Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastic Fibers
2.2. Soil
2.3. Experiment 1—Wet-Dry Cycles
2.4. Experiment 2—Soil microbes
2.5. Soil Aggregation Measurements
2.6. Statistics
3. Results
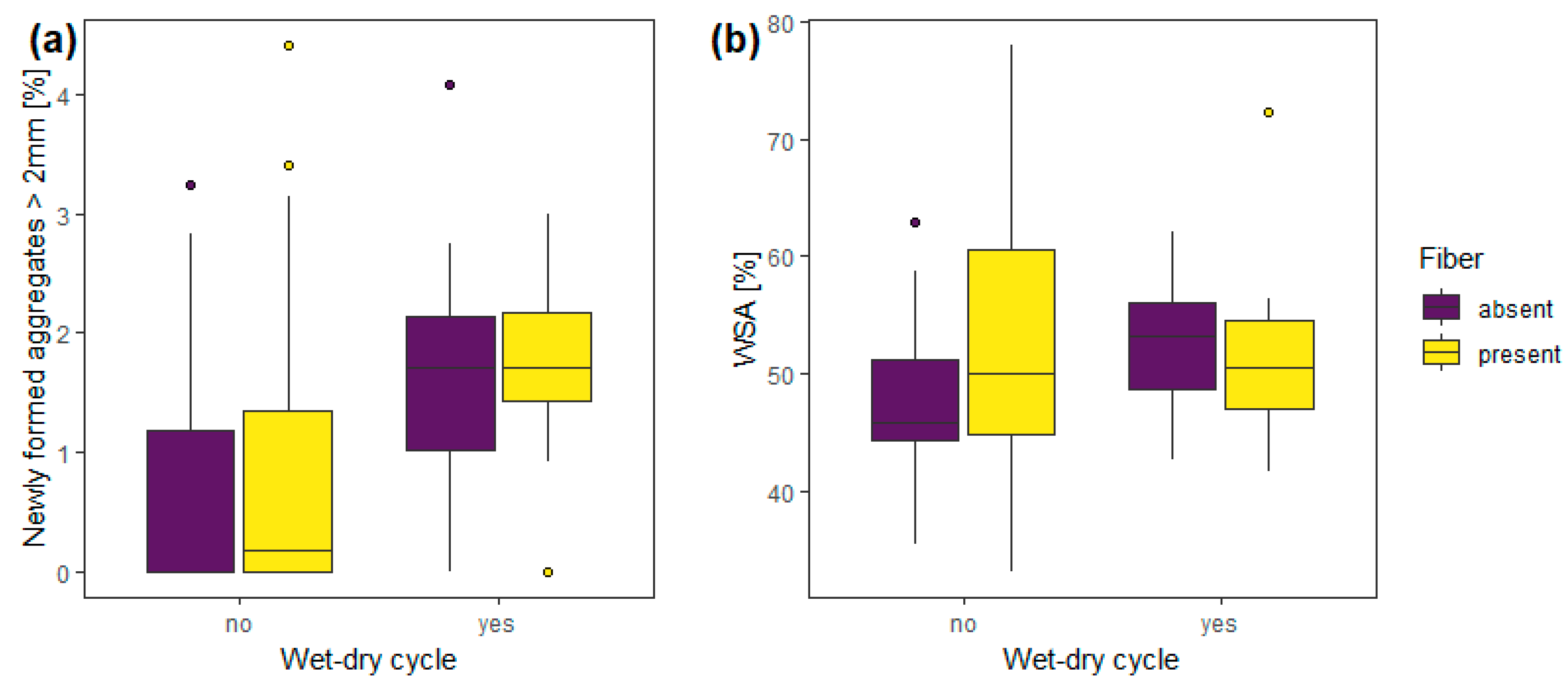
3.1. Experiment 1—Wet-Dry Cycles
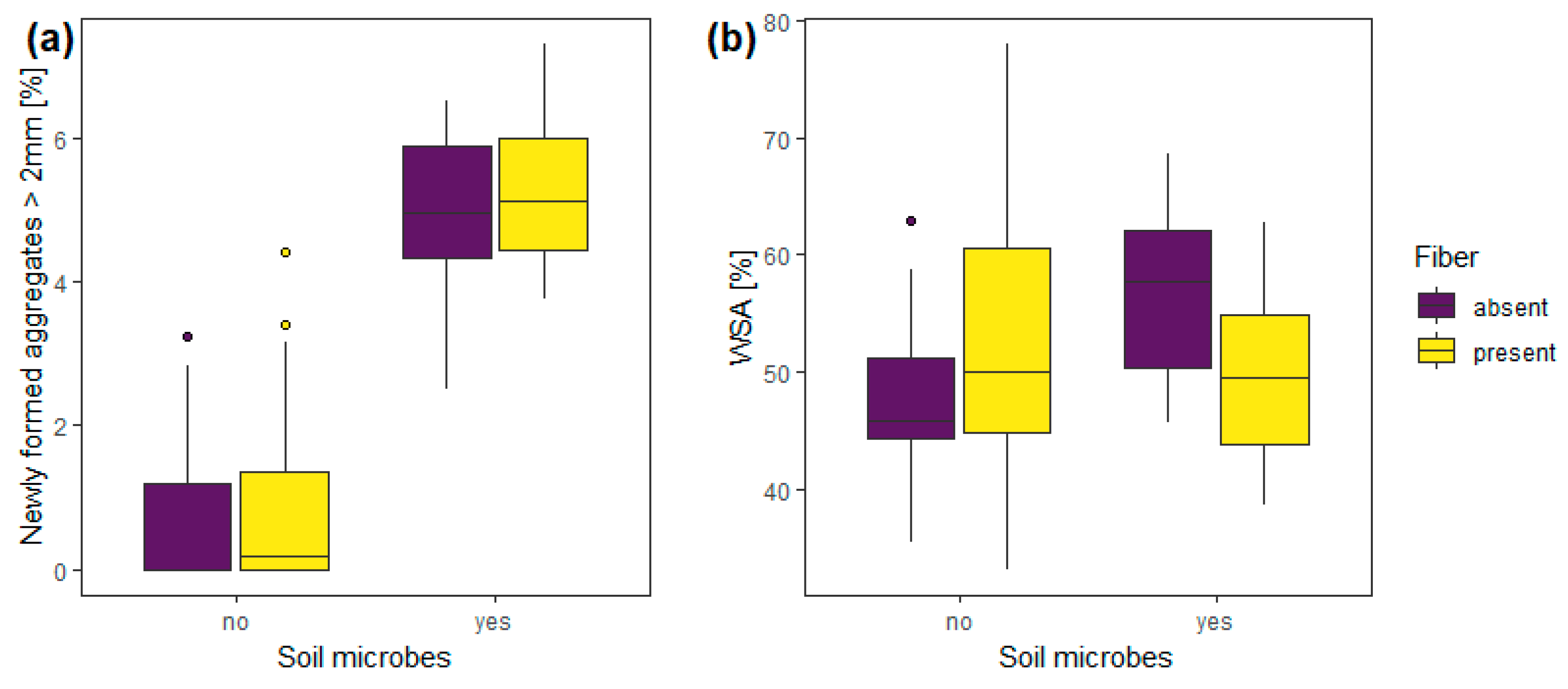
3.2. Experiment 2—Soil Microbes
4. Discussion
4.1. Wet-Dry Cycles and Soil Microbial Effects on Soil Aggregation
4.2. Microfiber Effects on Soil Aggregation
4.3. Interactive Effects of Microplastic and Biotic and Abiotic Factors
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.A.D.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.A.D.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Thapa, B.; Yang, X.M.; Gertsen, H.; Salanki, T.; Geissen, V.; Garbeva, P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: A potential for soil restoration. Sci. Total Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Maass, S.; Daphi, D.; Lehmann, A.; Rillig, M.C. Transport of microplastics by two collembolan species. Environ. Pollut. 2017, 225, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mardatin, N.F.; Leifheit, E.F.; Antunes, P.M. Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol. Biochem. 2010, 42, 1189–1191. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis. Part I—Physical and Mineralogical Methods, 2nd ed.; Lute, A., Ed.; SSSA: Madison, MI, USA, 1986; pp. 425–443. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-137. 2018.
