Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimentation and Sample Collection
2.1.1. Pot Studies
2.1.2. Field Studies
2.2. Porewater Collection and Analysis
2.3. Fe Plaque Characterization
2.4. Plant Analysis
2.5. Field Study Soil Fe, Mn, and As Extractions
2.6. Statistical Analyses
3. Results
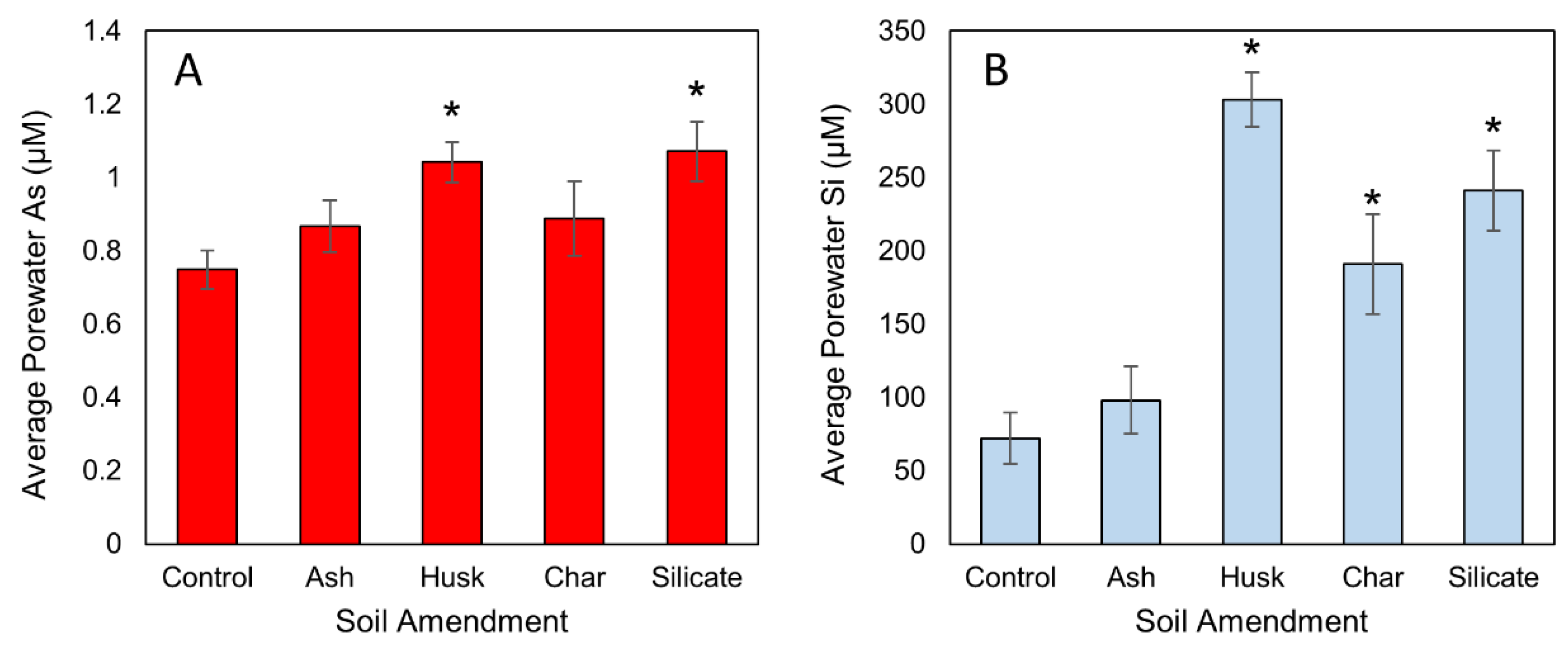
3.1. Influence of Si Amendment on Porewater Si and As Concentrations
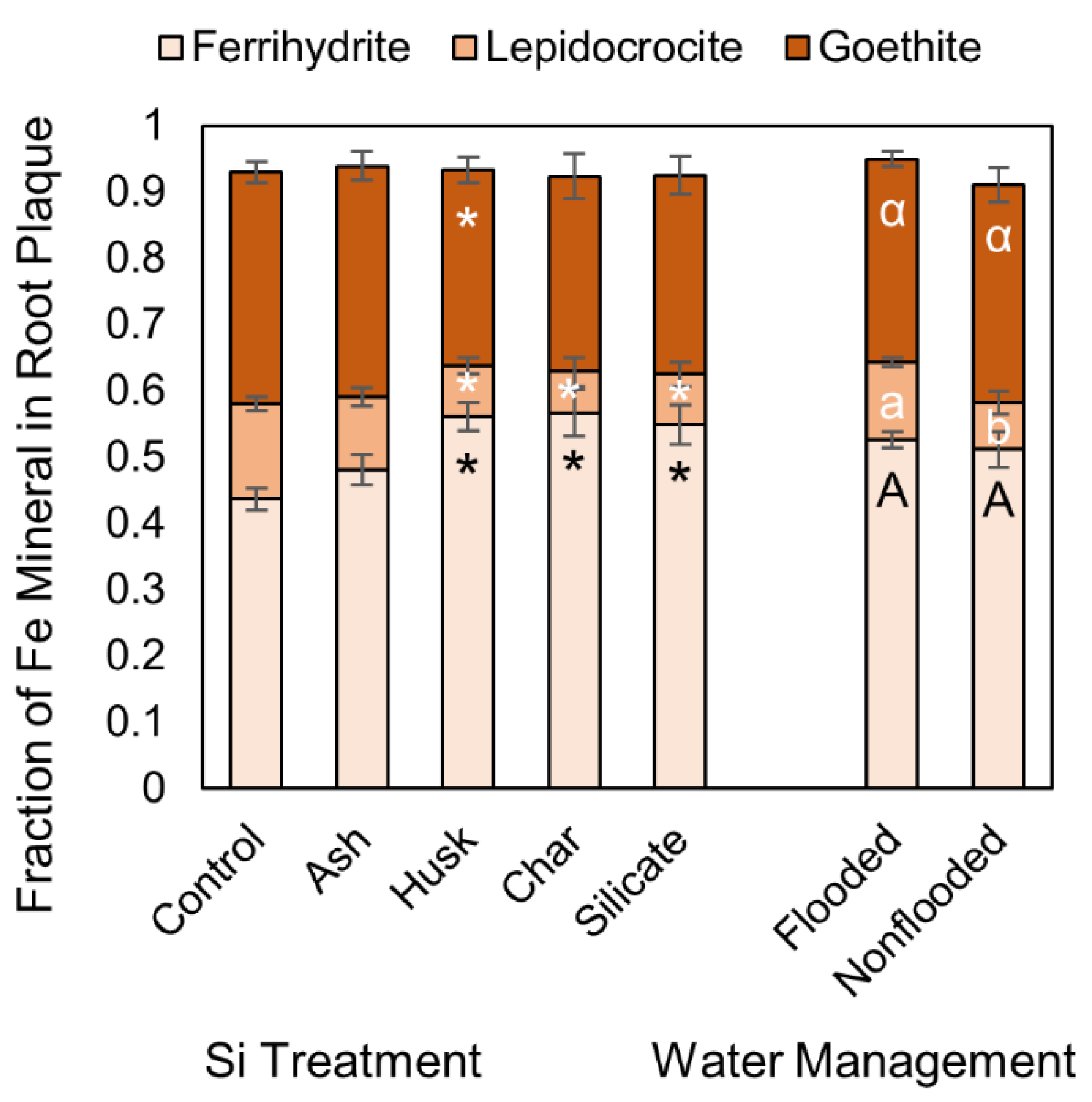
3.2. Impacts of Si and Flooding Extent on Root Fe Plaque
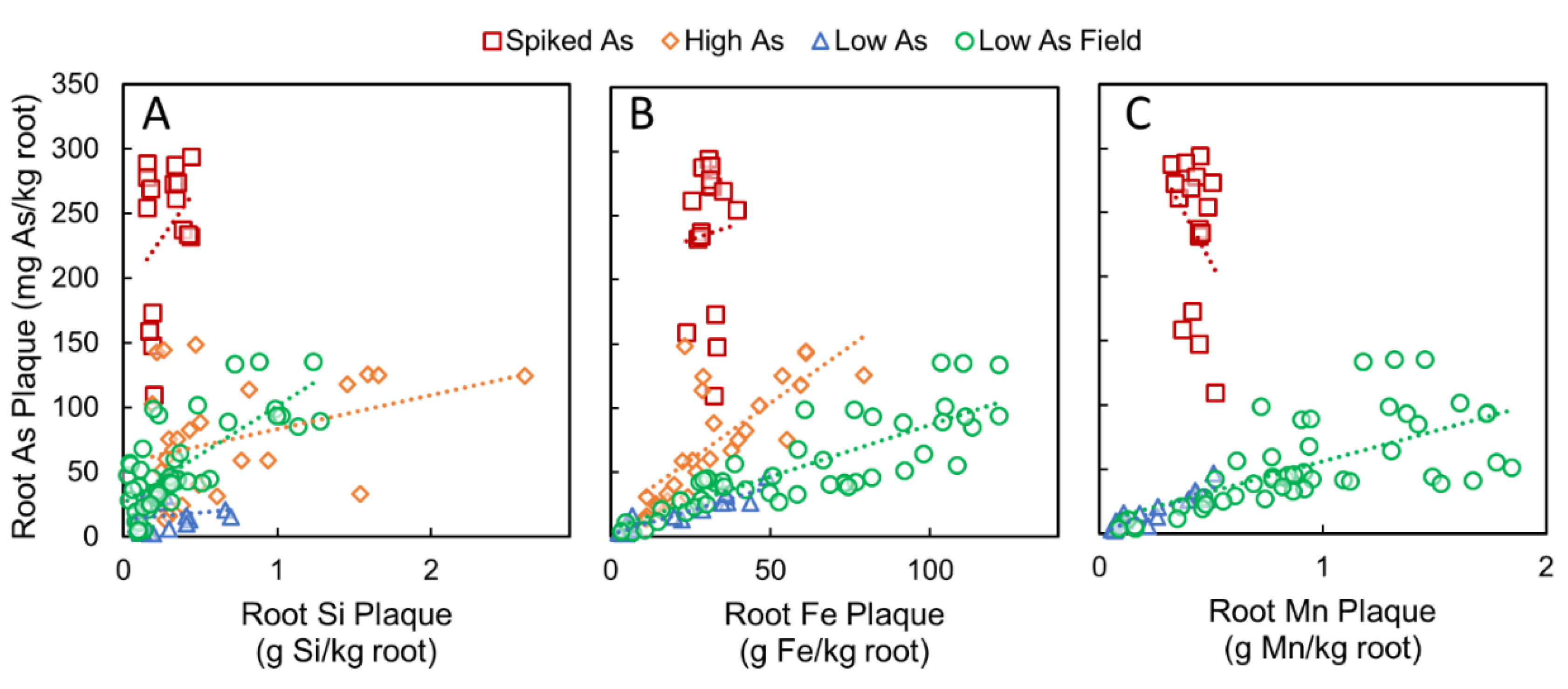
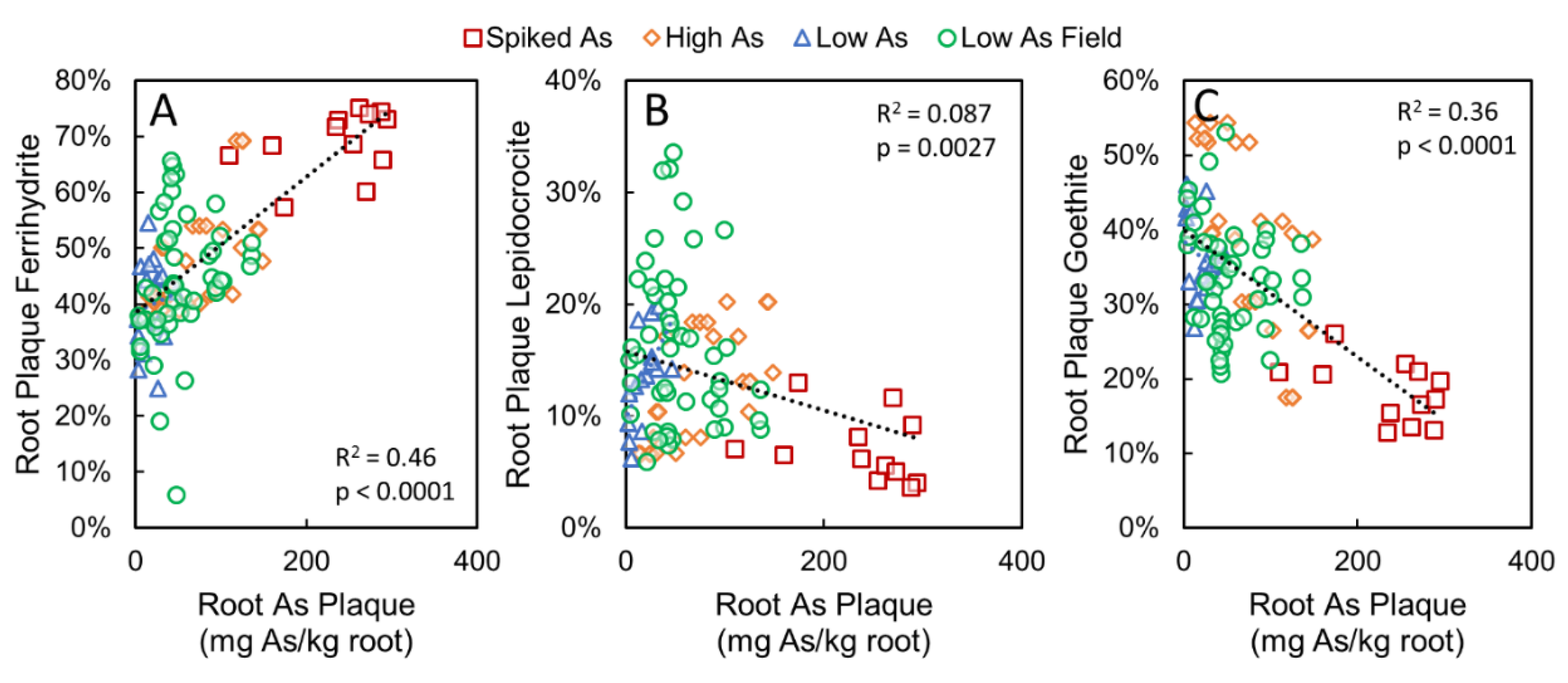
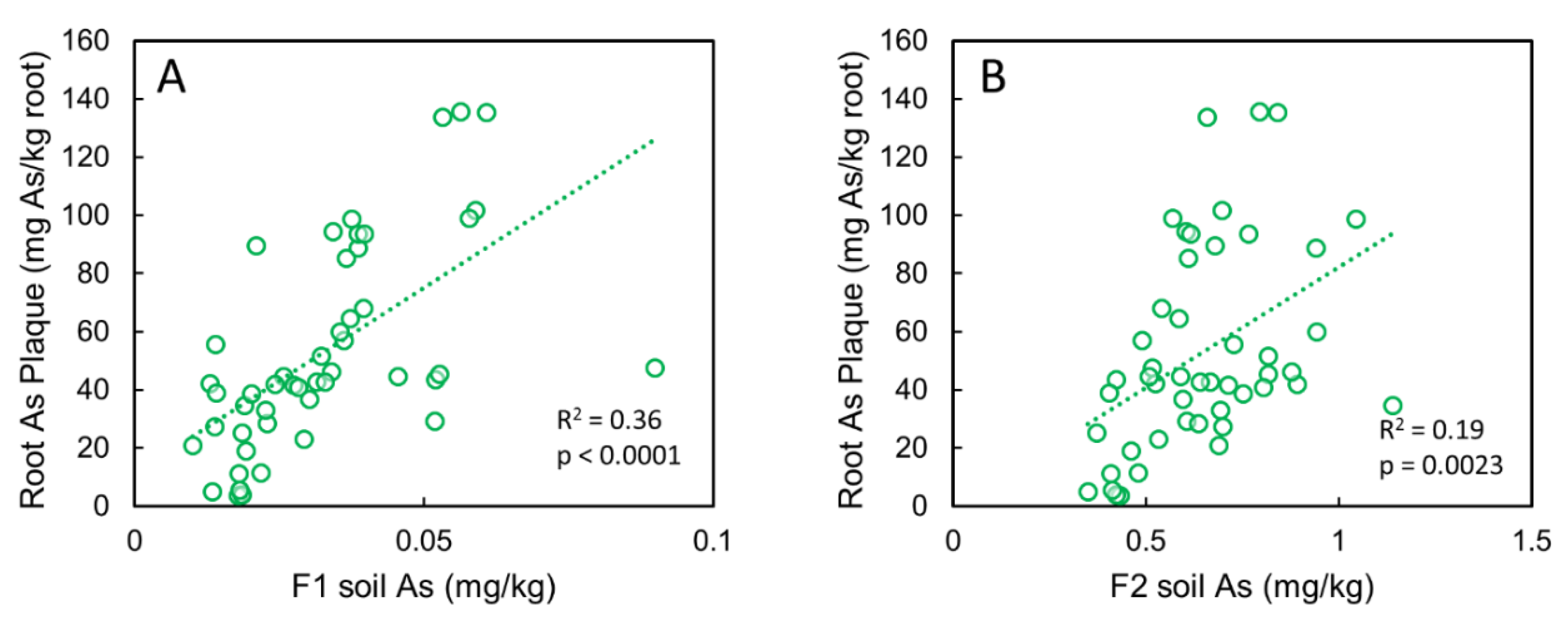
3.3. Correlations between Root Plaque As, Plaque Composition, and Soil As Pools
3.4. Relations between Porewater Si, Oxalate-Extractable Fe in Bulk Soil, and Plant-Available As
3.5. Influence of Soil As Pools on As Porewater Concentrations
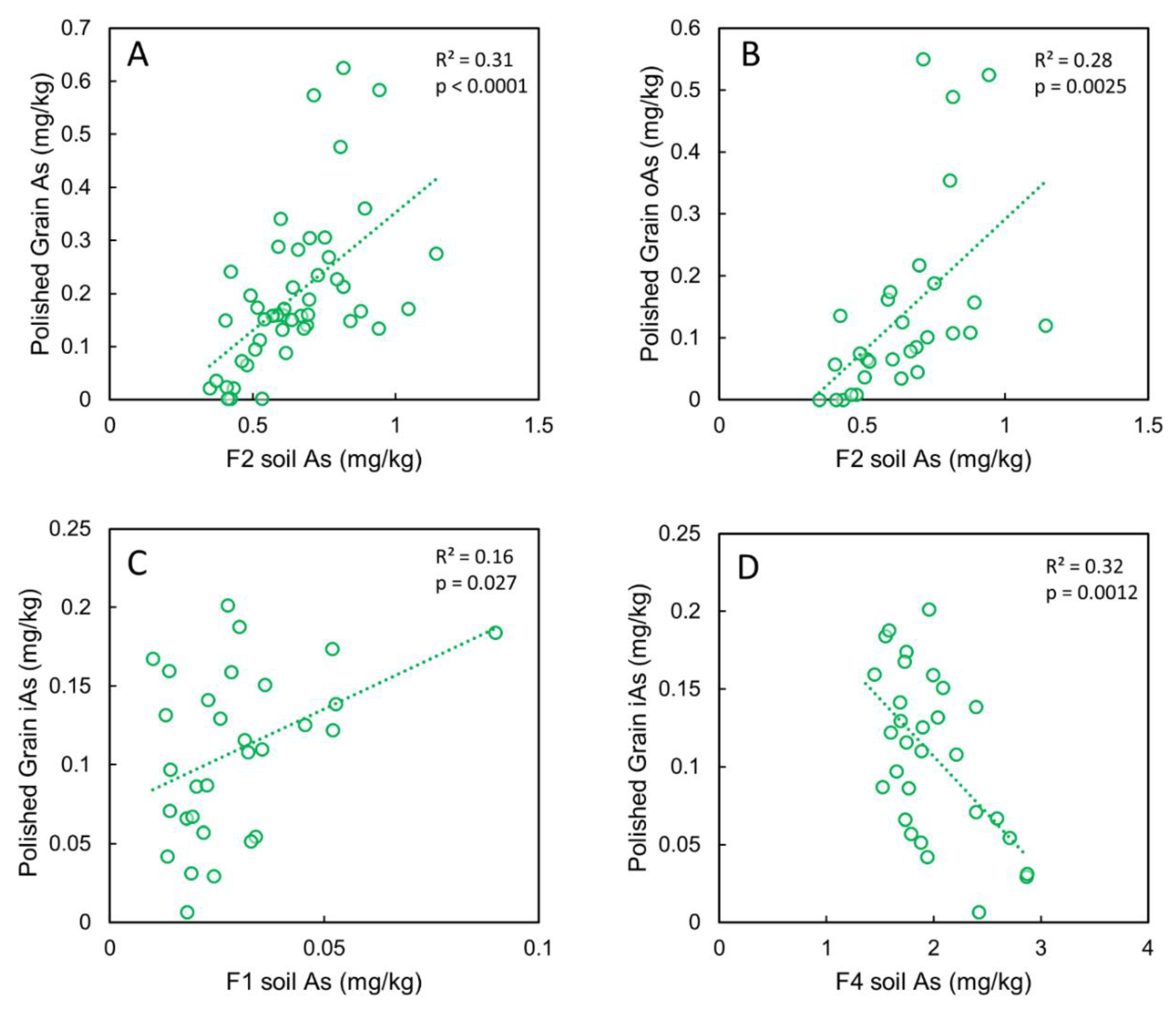
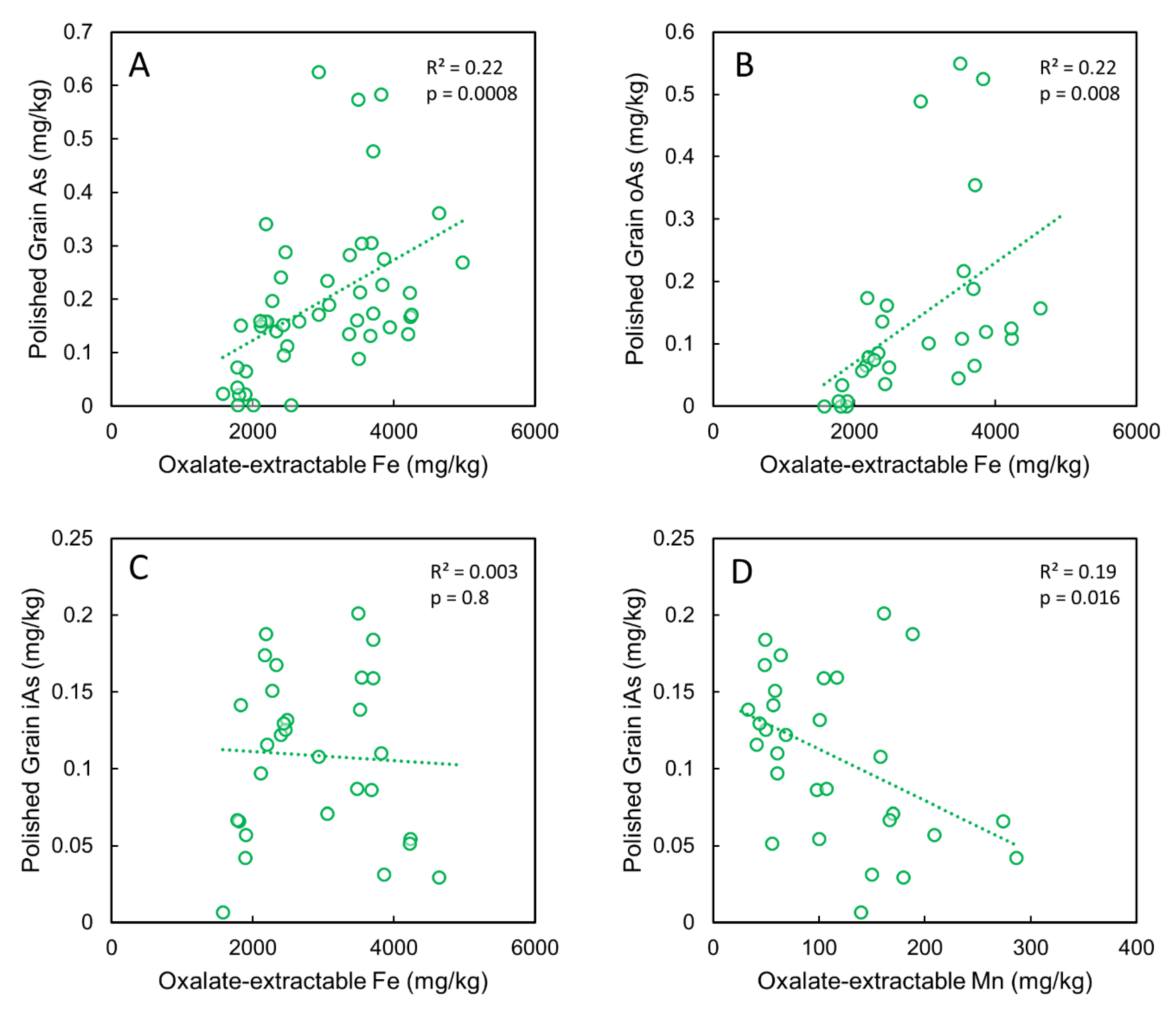
3.6. Correlations between Grain As and Soil As, Fe and Mn Pools
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duxbury, J.M.; Mayer, A.B.; Lauren, J.G.; Hassan, N. Food chain aspects of arsenic contamination in Bangladesh: Effects on quality and productivity of rice. J. Environ. Sci. Health Part A 2003, 38, 61–69. [Google Scholar] [CrossRef]
- Meharg, A.A. Arsenic in rice-understanding a new disaster for South-East Asia. Trends Plant Sci. 2004, 9, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef]
- Panaullah, G.M.; Alam, T.; Hossain, M.B.; Loeppert, R.H.; Lauren, J.G.; Meisner, C.A.; Ahmed, Z.U.; Duxbury, J.M. Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 2009, 317, 31–39. [Google Scholar] [CrossRef]
