Abstract
The intensity and frequency of ecosystem disturbances are shifting with climate change, and multiple disturbances in close succession have the potential to compound their independent effects and strongly alter ecosystem structure and function. In this paper, we examine the effects of an extreme precipitation event on a montane forest landscape that was previously decimated by wildfire (37 months prior) relative to an unburned site in the same ecosystem. We assessed responses in soil edaphic properties, bacterial community composition and assembly, and soil enzyme activities involved in carbon (C) and nitrogen (N) acquisition. Our research reveals that previously burned landscapes are susceptible to a subsequent extreme precipitation event via significant increases in soil pH where unburned soils are not. Beta- and Delta-proteobacteria associated with early succession increased and shifts were observed in N- vs. C-acquiring extracellular enzymes within burned soils after the extreme precipitation event. Finally, we connected variation in ecological selective pressures on bacterial communities associated with pH change to these differences in microbial mediated soil enzyme activity. Thus, this research demonstrates how multiple, compounding disturbances drive distinct changes relative to systems experiencing a single disturbance and suggests that changes in bacterial community assembly process with disturbance may underlie this response.
1. Introduction
With the effects of climate change resulting in increased forest wildfire activity in the western United States, large areas of forest landscapes have and will experience high severity fires [1,2,3]. Research efforts not only are expanding our understandings of how fires influence ecosystem properties including soil edaphic properties, microbial community structure, and ecosystem function [4,5,6,7,8,9], but also are better demonstrating how forest ecosystems recover from fire over time [10,11,12]. While efforts to understand fire disturbance are central in our ability to model ecosystem responses and better manage forest ecosystems both before and after fires [13,14,15,16], various other disturbance types are also increasing in frequency, intensity, and scale, ultimately increasing the potential for burned landscapes to encounter multiple disturbances [17,18]. Knowledge of how multiple disturbances compound through time to influence soil biogeochemistry is critical to resolving uncertainty in process-based models.
Forest fires alone modify physical, chemical, and biological soil properties [19,20,21,22,23,24]. These fire-induced changes can be short-term or long-term, depending on the severity and frequency of fire, and post-fire climatic conditions [19,23]. During wildfires soils experience extreme temperatures, ranging from less than 100 °C to well over 400 °C [25], that change soil carbon (C) chemistry, nutrient pools, pH, and erosion potential, among other properties.
In general, fire disrupts soil C and nitrogen (N) pools and cycling by combusting soil organic matter and increasing inorganic N content [22,26,27]. Changes in organic matter (OM) content are dependent on fire type, severity, and slope [28], but in most cases, OM content decreases during a fire. Frequently, a significant portion of available organic N in the soil is also lost through volatilization or mineralization [23]. N mineralization generates inorganic N (primarily ammonium) that enhances nitrification and often creates a delayed pulse of nitrate (NO3−) [23,29,30,31]. In a recent global study of frequently burned sites, research showed overall increases in soil C and N of temperate needleleaf forests over years to decades [19], highlighting the importance of the temporal scale in considering effects of fire disturbance. Generally, the immediate effects of fire disturbance on soil nutrient availability and solubility can alter soil mineralization rates for at least 2 years after a wildfire and, as time proceeds, N and C in the remainder ash fractions are ultimately incorporated into the soil profile [32,33].
Fires also impact soil chemical properties. For example, extreme temperatures lead to organic acid denaturation, production of hydroxides, and the consequent release of bases such as hydroxides and carbonates [34,35] that directly impact the pH of fire affected soil [23,36,37]. Higher pH in burned soils is typically observed due to production and accumulation of ash, as well as loss of organic acids [38,39]. Additionally, fire severity has been shown to impact soil pH differently, whereby pH increases as a function of fire severity [8,40], a factor known also to strongly influence microbial community structure and function [41].
Given that both nutrient availability and pH are major known controls on soil bacterial communities [41,42], fires can have a large impact on microbial community structure and function. Fires alter bacterial communities responsible for C and N cycling through a variety of direct and indirect effects, including destruction of microbial biomass, changes in aboveground communities, and alterations in nutrient pools and pH [6,8,11,12,43,44]. Indeed, the implications of fires on microbial mediated ecosystem function has been seen in studies of microbial extracellular soil enzyme production and potential [10,21,45]. Examination of extracellular soil enzyme potential has been successfully used in post-fire landscapes to understand changes in microbial nutrient limitation and the relative shifts in the cycling of C and N, which are strongly impacted by fire and have been shown to influence subsequent bacterial communities [7,8,10,12].
Fires may also strongly impact physical properties of soils. Burnt soils generally have higher water repellence and bulk soil conductivity, which significantly decreases the permeability and water infiltration capacity of the soil, making the system more susceptible to erosion and higher runoff potential.
In the western mountain regions of the United States (e.g., Colorado Front Range), extreme precipitation events have the potential to compound with fire disturbances in their effects on soils and ecosystems. In these ecosystems post-fire runoff, erosion, and flooding are primarily episodic, mostly dominated by high intensity summer thunderstorms with infiltration excess and overland flow [46,47,48]. A post-fire flood event may lead to surface soil erosion, amplified runoff and ash-dissolution products such as soluble hydroxides and carbonate compounds [49,50] that may also raise soil pH substantially. At the same time rain associated with such events may decrease soil pH, and this too has been observed in recently burned landscapes that encounter extreme precipitation events [51].
Extreme precipitation events coupled to fires result in redistribution of nutrients, further influencing C and N cycles in poorly understood ways [49,52,53,54]. Some work has shown that intense rainfall on fire-affected soils, while influencing other edaphic properties, does not significantly alter total percent nitrogen and total percent carbon content [51].
Although changes in soil properties and bacterial communities after fires have been shown in many studies and the susceptibility of montane forest ecosystems to precipitation events is generally known, the compounding effects of fire and extreme precipitation event disturbances on soil microbial communities and the related nutrient cycling they mediate has been understudied. Although flooding events have been known to create anaerobic soil conditions, aerobic microorganisms have also been found to recover quickly after flooding [55]. With the loss of soil oxygen, facultative bacteria might have the opportunity to shift to an alternative electron acceptor. How legacy effects on soil microorganisms from prior fires may influence responses to secondary extreme precipitation events remains largely unknown.
