Plant Secondary Metabolites—Missing Pieces in the Soil Organic Matter Puzzle of Boreal Forests
Abstract
1. Introduction
2. SOM Decomposition in Boreal Forests
3. Effects of PSM on SOM Transformations
3.1. Plant Synthesis of PSM and Soil Concentrations
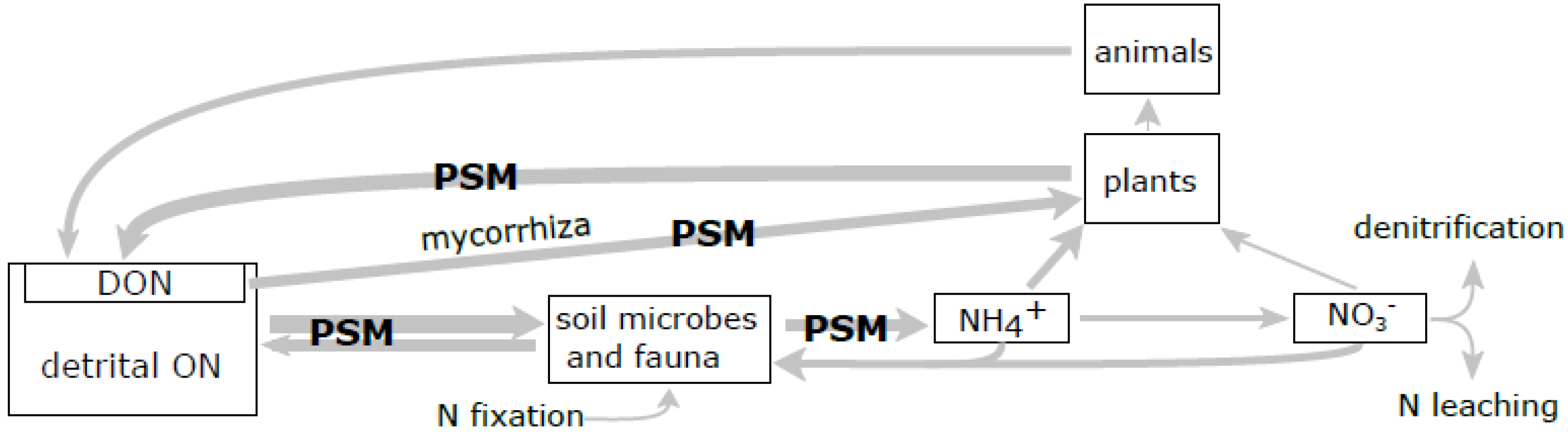
3.2. Influence of PSM on SOM Transformations
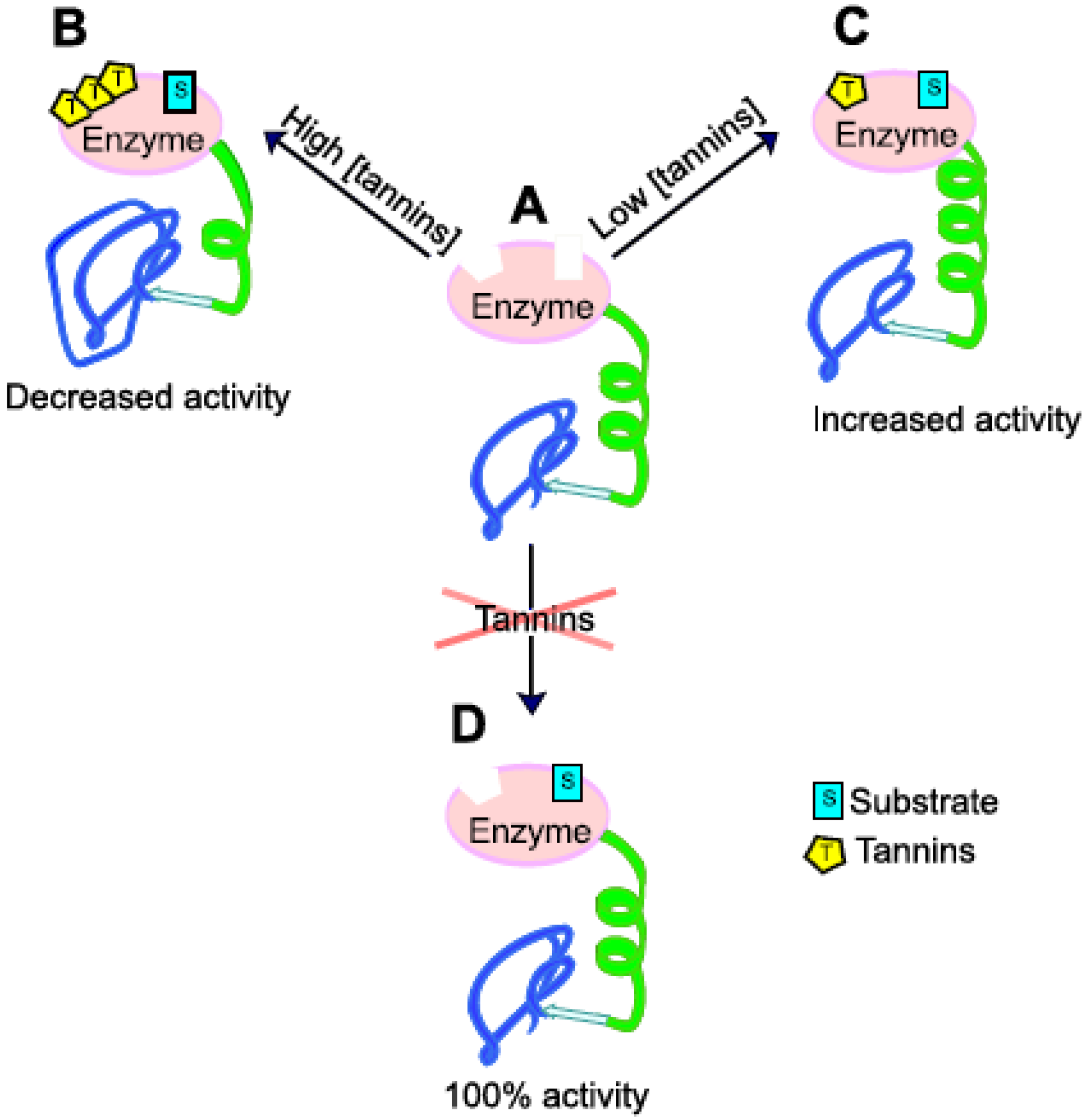
3.3. Effects of PSM on SOM Degradation via Regulation of Enzymatic Activity
4. Conclusions and Directions of Future Studies
Acknowledgments
Author Contributions
Conflict of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Korhonen, J.F.J.; Pihlatie, M.; Pumpanen, J.; Aaltonen, H.; Hari, P.; Levula, J.; Kieloaho, A.-J.; Nikinmaa, E.; Vesala, T.; Ilvesniemi, H. Nitrogen balance of a boreal Scots pine forest. Biogeosciences 2013, 10, 1083–1095. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Fournier, E.; Loreau, M. Respective roles of recent hedges and forest patch remnants in the maintenance of ground-beetle (Coleoptera: Carabidae) diversity in an agricultural landscape. Landsc. Ecol. 2001, 16, 17–32. [Google Scholar] [CrossRef]
- Sulman, B.N.; Phillips, R.P.; Oishi, A.C.; Shevliakova, E.; Pacala, S.W. Microbe-driven turnover offsets mineral-mediated turnover offsets mineral-mediated storage under elevated elevated CO storage of of soil soil carbon carbon under elevated CO2. Nat. Clim. Chang. 2014, 4, 1099–1102. [Google Scholar] [CrossRef]
- Hobbie, S.E. Plant species effects on nutrient cycling: Revisiting litter feedbacks. Trends Ecol. Evol. 2015, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. Perspective The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Högberg, P.; Näsholm, T.; Franklin, O.; Högberg, M.N. Tamm Review: On the nature of the nitrogen limitation to plant growth in Fennoscandian boreal forests. For. Ecol. Manag. 2017, 403, 161–185. [Google Scholar] [CrossRef]