- Meharg, A.A.; Deacon, C.; Campbell, R.C.; Carey, A.M.; Williams, P.N.; Feldmann, J.; Raab, A. Inorganic arsenic levels in rice milk exceed EU and US drinking water standards. J. Environ. Monit. 2008, 10, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Sun, G.; Williams, P.N.; Adomako, E.; Deacon, C.; Zhu, Y.G.; Feldmann, J.; Raab, A. Inorganic arsenic levels in baby rice are of concern. Environ. Pollut. 2008, 152, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Raab, A. Getting to the bottom of arsenic standards and guidelines. Environ. Sci. Technol. 2010, 44, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Polizzotto, M.L.; Kocar, B.D.; Benner, S.G.; Sampson, M.; Fendorf, S. Near-surface wetland sediments as a source of arsenic release to ground water in Asia. Nature 2008, 454, 505–508. [Google Scholar] [CrossRef]
- Fendorf, S.; Michael, H.A.; Geen, A.V. Spatial and Temporal Variations of Groundwater Arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erban, L.E.; Gorelick, S.M.; Zebker, H.A.; Fendorf, S. Release of arsenic to deep groundwater in the Mekong Delta, Vietnam, linked to pumping-induced land subsidence. Proc. Natl. Acad. Sci. USA 2013, 110, 13751–13756. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, M.V.; Ying, S.C.; Benner, S.G.; Duan, Y.; Wang, Y.; Fendorf, S. Aquifer Arsenic Cycling Induced by Seasonal Hydrologic Changes within the Yangtze River Basin. Environ. Sci. Technol. 2016, 50, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.V.; Guo, X.; Gan, Y.; Benner, S.G.; Griffin, A.M.; Gorski, C.A.; Wang, Y.; Fendorf, S. Redox controls on arsenic enrichment and release from aquifer sediments in central Yangtze River Basin. Geochim. Cosmochim. Acta 2017, 204, 104–119. [Google Scholar] [CrossRef] [Green Version]