Despite the expected increase in extreme precipitation events both globally and for the western United States [56,57], the potential for this post-fire disturbance to impact burned landscapes with a secondary disturbance has received relatively little attention. It is well known that burned landscapes are susceptible to erosion and nutrient export from normal precipitation patterns [23,58,59,60], but the relative infrequency of extreme precipitation events in itself limits opportunity to study these disturbances. Furthermore, although some work has examined the effect of extreme precipitation events on forest soil edaphic properties [61,62], little work has sought to assess the effect of multiple disturbances on a more comprehensive view of soils [51]: Edaphic properties, microbiomes, and enzyme activity. Herein, we examine the effects of an extreme precipitation event on edaphic properties and bacterial structure and function in previously burned soils of Colorado montane forest. Our work demonstrates that prior perturbation, disturbance legacy, strongly impacts ecosystem responses to secondary disturbances.
2. Materials and Methods
2.1. Site and Sampling Scheme
Samples were collected in the Four Mile Canyon, Boulder County, CO, USA. Samples for this study were collected 33-months (June 2013) and 37-months (October 2013) after a major wildfire, which ignited in September 2010 (0 months). Soil samples (37-month) were collected approximately one month following an extreme precipitation event that resulted in major rains and flooding. An extreme precipitation event occurred between September 9 and 16, 2013 (36 months after fire), with rainfall exceeding 400 mm in the area during this time [63].
At both sample times, samples were collected from an undisturbed (reference) forest landscape and an adjacent fire-disturbed (burned) landscape. Replicate samples were collected across both of these landscapes, which had similar slope–aspect (northeaster facing) and elevation (~2100 to 2300 masl.). Sampling transects within each plot covered approximately 650 m, and burn and unburned landscapes were located approximately 300 m apart. The site was located in the Fourmile Fire (latitude: 40.036153, longitude: −105.400537); a map is provided in Knelman et al. 2017 [10]. Forests in both the reference and burned sites were comprised of Ponderosa pine and Douglas fir trees, and all trees in the burned site exhibited high fire severity burn (all canopy trees killed and needles consumed). Understory vegetation was present at both sampling time points with sampling that occurred within a single growing season; qualitatively no major changes were observed in composition.
Ten replicates for both burned and forested undisturbed soils were collected before and after the extreme precipitation event at a depth of 5 cm, avoiding belowground plant material. In reference site samples, the organic layer was removed prior to sampling; no organic layer was present in burned soils, although any vegetation debris was removed. Areas that showed clear physical perturbation after the extreme precipitation event were avoided; sampled areas still had intact understory vegetation that was qualitatively similar in composition to before the extreme precipitation event. Aseptic technique was used to collect soils; implements were alcohol washed between sampling. Samples were collected from ~1 m from the base of either a living (reference samples) or dead (burned samples) tree. At both of the sampled time points the burned soils were revegetated with understory herbaceous plants introduced by seeding.
All samples were processed according to the site description and collection methods enumerated in Ferrenberg et al. (2013) [9]. Briefly, samples were transported back to labs at the Institute of Arctic and Alpine Research within 3 h of sampling completion. Soils were sieved through a 2 mm mesh size and a subsample of each replicate sample was stored in a −70 °C freezer for molecular analysis or at 4 °C for enzyme analysis and soil chemistry assays.
2.2. Soil Analyses
Soil moisture, pH, percent nitrogen (%N), and percent carbon (%C) were measured for all samples. A subsample of each soil was dried at 100 °C for 48 h to determine gravimetric soil moisture and all edaphic properties were reported on a dry weight basis. Dried soils of all samples were ground with pestle and mortar and 50 mg were packed into tin capsules for %C and %N analysis using a Thermo Finnigan EA 1112 Series Flash Elemental Analyzer; (Thermo Fisher Scientific, Inc., Waltham, MA, USA) [64]. Atropine analytical standards were used for calibration and generating a standard curve. Soil pH was measured with a ratio of 2 mg dry soil: 4 mL water for all samples. Soil slurries were shaken at 250 rpm for one hour, allowed to equilibrate, and then measured on a pH meter.
2.3. DNA Extraction, PCR, and Sequencing
DNA was extracted using MoBio’s PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA), according to the manufacturer’s protocol and final DNA was eluted in TE buffer. PCR was performed on all samples and negative controls using 515F/806R primers, targeting the V4 region of the 16S rRNA. The forward primer was comprised of the 5′ Illumina adaptor, a forward primer pad, forward primer linker, and forward 515 primer. The reverse primer was comprised of the reverse complement of the 3′ adaptor, golay barcode, reverse primer pad, reverse primer linker, and the reverse 806 primer. PCR was run with a reaction mixture of 25.0 μL including 8.6 μL PCR Grade H2O, 12.5 μL FideliTaq Master Mix, 1.0 μL Primer 515 192 F (10 μM), 1.0 μL Primer 806R (10 μM), 0.11 μL MgCl2 (25 mM), and 2.79 μL template DNA (all samples normalized to 3.583 ng/μL). Reaction mixtures were amplified in triplicate for each sample with an initial denaturation at 94 °C for 2 min and then 25 cycles of 94 °C for 45 s, 50 °C for 60 s, and 68 °C for 45 s. A final extension of 5 min at 68 °C concluded all reactions. To eliminate primer dimer contamination, barcoded PCR product was purified using a QIAquick Gel Extraction Kit, according to the manufacturer’s protocol. DNA purity and quality was determined on a NanoDrop800 and sample DNA concentration was determined using the PicoGreen method on a microplate reader according to the manufacturer’s protocol. Samples, including negative controls, were pooled and purified using the UltraClean PCR Clean-up Kit, according to the manufacturer’s protocol. This final multiplexed DNA was sequenced at the University of Colorado (BioFrontiers Institute, Boulder, CO, USA) on an Illumina MiSeq with the MiSeq Reagent Kit v2, 300 cycles.
2.4. Enzyme Analysis
Enzyme activity was measured via fluorometric microplate methods [65,66] for β-1,4-glucosidase (BG), and β -1,4-N-acetylglucosaminidase (NAG). The methods were employed using a 96-well assay plate method setup with 1M sodium acetate buffer titrated to a pH of 7, and 4-methylumbelliferone standards [66,67]. Each sample was prepared from ~1 g of refrigerated soil mixed in buffer with a tissue homogenizer for uniformity. Each sample was run with 16 analytical replicates, quench corrections, standards, and negative controls. Fluorescence was measured on a microplate reader (Thermo Labsystems, Franklin, MA, USA) at 365 nm excitation and 460 nm emission to calculate nmol activity h−1 g soil−1.
