Nitrogen Dynamics in an Established Alfalfa Field under Low Biochar Application Rates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Biochars
2.3. Experimental Design
2.4. Plant and Soil Samples Collection
2.5. Plant and Soil Analyses
2.6. Enzyme Analyses
2.7. DNA Analyses
2.8. Data Analyses
3. Results
3.1. Plant and Soil N Dynamics
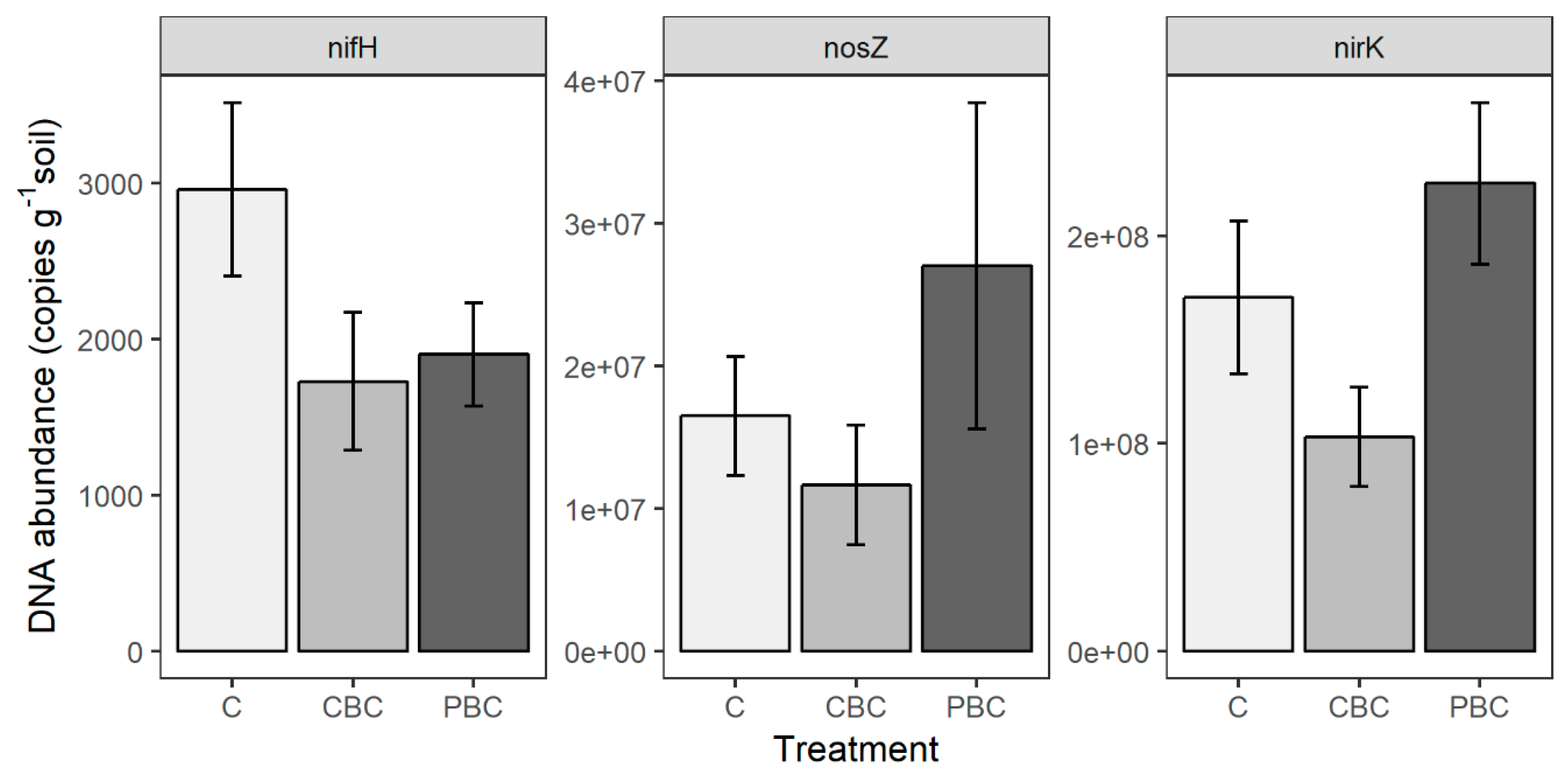
3.2. Microbial Measurements
3.3. Correlations between Measurements
4. Discussion
4.1. Lack of Biochar Addition Effects
4.2. Seasonal Trends in N Cycling
4.3. N Cycling Genes and SIN Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: A meta-analysis of crop yield and N dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- King, A.E.; Hofmockel, K.S. Diversified cropping systems support greater microbial cycling and retention of carbon and nitrogen. Agric. Ecosyst. Environ. 2017, 240, 66–76. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Earles, R. Sustainable Agriculture: An Introduction; National Sustainable Agriculture Information Service: Washington, DC, USA, 2005; pp. 1–8. [Google Scholar]
- Forge Legumes, University of Minnesota Extension. Available online: https://extension.umn.edu/forage-variety-selection/forage-legumes (accessed on 27 September 2019).