- Ying, S.C.; Schaefer, M.V.; Cock-Esteb, A.; Li, J.; Fendorf, S. Depth Stratification Leads to Distinct Zones of Manganese and Arsenic Contaminated Groundwater. Environ. Sci. Technol. 2017, 51, 8926–8932. [Google Scholar] [CrossRef] [PubMed]
- Gillispie, E.C.; Andujar, E.; Polizzotto, M.L. Chemical controls on abiotic and biotic release of geogenic arsenic from Pleistocene aquifer sediments to groundwater. Environ. Sci. Process. Impacts 2016, 18, 1090–1103. [Google Scholar] [CrossRef] [PubMed]
- Gillispie, E.C.; Matteson, A.R.; Duckworth, O.W.; Neumann, R.B.; Phen, N.; Polizzotto, M.L. Chemical variability of sediment and groundwater in a Pleistocene aquifer of Cambodia: Implications for arsenic pollution potential. Geochim. Cosmochim. Acta 2019, 245, 441–458. [Google Scholar] [CrossRef]
- Williams, P.N.; Zhang, H.; Davison, W.; Meharg, A.A.; Hossain, M.; Norton, G.J.; Brammer, H.; Islam, M.R. Organic matter-solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils. Environ. Sci. Technol. 2011, 45, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, C.; Wang, P.; Kretzschmar, R.; Zhao, F.J. Control of arsenic mobilization in paddy soils by manganese and iron oxides. Environ. Pollut. 2017, 231, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Jahiruddin, M.; Panaullah, G.M.; Loeppert, R.H.; Islam, M.R.; Duxbury, J.M. Spatial variability of arsenic concentration in soils and plants, and its relationship with iron, manganese and phosphorus. Environ. Pollut. 2008, 156, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Hering, J.G. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C.T. Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Jain, A.; Raven, K.P.; Loeppert, R.H. Arsenite and arsenate adsorption on ferrihydrite: Surface charge reduction and net OH- release stoichiometry. Environ. Sci. Technol. 1999, 33, 1179–1184. [Google Scholar] [CrossRef]
- Lafferty, B.J.; Loeppert, R.H. Methyl arsenic adsorption and desorption behavior on iron oxides. Environ. Sci. Technol. 2005, 39, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.C.; Kocar, B.D.; Fendorf, S. Oxidation and competitive retention of arsenic between iron- and manganese oxides. Geochim. Cosmochim. Acta 2012, 96, 294–303. [Google Scholar] [CrossRef]
- Armstrong, W. Oxidising activity of roots in waterlogged soils. Physiol. Plant. 1967, 20, 920–926. [Google Scholar] [CrossRef]
- Chen, C.C.; Dixon, J.B.; Turner, F.T. Iron coatings on rice roots-Morphology and models of development. Soil Sci. Soc. Am. J. 1980, 44, 1113–1119. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.G.; Liu, W.J.; Meharg, A.A. Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol. 2005, 165, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.H.; Zhu, Y.G.; Huang, Y.Z.; Hu, H.Q.; Christie, P. Sequestration of As by iron plaque on the roots of three rice (Oryza sativa L.) cultivars in a low-P soil with or without P fertilizer. Environ. Geochem. Health 2005, 27, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Zhu, Y.G.; Smith, F.A. Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 2005, 227, 127–138. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhu, Y.G.; Hu, Y.; Williams, P.N.; Gault, A.G.; Meharg, A.A.; Charnock, J.M.; Smith, F.A. Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ. Sci. Technol. 