2.5. Ecological Null Models
We implemented null modeling methodology developed by Stegen et al. (2012, 2015) [68,69] using R software (http://cran.r-project.org/) to disentangle community assembly processes [68,69]. To evaluate the strength of selection, pairwise phylogenetic turnover between communities was calculated using the mean-nearest-taxon-distance (βMNTD) metric in the R package ‘picante’. For comparison, a null distribution of βMNTD values was generated by calculating pairwise βMNTD values from 999 randomizations, in which species were shuffled across phylogenetic tips. Communities were evaluated for significantly less turnover than expected (βNTI < −2, homogeneous selection) or more turnover than expected (βNTI > 2, variable selection) by comparing observed βMNTD values to the mean of a null distribution of βMNTD values—and normalizing by its standard deviation—to yield βNTI [68]. Significance levels for βNTI are based on standard deviations—|βNTI| = 2 denotes two standard deviations from the mean of the null distribution. Inferences from βNTI have previously been shown to be robust [69,70,71,72].
2.6. Sequence and Statistical Analysis
UPARSE [73] and QIIME [74] software packages were used for sequence processing and analysis according to Knelman et al. (2015) [8]. Sequences quality filtered, chimera checked, picked for OTUs at a 97% identity level, and assembled into OTU tables by using UPARSE. OTU tables were rarefied to 13,000 sequences (the minimum sequencing depth for a given sample) for each sample. We used a closed reference OTU picking method with the uclust algorithm (Edgar 2010) and a 97% identity threshold against the Greengenes gg_13_5_otus file in QIIME to create a single OTU table. The relative abundances of major taxa (phyla level) were calculated. A Bray–Curtis dissimilarity matrix was generated including all samples.
The pgirmess and vegan packages in the R statistical environment (R Development Core Team 2013) was used to analyze data. All data were first evaluated for conformance with normal distribution before data analysis using the Shapiro-Wilk test. Accordingly, soil properties and bacterial phyla relative abundances were analyzed for significant differences between timepoints within burned/reference forests using Analysis of Variance (ANOVA) Tukey’s HSD or Kruskal–Wallis tests. Pearson Product–Moment Correlations were calculated among factors that were observed to change between pre and post-flood and major bacterial taxa as well as BG:NAG ratio. Differences in bacterial community composition were evaluated by applying the adonis Permutational ANOVA (PermANOVA) function to a dissimilarity matrix based on the Bray–Curtis metric in the R vegan package.
Trends in pH across successional periods were evaluated by combining the time points presented here with data collected at 1 month and 4 months from identical soils as reported by Ferrenberg et al. (2013) and Knelman et al. (2017) [9,10]. Quadratic regressions were fit to pH values from all before extreme precipitation event time points (up to 33 months) in burned vs. reference soils separately to determine successional trends in pH. Expected pH for burned and reference sites at 37 months was calculated by extrapolating linear models from 4 months to 33 months to predict 37-month pH values for an undisturbed successional trajectory. Observed pH values were compared to predicted values using one-sample t-tests.
Relationships between environmental change, extracellular enzyme activities, and ecological selection were evaluated by comparing pairwise differences in edaphic properties, BG, NAG, and BG:NAG to associated βNTI values using Mantel tests in the “vegan” package (999 permutations).
2.7. Data Availability
All data used for this study are contained in metadata, sequences, and mapping files made available at the DOI: https://doi.org/10.6084/m9.figshare.1556158.v1.
3. Results
3.1. Extracellular Enzyme Activities
BG:NAG ratios in burned soils significantly declined between 33- and 37-month soils, indicating a relative shift toward N cycling potential in these soils, while reference soils showed no statistically significant change (Table 1). Individually, extracellular enzyme activity related to carbon (BG) and nitrogen (NAG) cycling did not show significant differences between 33- and 37-month soils in either burned or unburned soils.

Table 1.
Edaphic property and enzyme activity means and standard deviation for both burned and reference forest soils, before and after extreme precipitation event. Letters denote significant differences as per Tukey’s HSD (All variables, except soil moisture) and Kruskal-Wallis contrasts (only soil moisture) to test differences before and after.
3.2. Soil Properties
Both reference and burned soils showed significant increases in soil moisture from before (33 month) to after (37 month) the extreme precipitation event (Table 1). Outside of this change, reference soils did not show any significant differences in measured edaphic properties across the two timepoints. Burned soils, however, also showed significant increases in pH between timepoints (Table 1). This increase in pH was inconsistent with background post-burn successional patterns observed at this site (immediate increase post fire, and declines over subsequent time) as well as the general trend in reference forests (Figure 1, one-sample t-test p = 0.002). After extreme precipitation event pH in reference forests was slightly below expectations (one-sample t-test p = 0.04).
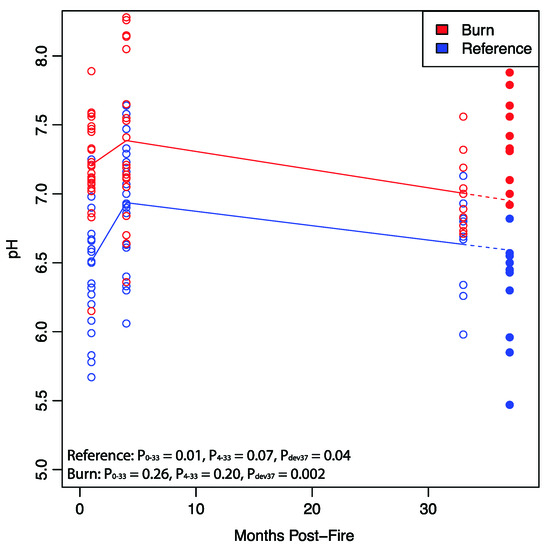
Figure 1.
Post-extreme precipitation event burned Soil pH differs from successional trajectory. Quadratic regressions revealed a decrease in reference soil pH from 4 to 33 months post-fire after an initial increase from 0 to 4 months. Burned soils followed a similar trajectory, though pH in burned soils was more variable. Subsequent to the extreme precipitation event, burned soils had higher pH relative to expectations while reference soils experienced a slight decrease in pH.
