9 ± 0.2 | 2.9 | 10.2 | 16.7 | 12.3 | 20.5 | 8.2 | 13.7 |
| 32 | Fungi | 5.7 | 53.6 ± 1.8 | 5.3 | 8.4 | 34.2 | 8.0 | 14.9 | 8.8 | 16.4 |
| 27 | Fungi | 2.6 | 48.0 ± 1.7 | 2.7 | 7.1 | 18.3 | 5.4 | 11.3 | 8.7 | 18.1 |
| 28 | Control | 1.9 | 40.7 ± 1.8 | 2.3 | 9.4 | 10.0 | 4.7 | 11.6 | 14.0 | 34.4 |
| 26 | P—high | 1.5 | 38.5 ± 0.9 | 1.9 | 6.9 | 10.7 | 2.8 | 7.1 | 11.0 | 28.6 |
| 13 | P—high | 2.9 | 32.0 ± NA | 4.5 | 5.8 | 24.8 | 5.6 | 17.4 | 6.1 | 18.9 |
| 30 | Nitrogen | 0.5 | 28.1 ± 0.4 | 1.0 | 4.6 | 6.0 | 0.3 | 1.1 | 8.9 | 31.6 |
| 31 | Compost | 1.3 | 27.2 ± 0.3 | 2.4 | 6.4 | 10.1 | 3.6 | 13.3 | 9.3 | 34.1 |
| 29 | P—low | 1.3 | 23.5 ± 1.1 | 2.8 | 1.2 | 55.7 | 0.2 | 1.0 | 2.2 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matzen, S.; Fakra, S.; Nico, P.; Pallud, C. Pteris vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion during Field-Scale Phytoextraction. Soil Syst. 2020, 4, 71. https://doi.org/10.3390/soilsystems4040071
Matzen S, Fakra S, Nico P, Pallud C. Pteris vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion during Field-Scale Phytoextraction. Soil Systems. 2020; 4(4):71. https://doi.org/10.3390/soilsystems4040071
Chicago/Turabian StyleMatzen, Sarick, Sirine Fakra, Peter Nico, and Céline Pallud. 2020. "Pteris vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion during Field-Scale Phytoextraction" Soil Systems 4, no. 4: 71. https://doi.org/10.3390/soilsystems4040071
APA StyleMatzen, S., Fakra, S., Nico, P., & Pallud, C. (2020). Pteris vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion during Field-Scale Phytoextraction. Soil Systems, 4(4), 71. https://doi.org/10.3390/soilsystems4040071