2006, 40, 5730–5736. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nakamura, T.; Dong, D.; Takahashi, Y.; Amachi, S.; Makino, T. Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere 2011, 83, 925–932. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ohkura, T.; Takahashi, Y.; Maejima, Y.; Arao, T. Arsenic Distribution and Speciation near Rice Roots Influenced by Iron Plaques and Redox Conditions of the Soil Matrix. Environ. Sci. Technol. 2014, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Webb, S.M.; Andrews, J.C.; Fendorf, S. Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L. ) roots having variable Fe coatings. Environ. Sci. Technol. 2010, 44, 8108–8113. [Google Scholar] [PubMed]
- Seyfferth, A.L.; Webb, S.M.; Andrews, J.C.; Fendorf, S. Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochim. Cosmochim. Acta 2011, 75, 6655–6671. [Google Scholar] [CrossRef]
- Amaral, D.C.; Lopes, G.; Guilherme, L.R.; Seyfferth, A.L. A new approach to sampling Iintact Fe plaque reveals Si-induced changes in Fe mineral composition and shoot As in rice. Environ. Sci. Technol. 2017, 51, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Teasley, W.A.; Limmer, M.A.; Seyfferth, A.L. How rice (Oryza sativa L) responds to elevated As under different Si-rich soil amendments. Environ. Sci. Technol. 2017, 51, 10335–10343. [Google Scholar] [CrossRef]
- Limmer, M.A.; Mann, J.; Amaral, D.C.; Vargas, R.; Seyfferth, A.L. Silicon-rich amendments in rice paddies: Effects on arsenic uptake and biogeochemistry. Sci. Total Environ. 2018, 624, 1360–1368. [Google Scholar] [CrossRef]