- R Development Core Team. R: A Language and Environment for Statistical Computing, version 3.4.1. 2014.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Cosentino, D.; Chenu, C.; Le Bissonnais, Y. Aggregate stability and microbial community dynamics under drying-wetting cycles in a silt loam soil. Soil Biol. Biochem. 2006, 38, 2053–2062. [Google Scholar] [CrossRef]
- Tisdall, J.; Cockroft, B.; Uren, N. The stability of soil aggregates as affected by organic materials, microbial activity and physical disruption. Aust. J. Soil Res. 1978, 16, 9–17. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Bossuyt, H.; Frey, S.D.; Elliott, E.T.; Merckx, R.; Paustian, K. Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem. 2001, 33, 1599–1611. [Google Scholar] [CrossRef]
- Dorioz, J.M.; Robert, M.; Chenu, C. The role of roots, fungi and bacteria on clay particle organization —An experimental approach. Geoderma 1993, 56, 179–194. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till Res 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.S.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar]
- Sutton, J.C.; Sheppard, B.R. Aggregation of sand-dune soil by endomycorrhizal fungi. Can. J. Bot./Rev. Can. Bot. 1976, 54, 326–333. [Google Scholar] [CrossRef]
- Molope, M.B.; Grieve, I.C.; Page, E.R. Contributions by fungi and bacteria to aggregate stability of cultivated soils. J. Soil Sci. 1987, 38, 71–77. [Google Scholar] [CrossRef]
- Roldan, A.; Garciaorenes, F.; Lax, A. An incubation experiment to determine factors involving aggregation changes in an arid soil receiving urban refuse. Soil Biol. Biochem. 1994, 26, 1699–1707. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: a review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Rillig, M.C. Mycorrhizas and soil aggregation. In Mycorrhizal Mediation of Soil-Fertility, Structure, and Carbon Storage, 1st ed.; Johnson, N., Gehring, C., Jansa, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 241–262. [Google Scholar]
- Blasing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Cole, M. A novel method for preparing microplastic fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Rillig, M.; de Souza Machado, A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2018. [Google Scholar] [CrossRef]
| Newly Formed Aggregates | WSA | ||||||
|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | ||
| Experiment 1 | wet-dry cycle | 1, 56 | 9.97 | 0.01 | 1, 56 | 2.56 | 0.12 |
| fibers | 1, 56 | 0.63 | 0.43 | 1, 56 | 2.03 | 0.16 | |
| wet-dry cycle: fibers | 1, 56 | 0.19 | 0.67 | 1, 56 | 1.13 | 0.29 | |
| Experiment 2 | Soil microbes | 1, 56 | 96.68 | <0.0001 | 1, 56 | 8.18 | 0.01 |
| fibers | 1, 56 | 0.63 | 0.43 | 1, 56 | 2.26 | 0.14 | |
| Soil microbes: fibers | 1, 56 | 0.14 | 0.71 | 1, 56 | 6.45 | 0.01 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, A.; Fitschen, K.; Rillig, M.C. Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Syst. 2019, 3, 21. https://doi.org/10.3390/soilsystems3010021
Lehmann A, Fitschen K, Rillig MC. Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Systems. 2019; 3(1):21. https://doi.org/10.3390/soilsystems3010021
Chicago/Turabian StyleLehmann, Anika, Katharina Fitschen, and Matthias C. Rillig. 2019. "Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation" Soil Systems 3, no. 1: 21. https://doi.org/10.3390/soilsystems3010021
APA StyleLehmann, A., Fitschen, K., & Rillig, M. C. (2019). Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Systems, 3(1), 21. https://doi.org/10.3390/soilsystems3010021