3.3. Soil Bacterial Communities
PermANOVA analysis did not reveal differences in bacterial community dissimilarities (Bray–Curtis) according to burned vs. reference or to pre vs. post-extreme precipitation event. However, the interaction between the two factors was significant (Table 2). At the phyla level, burned soils experienced significant shifts in three major bacterial taxa from before to after the extreme precipitation event: Significant increases in relative abundances of Betaproteobacteria, and Deltaproteobacteria, and significant declines in the relative abundance of Alphaproteobacteria (Table 3). Reference soils did not show shifts in any of these bacterial taxa, but did show a significant decrease in Actinobacteria between the two sampling dates (Table 3).

Table 2.
Results from PERMANOVA analysis of bacterial community composition differences as per burned/reference forest soils, before/after extreme precipitation event. Analysis based on Bray–Curtis distance among all samples.

Table 3.
Bacterial phyla relative abundance means and standard deviation for both burned and reference forest soils, before and after the extreme precipitation event. Letters denote significant differences based on Tukey’s HSD (P < 0.05) to test differences before and after extreme precipitation event within burned and reference soils.
3.4. Soil Properties and Bacterial Community Structure and Function
For those bacterial phyla that significantly changed in relative abundance between timepoints within burned and unburned soils respectively, correlations were examined with edaphic properties of moisture and pH, which showed significant changes between the two timepoints themselves. Across all burned soil bacterial communities before and after the extreme precipitation event, correlations were found between bacterial taxa that changed significantly and soil moisture: Alphaproteobacteria (r = −0.598, p < 0.05), Betaproteobacteria (r = 0.693, p < 0.05), and Deltaproteobacteria (r = 0.529, p < 0.05).
3.5. Ecological Selection and Relationship to Soil Biogeochemistry
All bacterial communities were dominated by similar selective pressures (homogenous selection (βNTI < −2) 89.3%). Importantly, changes in microbial selection corresponded to after extreme precipitation event shifts in pH and enzyme activity in burned soils. Pairwise differences in pH (p = 0.001, Mantel r = 0.22), BG (p = 0.001, r = 0.27), NAG (p = 0.001, r = 0.42), and BG:NAG (p = 0.001, r = 0.33) positively correlated with βNTI values, demonstrating a linear divergence in selective pressures as differences in pH and enzyme activities increased across soils (Figure 2).
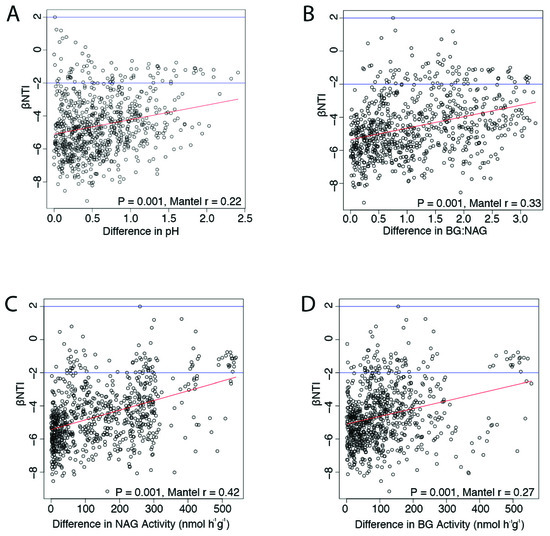
Figure 2.
Changes in selective pressures correspond to soil pH and microbial enzyme activities. Ecological null models were used to infer pairwise differences in selection across soil bacterial communities (βNTI). βNTI values below -2 indicated homogeneous selective pressures between samples, while βNTI greater than 2 indicated statistically dissimilar selective pressures between two samples. Therefore, positive correlations between βNTI and differences in soil or bacterial properties indicated that selective pressures diverged as these differences increased. We found that differences in (A) pH, (B) β-1,4-glucosidase (BG):β -1,4-N-acetylglucosaminidase (NAG), (C) NAG, and (D) BG across samples corresponded to increasingly different selective pressures, thereby linking edaphic properties, changes in ecological assembly processes, and microbial investment in carbon (C)- vs. nitrogen (N)-acquiring enzymes.
4. Discussion
4.1. Soil Property Responses to Compounding Disturbance
With climatic changes and shifts in disturbance regimes, research is increasingly uncovering the implications of multiple, interacting disturbances [13,14,75,76,77]. In this work we show how montane landscapes that have previously experienced fire disturbance respond to extreme precipitation events in soil edaphic properties, bacterial community assembly, and ecosystem process (C and N cycling) as compared to adjacent unburned forest soils. Overall, our work shows that post-fire soils experience increases in pH after an extreme precipitation event, which importantly alters selective pressures on bacterial communities and their investment in C and N cycling activities. While we establish a coordinated shift in selection on bacterial communities and C and N-cycling enzymes, we also acknowledge that these shifts may relate in part to changes in resource environments, for example leaching of nitrate with extreme precipitation. While we acknowledge that a variety of other unmeasured edaphic properties, such as charcoal content or carbon/nitrogen chemistry etc., may also respond to compounding disturbance with implications for microbial communities and requires further study, in this paper we focus on core edaphic properties that are well known to effect bacterial community structure and function.
Significant increases in soil moisture were, unsurprisingly, observed in both burned and unburned soils. Yet, burned soils also saw a statistically significant increase in pH, while unburned soils did not (Table 1). Although it is possible that this observed change in pH is simply due to successional processes associated with post-fire soil succession outside of the extreme precipitation event, pH in burned soils deviated from pH values predicted from successional trajectories (Figure 1). Furthermore, previous work at this site along with wide-ranging studies of post-fire succession have shown that after an initial spike in pH associated with fire, post-fire soils see a decline in soil pH over time [10,23] (Figure 1). Overall, previous work at this site and at others strongly suggest that the observed increases in pH result directly from the extreme precipitation event rather than processes of post-fire succession where declines in pH are typical over time [9,10,22,23].