- Seyfferth, A.L. Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant Soil 2015, 397, 43–61. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Morris, A.H.; Gill, R.; Kearns, K.A.; Mann, J.N.; Paukett, M.; Leskanic, C. Soil incorporation of silica-rich rice husk decreases inorganic arsenic in rice grain. J. Agric. Food Chem. 2016, 64, 3760–3766. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Fendorf, S. Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 13176–13183. [Google Scholar] [CrossRef]
- Schwertmann, U.; Taylor, R.M. Influence of silicate on transformation of lepidocrocite to goethite. Clays Clay Miner. 1972, 20, 159–164. [Google Scholar] [CrossRef]
- Schwertmann, U.; Thalmann, H. Influence of Fe (II), Si, and pH on formation of lepidocrocite and ferrihydrite during oxidation of aqueous FeCl2 solutions. Clay Miner. 1976, 11, 189–200. [Google Scholar] [CrossRef]
- Anderson, P.R.; Benjamin, M.M. Effects of silicon on the crystallization and adsorption properties of ferric oxides. Environ. Sci. Technol. 1985, 19, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Luxton, T.P.; Tadanier, C.J.; Eick, M.J. Mobilization of arsenite by competitive interaction with silicic acid. Soil Sci. Soc. Am. J. 2006, 70, 204–214. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Limmer, M.A.; Dykes, G.E. On the use of silicon as an agronomic mitigation strategy to decrease arsenic uptake by rice. Adv. Agron. 2018, 149, 49–91. [Google Scholar]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Amaral, D.; Limmer, M.A.; Guilherme, L.R. Combined impacts of Si-rich rice residues and flooding extent on grain As and Cd in rice. Environ. Int. 2019, 128, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kraska, J.E.; Breitenbeck, G.A. Simple, Robust Method for Quantifying Silicon in Plant Tissue. Commun.Soil Sci. Plant Anal. 2010, 41, 2075–2085. [Google Scholar] [CrossRef]