- Davis, A.S.; Hill, J.D.; Chase, C.A.; Johanns, A.M.; Liebman, M. Increasing Cropping System Diversity Balances Productivity, Profitability and Environmental Health. PLoS ONE 2012, 7, e47149. [Google Scholar] [CrossRef]
- Spargo, J.T.; Cavigelli, M.A.; Mirsky, S.B.; Maul, J.E.; Meisinger, J.J. Mineralizable soil nitrogen and labile soil organic matter in diverse long-term cropping systems. Nutr. Cycl. Agroecosyst. 2011, 90, 253–266. [Google Scholar] [CrossRef]
- Rubio, L.M.; Ludden, P.W. Biosynthesis of the Cofactor of Nitrogenase. Annu. Rev. Microbiol. 2008, 62, 93–111. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management Science, Technology and Implementation; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community strucutre changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Fogle, E.J.; Cotrufo, M.F. Sorption to Biochar Impacts β-Glucosidase and Phosphatase Enzyme Activities. Agricuture 2018, 8, 158. [Google Scholar] [CrossRef]
- Nelissen, V.; Ruysschaert, G.; Muller-Stover, D.; Bodé, S.; Cook, J.; Ronsse, F.; Shackley, S.; Boeckx, P.; Hauggaard-Nielsen, H. Short-Term Effect of Feedstock and Pyrolysis Temperature on Biochar Characteristics, Soil and Crop Response in Temperate Soils. Agronomy 2014, 4, 52–73. [Google Scholar] [CrossRef]
- Nelissen, V.; Rutting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Güereña, D.T.; Lehmann, J.; Thies, J.E.; Enders, A.; Karanja, N.; Neufeldt, H. Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol. Fertil. Soils 2015, 51, 479–491. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yan, X.; Li, J.; Jiao, N.; Hu, S. Biochar amendments increase the yield advantage of legume-based intercropping systems over monoculture. Agric. Ecosyst. Environ. 2017, 237, 16–23. [Google Scholar] [CrossRef]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Oram, N.J.; Van de Voorde, T.F.J.; Ouwehand, G.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Van Groenigen, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Quilliam, R.S.; DeLuca, T.H.; Jones, D.L. Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 2013, 366, 83–92. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Rose, T.; Herridge, D.; Kimber, S.; Rust, J.; Cowie, A. Enhanced biological N2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: Dissection of causal mechanisms. Plant Soil 2015, 395, 7–20. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Enhanced biological nitrogen fixation and competitive advantage of legumes in mixed pastures diminish with biochar aging. Plant Soil 2018, 424, 639–651. [Google Scholar] [CrossRef]
- Galinato, S.P.; Yoder, J.K.; Granatstein, D. The economic value of biochar in crop production and carbon sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- Foster, E.J.; Baas, P.; Wallenstein, M.D.; Cotrufo, M.F. Prescision biochar and innoculum applications shift bacterial community structure and increase specific nutrient availability and maize yield. Appl. Soil Ecol. 2019. in revision. [Google Scholar]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E.; Russelle, M.P. Understanding the variability in soybean nitrogen fixation across agroecosystems. Plant Soil 2010, 329, 379–397. [Google Scholar] [CrossRef]