- Schimel, J.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Blaško, R.; Holm Bach, L.; Yarwood, S.A.; Trumbore, S.E.; Högberg, P.; Högberg, M.N. Shifts in soil microbial community structure, nitrogen cycling and the concomitant declining N availability in ageing primary boreal forest ecosystems. Soil Biol. Biochem. 2015, 91, 200–211. [Google Scholar] [CrossRef]
- Kieloaho, A.-J.; Pihlatie, M.; Dominguez Carrasco, M.; Kanerva, S.; Parshintsev, J.; Riekkola, M.-L.; Pumpanen, J.; Heinonsalo, J. Stimulation of soil organic nitrogen pool: The effect of plant and soil organic matter degrading enzymes. Soil Biol. Biochem. 2016, 96, 97–106. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Linkins, A.E.; Ricca, A.; Fry, B.; Nadelhoffer, K.J. Carbon and nitrogen dynamics along the decay continuum: Plant litter to soil organic matter. Plant Soil 1989, 115, 189–198. [Google Scholar] [CrossRef]
- Näsholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Högberg, M.; Högberg, P. Boreal forest plants take up organic nitrogen. Nature 1998, 392, 914–917. [Google Scholar] [CrossRef]
- Chapman, S.K.; Langley, J.A.; Hart, S.C.; Koch, G.W. Plants actively control nitrogen cycling: Uncorking the microbial bottleneck. New Phytol. 2006, 169, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Inselsbacher, E.; Näsholm, T. The below-ground perspective of forest plants: Soil provides mainly organic nitrogen for plants and mycorrhizal fungi. New Phytol. 2012, 195, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Smolander, A.; Kitunen, V.; Godlewski, M. Proteins as nitrogen source for plants. Plant Signal. Behav. 2010, 5, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- McClaugherty, C.A.; Pastor, J.; Aber, J.D.; Melillo, J.M. Forest Litter Decomposition in Relation to Soil Nitrogen Dynamics and Litter Quality. Ecology 1985, 66, 266–275. [Google Scholar] [CrossRef]