- Taylor, G.J.; Crowder, A.A. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am. J. Bot. 1983, 70, 1254–1257. [Google Scholar] [CrossRef]
- Maher, W.; Foster, S.; Krikowa, F.; Donner, E.; Lombi, E. Measurement of inorganic arsenic species in rice after nitric acid extraction by HPLC-ICPMS: Verification using XANES. Environ. Sci. Technol. 2013, 47, 5821–5827. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.P. Fast ion chromatography-ICP-QQQ for arsenic speciation. J. Anal. At. Spectrom. 2015, 30, 1405–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis. Part 3, Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Cornell, M.R.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Ehlert, K.; Mikutta, C.; Kretzschmar, R. Impact of Birnessite on Arsenic and Iron Speciation during Microbial Reduction of Arsenic-Bearing Ferrihydrite. Environ. Sci. Technol. 2014, 48, 11320–11329. [Google Scholar] [CrossRef] [PubMed]
- Ona-Nguema, G.; Morin, G.; Juillot, F.; Calas, G.; Brown, G.E. EXAFS analysis of arsenite adsorption onto two-line ferrihydrite, hematite, goethite, and lepidocrocite. Environ. Sci. Technol. 2005, 39, 9147–9155. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Wu, S.C.; Wu, F.Y.; Deng, H.; Wong, M.H. Effects of root anatomy and Fe plaque on arsenic uptake by rice seedlings grown in solution culture. Environ. Pollut. 2010, 158, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limmer, M.A.; Wise, P.; Dykes, G.E.; Seyfferth, A.L. Silicon decreases dimethylarsinic acid concentration in rice grain and mitigates straighthead disorder. Environ. Sci. Technol. 2018, 52, 4809–4816. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209. [Google Scholar] [CrossRef]