4.2. Bacterial Community Responses to Compounding Disturbance
We also show that changes in bacterial communities are influenced by the interaction of fire disturbance history with the extreme precipitation event. While it is interesting that bacterial taxa that shifted in relative abundance before and after the extreme precipitation event generally showed correlations with increases in soil moisture, a factor associated with extreme precipitation event, we cannot disentangle confounding, cooccurring variables associated with disturbance that likely accompany these changes in soil moisture. Interestingly, Betaproteobacteria, which increased most strongly in burned soils between pre- and post- precipitation timepoints, are known to be associated with highly disturbed, early successional environments, whereas Alphaproteobacteria, which decline, have been associated with later stages in succession [9,78,79,80]. Thus, the secondary disturbance may create conditions that favor early successional taxa once again. More generally, past work has shown all of these taxa are known to be dynamic in the context of fire disturbed soils [44,81,82]. Additionally, although little variation in overall community structure was independently explained by burn history or by the precipitation event, the interaction of burn history and precipitation significantly influenced bacterial communities (Table 2). That is, whether bacterial community structure differed before and after the extreme precipitation depended on whether the soil was unburned or burned, supporting the interpretation that the fire disturbance context interacts with the extreme precipitation event to impact bacterial community structure.
Interestingly, changes in soil pH corresponded to shifts in bacterial selection that altered microbial investment in C- vs. N-acquiring enzymes. Overall, selective pressures on bacterial communities were similar in burned and reference soils, as over 89% of assembly processes were categorized as homogeneous selection. Given the close spatial proximity and the similarity in many edaphic properties, homogeneous selection appears to be dictated by environmental conditions including unmeasured variables such as soil mineralogy and physical matrix structure. However, selective pressures on bacterial communities diverged with differences in pH across soils (Figure 2). Fire and extreme precipitation events can each impact soil pH values, respectively through the release of bases such as hydroxides and carbonates [23,34,35,36,37] and amplified run-off [49,50]; and, we show that such changes propagate into shift in soil microbiomes. We also linked selective pressures directly to changes in microbial C- and N-cycling activities, demonstrating connectivity between edaphic properties, soil microbiomes, and ecosystem function. We observed that divergences in selective pressures between soils corresponded to similar divergences in rates of BG, NAG, and BG:NAG activities.
This study suggests the possibility that shifts in assembly processes with disturbance may contribute to differences in enzyme activity, though we also acknowledge that other factors, including resource controls and changes in fungal community composition may also have an impact [12]. While this work does not claim to comprehensively describe the mechanism by which this ecosystem responds to compounding disturbance, this work posits the need to better understand shifts in assembly processes after multiple disturbances and their connection to function in these contexts.
Additionally, while we note that the single site nature of this study limits the generalizability of these results, these very types of studies across temporal scales have long been testing grounds for important advancements in ecological theory [67,78,83,84,85,86,87]. Overall, we propose that increases in pH in burned soils experiencing an extreme precipitation event influence the selective pressures on bacterial communities that in turn may alter microbial enzyme allocation to C- and N-cycling processes (Figure 3), yet further research is needed. We intend for the proposed conceptual model to spur consideration of how changes in selective pressures on assembly of microbial communities may shift with compounding disturbance with implication for ecosystem structure and function.
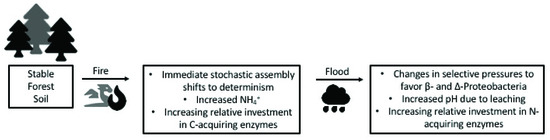
Figure 3.
The proposed conceptual model synthesizes this research and previous work at this site, noting the mechanistic underpinnings of multiple, compounding disturbances on soil edaphic properties and bacterial community assembly process and function through secondary succession.
Overall, our work indicates that the unique susceptibility of post-fire soil pH to an extreme precipitation event may have cascading effects on microbial community assembly and related biogeochemical function. This research supports the idea that disturbed ecosystems may be more susceptible to further perturbations through compounding disturbances [88].
5. Conclusions
This work suggests the susceptibility of recently burned soils (~3 years since burn) to extreme precipitation events in contrast to unburned forest soils. While consistent with other work that shows a response in soil pH in similar contexts of fire and extreme precipitation [51], differences in the directionality and magnitude of the observed soil response from previous work suggests the need to better understand how specifics of the fire (e.g., severity) and the extreme precipitation event (e.g., timing after fire) may ultimately moderate this response in soil pH. Beyond susceptibility in edaphic properties, our research importantly describes how soil bacterial community assembly and associated biogeochemistry (soil enzyme potential) is altered by this shift in pH. While more research is needed to examine how the findings at this site may be generalizable or not, this work highlights the importance of better understanding ecosystem responses to compounding disturbances, a topic of particular importance with shifting disturbance regimes now occurring alongside global climatic changes. As a whole, our work demonstrates that understanding how coupled disturbances interact in governing soil biogeochemistry has the potential to improve predictions of future ecosystem function.
Author Contributions
Conceptualization, J.E.K., S.K.S., V.G.-C., S.K. and E.B.G.; Data curation, J.E.K.; Formal analysis, J.E.K., and E.B.G.; Funding acquisition, S.K.S.; Investigation, J.E.K., S.K.S., and E.B.G.; Resources, J.E.K., S.K.S., and E.B.G.; Supervision, E.B.G.; Writing—original draft, J.E.K., V.G.-C., S.K., and E.B.G. Writing—review and editing, J.E.K., S.K.S., V.G.-C., S.K., and E.B.G.
Funding
The research was supported by the National Science Foundation of the USA through grant DEB-1258160 to Diana Nemergut and SKS.