- Crane-Droesch, A.; Abiven, S.; Jeffery, S.; Torn, M.S. Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environ. Res. Lett. 2013, 8, 044049. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modi fi cation. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Mayer, Z.A.; Eltom, Y.; Stennett, D.; Schr, E.; Apfelbacher, A. Characterization of Engineered Biochar for Soil Management. Environ. Prog. Sustain. Energy 2014, 33, 490–496. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Chalk, P.M. Dynamics of biologically fixed N in legume-cereal rotations: A review. Aust. J. Agric. Res. 1998, 49, 303–316. [Google Scholar] [CrossRef]
- Malik, K.A.; Bilal, R.; Rasul, G.; Mahmood, K.; Sajjad, M.I. Associative N2-fixation in plants growing in saline sodic soils and its relative quantification based on 15N natural abundance. Plant Soil 1991, 137, 67–74. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Menge, D.N.L.; Reed, S.C.; Cleveland, C.C. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130119. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; Vilgalys, R.J. Quantitative analyses of nitrogen cycling genes in soils. Pedobiologia 2005, 49, 665–672. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Ramlow, M.; Foster, E.J.; del Grosso, S.J.; Cotrufo, M.F. Broadcast woody biochar provides limited benefits to deficit irrigation maize in Colorado. Agric. Ecosyst. Environ. 2019, 269, 71–81. [Google Scholar] [CrossRef]
- Rochette, P.; Janzen, H.H. Towards a revised coefficient for estimating N2O emissions from legumes. Nutr. Cycl. Agroecosyst. 2005, 73, 171–179. [Google Scholar] [CrossRef]
- Soil Science Division Staff. Soil Science Division Staff. Soil survey manual. In USDA Handbook 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 1993. [Google Scholar]
- Cool Terra, Cool Planet. Available online: https://www.coolplanet.com/cool-terra/types-of-use/ (accessed on 27 September 2019).
- Chalk, P.M. Estimation of N2 fixation by isotope dilution. Soil Biol. Biochem. 1985, 17, 389–410. [Google Scholar] [CrossRef]
- Longyear, B.O. The Dandelion in Colorado; The Agricultural Experiment Station: Fort Collins, CO, USA, 1918. [Google Scholar]
- Rodriguez, A. Will Cutting Dandelion Root Off an Inch Below the Surface of the Ground Kill It? Home Guides, SF Gate. Available online: http://homeguides.sfgate.com/cutting-dandelion-root-off-inch-below-surface-ground-kill-it-81173.html (accessed on 22 April 2019).
- Weaver, J.E. Root Development of Field Crops; McGraw-Hill: New York, NY, USA, 1926. [Google Scholar]
- McTaggart, I.P.; Smith, K.A. Estimation of potentially mineralisable nitrogen in soil by KCI extraction. Plant Soil 1992, 157, 175–184. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; Mcmahon, S.K.; Wallenstein, M.D. High-throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. J. Vis. Exp. 2013, 81, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Terakado-Tonooka, J.; Ando, S.; Ohwaki, Y.; Yoneyama, T. NifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane and sweet potatoes. In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 437–444. [Google Scholar]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version 1.1, Russell Lenth; Iowa City, IA, USA, 2018; Available online: https://CRAN.R-project.org/package=emmeans (accessed on 27 September 2019).