- Garnier, J.M.; Travassac, F.; Lenoble, V.; Rose, J.; Zheng, Y.; Hossain, M.S.; Chowdhury, S.H.; Biswas, A.K.; Ahmed, K.M.; Cheng, Z.; et al. Temporal variations in arsenic uptake by rice plants in Bangladesh: The role of iron plaque in paddy fields irrigated with groundwater. Sci. Total Environ. 2010, 408, 4185–4193. [Google Scholar] [CrossRef] [Green Version]




| Log10 Grain As | Log10 Straw As | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate (SE) | p | Partial R2 | Parameter | Estimate (SE) | p | Partial R2 |
| log10 PW As | 0.45 (0.042) | <0.0001 | 0.56 | log10 PW As | 0.98 (0.039) | <0.0001 | 0.87 |
| Plaque As | 0.0025 (0.00038) | <0.0001 | 0.34 | PW Si | −0.0010 (0.00021) | <0.0001 | 0.19 |
| Plaque Si | −0.00019 (0.000059) | 0.0023 | 0.10 | Plaque lepidocrocite | 1.3 (0.44) | 0.0053 | 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyfferth, A.L.; Limmer, M.; Wu, W. Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice. Soil Syst. 2019, 3, 58. https://doi.org/10.3390/soilsystems3030058
Seyfferth AL, Limmer M, Wu W. Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice. Soil Systems. 2019; 3(3):58. https://doi.org/10.3390/soilsystems3030058
Chicago/Turabian StyleSeyfferth, Angelia L., Matt Limmer, and Weida Wu. 2019. "Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice" Soil Systems 3, no. 3: 58. https://doi.org/10.3390/soilsystems3030058
APA StyleSeyfferth, A. L., Limmer, M., & Wu, W. (2019). Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice. Soil Systems, 3(3), 58. https://doi.org/10.3390/soilsystems3030058