Acknowledgments
This work was truly facilitated and motivated by Diana Nemergut—who we keep in our heads and hearts and who is ever-present in our continued work. We also acknowledge support from supported by the US Department of Energy (DOE), Office of Biological and Environmental Research (BER), as part of Subsurface Biogeochemical Research Program's Scientific Focus Area (SFA) at the Pacific Northwest National Laboratory (PNNL). PNNL is operated for DOE by Battelle under contract DE‐AC06‐76RLO 1830. We thank Janet Prevéy for insights on reseeding and equipment retrieval attempts at the field site. We appreciate the expertise of Holly Hughes in analytical chemistry. We are grateful to both Pam Katcha, Winnetka Garden Club, and Suzanne Knelman of Farfellow Farms, for ongoing conversations on the topic of soil fertility.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Westerling, A.L. Increasing western US forest wildfire activity: Sensitivity to changes in the timing of spring. Philos. Trans. R. Soc. B 2016, 371, 20150178. [Google Scholar] [CrossRef] [PubMed]
- Gergel, D.R.; Nijssen, B.; Abatzoglou, J.T.; Lettenmaier, D.P.; Stumbaugh, M.R. Effects of climate change on snowpack and fire potential in the western USA. Clim. Chang. 2017, 141, 287–299. [Google Scholar] [CrossRef]
- Rocca, M.E.; Brown, P.M.; MacDonald, L.H.; Carrico, C.M. Climate change impacts on fire regimes and key ecosystem services in Rocky Mountain forests. For. Ecol. Manag. 2014, 327, 290–305. [Google Scholar] [CrossRef]
- Miesel, J.R.; Boerner, R.E.J.; Skinner, C.N. Soil nitrogen mineralization and enzymatic activities in fire and fire surrogate treatments in California. Can. J. Soil Sci. 2011, 91, 935–946. [Google Scholar] [CrossRef]
- Boerner, R.E.J.; Giai, C.; Huang, J.; Miesel, J.R. Initial effects of fire and mechanical thinning on soil enzyme activity and nitrogen transformations in eight North American forest ecosystems. Soil Biol. Biochem. 2008, 40, 3076–3085. [Google Scholar] [CrossRef]
- Docherty, K.M.; Balser, T.C.; Bohannan, B.J.M.; Gutknecht, J.L.M. Soil microbial responses to fire and interacting global change factors in a California annual grassland. Biogeochemistry 2012, 109, 63–83. [Google Scholar] [CrossRef]
- López-Poma, R.; Bautista, S. Plant regeneration functional groups modulate the response to fire of soil enzyme activities in a Mediterranean shrubland. Soil Biol. Biochem. 2014, 79, 5–13. [Google Scholar] [CrossRef]
- Knelman, J.E.; Graham, E.B.; Trahan, N.A.; Schmidt, S.K.; Nemergut, D.R. Fire severity shapes plant colonization effects on bacterial community structure, microbial biomass, and soil enzyme activity in secondary succession of a burned forest. Soil Biol. Biochem. 2015, 90, 161–168. [Google Scholar] [CrossRef]
- Ferrenberg, S.; O’Neill, S.P.; Knelman, J.E.; Todd, B.; Duggan, S.; Bradley, D.; Robinson, T.; Schmidt, S.K.; Townsend, A.R.; Williams, M.W.; et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 2013, 7, 1102–1111. [Google Scholar] [CrossRef]
- Knelman, J.E.; Graham, E.B.; Ferrenberg, S.; Lecoeuvre, A.; Labrado, A.; Darcy, J.L.; Nemergut, D.R.; Schmidt, S.K. Rapid Shifts in Soil Nutrients and Decomposition Enzyme Activity in Early Succession Following Forest Fire. Forests 2017, 8, 347. [Google Scholar] [CrossRef]
- Treseder, K.K.; Mack, M.C.; Cross, A. Relationships Among Fires, Fungi, and Soil Dynamics in Alaskan Boreal Forests. Ecol. Appl. 2004, 14, 1826–1838. [Google Scholar] [CrossRef]
- Holden, S.R.; Gutierrez, A.; Treseder, K.K. Changes in Soil Fungal Communities, Extracellular Enzyme Activities, and Litter Decomposition Across a Fire Chronosequence in Alaskan Boreal Forests. Ecosystems 2013, 16, 34–46. [Google Scholar] [CrossRef]
- Bradford, J.B.; Fraver, S.; Milo, A.M.; D’Amato, A.W.; Palik, B.; Shinneman, D.J. Effects of multiple interacting disturbances and salvage logging on forest carbon stocks. For. Ecol. Manag. 2012, 267, 209–214. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate Change and Forest DisturbancesClimate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Graham, E.B.; Wieder, W.R.; Leff, J.W.; Weintraub, S.R.; Townsend, A.R.; Cleveland, C.C.; Philippot, L.; Nemergut, D.R. Do we need to understand microbial communities to predict ecosystem function? A comparison of statistical models of nitrogen cycling processes. Soil Biol. Biochem. 2014, 68, 279–282. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E.; Schindlbacher, A.; Siciliano, S.; Breulmann, M.; Yannarell, A.; Beman, J.M.; Abell, G.; Philippot, L.; Prosser, J.; et al. Microbes as Engines of Ecosystem Function: When Does Community Structure Enhance Predictions of Ecosystem Processes? Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and landscape dynamics in a changing world1. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Buma, B.; Wessman, C.A. Disturbance interactions can impact resilience mechanisms of forests. Ecosphere 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Hedin, L.O.; Staver, A.C.; Govender, N. Fire alters ecosystem carbon and nutrients but not plant nutrient stoichiometry or composition in tropical savanna. Ecology 2015, 96, 1275–1285. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Hobbie, S.E.; Reich, P.B.; Jumpponen, A.; Brookshire, E.N.J.; Caprio, A.C.; Coetsee, C.; Jackson, R.B. Top-down and bottom-up controls on soil carbon and nitrogen cycling with repeated burning across four ecosystems. bioRxiv 2019, 565135. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Arcenegui, V.; Mataix-Solera, J.; Guerrero, C.; Zornoza, R.; Mayoral, A.M.; Morales, J. Factors controlling the water repellency induced by fire in calcareous Mediterranean forest soils. Eur. J. Soil Sci. 2007, 58, 1254–1259. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; Kobziar, L.N. The myth of the biological threshold: A review of biological responses to soil heating associated with wildland fire. For. Ecol. Manag. 2019, 432, 1022–1029. [Google Scholar] [CrossRef]