- Box, G.E.P.; Cox, D.R. An analysis of transforamtions. J. R. Stat. Soc. 1964, 26, 211–243. [Google Scholar]
- Borchard, N.; Schirrmann, M.; Luz, M.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Verhoeven, E.; Six, J. Biochar does not mitigate field-scale N2O emissions in a Northern California vineyard: An assessment across two years. Agric. Ecosyst. Environ. 2014, 191, 27–38. [Google Scholar] [CrossRef]
- Ducey, T.F.; Ippolito, J.A.; Cantrell, K.B.; Novak, J.M.; Lentz, R.D. Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl. Soil Ecol. 2013, 65, 65–72. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Bai, S.H.; Reverchon, F.; Xu, C.Y.; Xu, Z.; Blumfield, T.J.; Zhao, H.; Van Zwieten, L.; Wallace, H.M. Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Peoples, M.B.; Herridge, D.F.; Alves, B.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; Sampet, C.; Rerkasem, B.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Joëlle, F.; Fabien, L.; Stéphanie, M.; Jean-Bernard, C. Nitrogen rhizodeposition of legumes. A review. Agron. Sustain. Dev. 2010, 30, 57–58. [Google Scholar]
- Huang, Y.; Rickerl, D.H.; Kephart, K.D. Recovery of Deep-Point Injected Soil Nitrogen-15 by Switchgrass, Alfalfa, Ineffective Alfalfa, and Corn. J. Environ. Qual. 1996, 25, 1394–1400. [Google Scholar] [CrossRef]
- Wartiainen, I.; Eriksson, T.; Zheng, W.; Rasmussen, U. Variation in the active diazotrophic community in rice paddy nifH PCR-DGGE analysis of rhizophere and bulk soil. Appl. Soil Ecol. 2008, 39, 65–75. [Google Scholar] [CrossRef]
- Clark, I.M.; Buchkina, N.; Jhurreea, D.; Goulding, K.W.T.; Hirsch, P.R. Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1235–1244. [Google Scholar] [CrossRef]
- Kaiser, E.-A.; Mueller, T.; Joergensen, R.G.; Insam, H.; Heinemeyer, O. Evaluation of methods to estimate the soil microbial biomass and the relationship with soil texture and organic matter. Soil Biol. Biochem. 1992, 24, 675–683. [Google Scholar] [CrossRef]




| Biochar | Organic C (%) | H:Corg (Molar Ratio) | C:N (Mass Ratio) | Ash (%) | pH | Surface Area (m2/g) | Moisture (%) | Ece (mmhos/cm) |
|---|---|---|---|---|---|---|---|---|
| PBC | 72.07* | 0.659* | 30* | 1.02* | 6.13 | 97–110 | 2.25 | 1.88 |
| CBC | 62.25 | 0.418 | 148 | 1.88 | 7.61 | 200–300 | 4.29 | 1.92 |
| Measurement | Units | Biochar Type | ||
|---|---|---|---|---|
| C | CBC | PBC | ||
| Bacterial DNA abundance | copies g−1 soil | 7.95 × 109 ± 3.91 × 109 | 1.49 × 109 ± 3.24 × 108 | 3.57 × 109 ± 7.92 × 108 |
| Fungal DNA abundance | copies g−1 soil | 2.33 × 1011 ± 1.03 × 1011 | 3.52 × 1011 ± 1.62 × 1011 | 1.78 × 1011 ± 9.77 × 1010 |
| LAP | mmol g−1 soil | 4.70 ± 0.81 | 2.87 ± 1.07 | 5.54 ± 1.50 |
| NAG | mmol g−1 soil | 7.93 ± 4.28 | 3.65 ± 0.83 | 3.55 ± 1.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocci, K.S.; Fonte, S.J.; von Fischer, J.C.; Cotrufo, M.F. Nitrogen Dynamics in an Established Alfalfa Field under Low Biochar Application Rates. Soil Syst. 2019, 3, 77. https://doi.org/10.3390/soilsystems3040077
Rocci KS, Fonte SJ, von Fischer JC, Cotrufo MF. Nitrogen Dynamics in an Established Alfalfa Field under Low Biochar Application Rates. Soil Systems. 2019; 3(4):77. https://doi.org/10.3390/soilsystems3040077
Chicago/Turabian StyleRocci, Katherine S., Steven J. Fonte, Joseph C. von Fischer, and M. Francesca Cotrufo. 2019. "Nitrogen Dynamics in an Established Alfalfa Field under Low Biochar Application Rates" Soil Systems 3, no. 4: 77. https://doi.org/10.3390/soilsystems3040077
APA StyleRocci, K. S., Fonte, S. J., von Fischer, J. C., & Cotrufo, M. F. (2019). Nitrogen Dynamics in an Established Alfalfa Field under Low Biochar Application Rates. Soil Systems, 3(4), 77. https://doi.org/10.3390/soilsystems3040077