- Belillas, C.M.; Feller, M.C. Relationships Between Fire Severity and Atmospheric and Leaching Nutrient Losses in British Columbia’s Coastal Western Hemlock Zone Forests. Int. J. Wildland Fire 1998, 8, 87–101. [Google Scholar] [CrossRef]
- Newland, J.A.; DeLuca, T.H. Influence of fire on native nitrogen-fixing plants and soil nitrogen status in ponderosa pine—Douglas-fir forests in western Montana. Can. J. For. Res. 2000, 30, 274–282. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- White, C.S. Effects of prescribed fire on rates of decomposition and nitrogen mineralization in a ponderosa pine ecosystem. Biol. Fertil. Soils 1986, 2, 87–95. [Google Scholar] [CrossRef]
- Kaye, J.P.; Hart, S.C. Ecological Restoration Alters Nitrogen Transformations in a Ponderosa Pine–Bunchgrass Ecosystem. Ecol. Appl. 1998, 8, 1052–1060. [Google Scholar]
- Gundale, M.J.; DeLuca, T.H.; Fiedler, C.E.; Ramsey, P.W.; Harrington, M.G.; Gannon, J.E. Restoration treatments in a Montana ponderosa pine forest: Effects on soil physical, chemical and biological properties. For. Ecol. Manag. 2005, 213, 25–38. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Boot, C.M.; Haddix, M.; Paustian, K.; Cotrufo, M.F. Distribution of black carbon in ponderosa pine forest floor and soils following the High Park wildfire. Biogeosciences 2015, 12, 3029–3039. [Google Scholar] [CrossRef]
- DeByle, N.V. Fire, Logging, and Debris Disposal Effects on Soil and Water in Northern Coniferous Forests; International Union of Forest Research Organizations: Vienna, Austria, 1976. [Google Scholar]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- Owensby, C.E.; Wyrill, J.B. Effects of range burning on Kansas Flint Hills soil. J. Range Manag. 1973, 26, 185–188. [Google Scholar] [CrossRef]
- Murphy, J.D.; Johnson, D.W.; Miller, W.W.; Walker, R.F.; Carroll, E.F.; Blank, R.R. Wildfire effects on soil nutrients and leaching in a tahoe basin watershed. J. Environ. Q. 2006, 35, 479–489. [Google Scholar] [CrossRef]
- Molina, M.; Fuentes, R.; Calderón, R.; Escudey, M.; Avendaño, K.; Gutiérrez, M.; Chang, A.C. Impact of Forest Fire Ash on Surface Charge Characteristics of Andisols. Soil Sci. 2007, 172, 820–834. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C. Forest Fire Effects on Soil Color and Texture. Soil Sci. Soc. Am. J. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- Heydari, M.; Rostamy, A.; Najafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in Zagros oak (Quercus brantii Lindl.) forests in Iran. J. For. Res. 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Holden, S.R.; Rogers, B.M.; Treseder, K.K.; Randerson, J.T. Fire severity influences the response of soil microbes to a boreal forest fire. Environ. Res. Lett. 2016, 11, 035004. [Google Scholar] [CrossRef]
- Cutler, N.A.; Arróniz-Crespo, M.; Street, L.E.; Jones, D.L.; Chaput, D.L.; DeLuca, T.H. Long-Term Recovery of Microbial Communities in the Boreal Bryosphere Following Fire Disturbance. Microb. Ecol. 2017, 73, 75–90. [Google Scholar] [CrossRef]
- Choromanska, U.; DeLuca, T.H. Microbial activity and nitrogen mineralization in forest mineral soils following heating: Evaluation of post-fire effects. Soil Biol. Biochem. 2002, 34, 263–271. [Google Scholar] [CrossRef]
- Wilson, C.; Kampf, S.K.; Wagenbrenner, J.W.; MacDonald, L.H. Rainfall thresholds for post-fire runoff and sediment delivery from plot to watershed scales. For. Ecol. Manag. 2018, 430, 346–356. [Google Scholar] [CrossRef]
- Kampf, S.K.; Brogan, D.J.; Schmeer, S.; MacDonald, L.H.; Nelson, P.A. How do geomorphic effects of rainfall vary with storm type and spatial scale in a post-fire landscape? Geomorphology 2016, 273, 39–51. [Google Scholar] [CrossRef]
- Wagenbrenner, J.W.; MacDonald, L.H.; Coats, R.N.; Robichaud, P.R.; Brown, R.E. Effects of post-fire salvage logging and a skid trail treatment on ground cover, soils, and sediment production in the interior western United States. For. Ecol. Manag. 2015, 335, 176–193. [Google Scholar] [CrossRef]
- Carroll, E.M.; Miller, W.W.; Johnson, D.W.; Saito, L.; Qualls, R.G.; Walker, R.F. Spatial Analysis of a Large Magnitude Erosion Event Following a Sierran Wildfire. J. Environ. Q. 2007, 36, 1105–1111. [Google Scholar] [CrossRef]
- Pierson, F.B.; Jason Williams, C.; Hardegree, S.P.; Clark, P.E.; Kormos, P.R.; Al-Hamdan, O.Z. Hydrologic and Erosion Responses of Sagebrush Steppe Following Juniper Encroachment, Wildfire, and Tree Cutting. Rangel. Ecol. Manag. 2013, 66, 274–289. [Google Scholar] [CrossRef]
- Francos, M.; Pereira, P.; Alcañiz, M.; Mataix-Solera, J.; Úbeda, X. Impact of an intense rainfall event on soil properties following a wildfire in a Mediterranean environment (North-East Spain). Sci. Total Environ. 2016, 572, 1353–1362. [Google Scholar] [CrossRef]
- Gimeno-García, E.; Andreu, V.; Rubio, J.L. Changes in organic matter, nitrogen, phosphorus and cations in soil as a result of fire and water erosion in a Mediterranean landscape. Eur. J. Soil Sci. 2000, 51, 201–210. [Google Scholar] [CrossRef]
- Abney, R.B.; Sanderman, J.; Johnson, D.; Fogel, M.L.; Berhe, A.A. Post-wildfire Erosion in Mountainous Terrain Leads to Rapid and Major Redistribution of Soil Organic Carbon. Front. Earth Sci. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- García-Llamas, P.; Suárez-Seoane, S.; Taboada, A.; Fernández-Manso, A.; Quintano, C.; Fernández-García, V.; Fernández-Guisuraga, J.M.; Marcos, E.; Calvo, L. Environmental drivers of fire severity in extreme fire events that affect Mediterranean pine forest ecosystems. For. Ecol. Manag. 2019, 433, 24–32. [Google Scholar] [CrossRef]
- Gonzalez Mace, O.; Steinauer, K.; Jousset, A.; Eisenhauer, N.; Scheu, S. Flood-Induced Changes in Soil Microbial Functions as Modified by Plant Diversity. PLoS ONE 2016, 11, e0166349. [Google Scholar] [CrossRef]
- Fischer, E.M.; Knutti, R. Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nat. Clim. Chang. 2015, 5, 560–564. [Google Scholar] [CrossRef]
- Hagos, S.M.; Leung, L.R.; Yoon, J.-H.; Lu, J.; Gao, Y. A projection of changes in landfalling atmospheric river frequency and extreme precipitation over western North America from the Large Ensemble CESM simulations. Geophys. Res. Lett. 2016, 43, 1357–1363. [Google Scholar] [CrossRef]
- Cerda, A.; Imeson, A.C.; Calvo, A. Fire and aspect induced differences on the erodibility and hydrology of soils at La Costera, Valencia, southeast Spain. Catena 1995, 24, 289–304. [Google Scholar] [CrossRef]
- Inbar, M.; Tamir, M.; Wittenberg, L. Runoff and erosion processes after a forest fire in Mount Carmel, a Mediterranean area. Geomorphology 1998, 24, 17–33. [Google Scholar] [CrossRef]
- Shakesby, R.A. Post-wildfire soil erosion in the Mediterranean: Review and future research directions. Earth Sci. Rev. 2011, 105, 71–100. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. BioScience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, S.; Wan, X.; Jiang, C.; Song, X.; Wang, J. Effects of rainfall on soil moisture and water movement in a subalpine dark coniferous forest in southwestern China. Hydrol. Process. 2012, 26, 3800–3809. [Google Scholar] [CrossRef]
- Gochis, D.; Schumacher, R.; Friedrich, K.; Doesken, N.; Kelsch, M.; Sun, J.; Ikeda, K.; Lindsey, D.; Wood, A.; Dolan, B.; et al. The Great Colorado Flood of September 2013. Bull. Am. Meteorol. Soc. 2014. [Google Scholar] [CrossRef]
- Matejovic, I. Determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun. Soil Sci. Plant Anal. 1997, 28, 1499–1511. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Carreiro, M.; Repert, D. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 2012, 114, 313–326. [Google Scholar] [CrossRef]
- Bueno de Mesquita, C.P.; Knelman, J.E.; King, A.J.; Farrer, E.C.; Porazinska, D.L.; Schmidt, S.K.; Suding, K.N. Plant colonization of moss-dominated soils in the alpine: Microbial and biogeochemical implications. Soil Biol. Biochem. 2017, 111, 135–142. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.B.; Crump, A.R.; Resch, C.T.; Fansler, S.; Arntzen, E.; Kennedy, D.W.; Fredrickson, J.K.; Stegen, J.C. Coupling Spatiotemporal Community Assembly Processes to Changes in Microbial Metabolism. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Graham, E.B.; Crump, A.R.; Resch, C.T.; Fansler, S.; Arntzen, E.; Kennedy, D.W.; Fredrickson, J.K.; Stegen, J.C. Deterministic influences exceed dispersal effects on hydrologically-connected microbiomes. Environ. Microbiol. 2017, 19, 1552–1567. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Disturbance Interactions Can Impact Resilience Mechanisms of Forests-Buma-2011-Ecosphere-Wiley Online Library. Available online: https://esajournals.onlinelibrary.wiley.com/doi/full/10.1890/ES11-00038.1 (accessed on 24 November 2018).
- Bigler, C.; Kulakowski, D.; Veblen, T.T. Multiple Disturbance Interactions and Drought Influence Fire Severity in Rocky Mountain Subalpine Forests. Ecology 2005, 86, 3018–3029. [Google Scholar] [CrossRef]
- Disturbance and Landscape Dynamics in a Changing World1-Turner-2010-Ecology-Wiley Online Library. Available online: https://esajournals.onlinelibrary.wiley.com/doi/full/10.1890/10-0097.1 (accessed on 24 November 2018).
- Knelman, J.E.; Graham, E.B.; Prevéy, J.S.; Robeson, M.S.; Kelly, P.; Hood, E.; Schmidt, S.K. Interspecific Plant Interactions Reflected in Soil Bacterial Community Structure and Nitrogen Cycling in Primary Succession. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Knelman, J.E.; Legg, T.M.; O’Neill, S.P.; Washenberger, C.L.; González, A.; Cleveland, C.C.; Nemergut, D.R. Bacterial community structure and function change in association with colonizer plants during early primary succession in a glacier forefield. Soil Biol. Biochem. 2012, 46, 172–180. [Google Scholar] [CrossRef]
- Sattin, S.R.; Cleveland, C.C.; Hood, E.; Reed, S.C.; King, A.J.; Schmidt, S.K.; Robeson, M.S.; Ascarrunz, N.; Nemergut, D.R. Functional shifts in unvegetated, perhumid, recently-deglaciated soils do not correlate with shifts in soil bacterial community composition. J. Microbiol. 2009, 47, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, S.; Liu, X.; Wang, J. Short-term response of the soil bacterial community to differing wildfire severity in Pinus tabulaeformis stands. Sci. Rep. 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Gonzalez-Perez, J.A.; Turmero, A.; Hernandez, M.; Ball, A.S.; Gonzalez-Vila, F.J.; Arias, M.E. Physico-chemical and microbial perturbations of Andalusian pine forest soils following a wildfire. Sci. Total Environ. 2018, 634, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Walker, L.R.; Fastie, C.L.; Sharman, L.C. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol. Monogr. 1994, 64, 149–175. [Google Scholar] [CrossRef]
- Schimel, J.P.; Cleve, K.V.; Cates, R.G.; Clausen, T.P.; Reichardt, P.B. Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: Implications for changes in N cycling during succession. Can. J. Bot. 1996, 74, 84–90. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Walker, L.R.; Whiteaker, L.D.; Matson, P.A. Nutrient limitations to plant growth during primary succession in Hawaii Volcanoes National Park. Biogeochemistry 1993, 23, 197–215. [Google Scholar] [CrossRef]
- Brown, S.P.; Jumpponen, A. Contrasting primary successional trajectories of fungi and bacteria in retreating glacier soils. Mol. Ecol. 2013, 23, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Walker, L.R. Impact of coloniser plant species on the development of decomposer microbial communities following deglaciation. Soil Biol. Biochem. 2004, 36, 555–559. [Google Scholar] [CrossRef]
- Buma, B. Disturbance interactions: Characterization, prediction, and the potential for cascading effects. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).