- Keplin, B.; Hüttl, R.F. Decomposition of root litter in Pinus sylvestris L. and Pinus nigra stands on carboniferous substrates in the Lusatian lignite mining district. Ecol. Eng. 2001, 17, 285–296. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Oleksyn, J.; Eissenstat, D.M.; Reich, P.B. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 2010, 162, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Viedenz, K.; Polle, A.; Thomas, F.M. Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 2010, 164, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Coq, S.; Weigel, J.; Butenschoen, O.; Bonal, D.; Hättenschwiler, S. Litter composition rather than plant presence affects decomposition of tropical litter mixtures. Plant Soil 2011, 343, 273–286. [Google Scholar] [CrossRef]
- Berg, B. Decomposition of root litter and some factors regulating the process: Long-term root litter decomposition in a scots pine forest. Soil Biol. Biochem. 1984, 16, 609–617. [Google Scholar] [CrossRef]
- Berg, B.; Johansson, M.; Meentemeyer, V.; Kratz, W. Decomposition of tree root litter in a climatic transect of coniferous forests in northern Europe: A synthesis. Scand. J. For. Res. 1998, 13, 402–412. [Google Scholar] [CrossRef]
- Guo, C.; Dannenmann, M.; Gasche, R.; Zeller, B.; Papen, H.; Polle, A.; Rennenberg, H.; Simon, J. Preferential use of root litter compared to leaf litter by beech seedlings and soil microorganisms. Plant Soil 2013, 368, 519–534. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Lindahl, B.D.; de Boer, W.; Finlay, R.D. Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. ISME J. 2010, 4, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Rineau, F.; Roth, D.; Shah, F.; Smits, M.; Johansson, T.; Canbäck, B.; Olsen, P.B.; Persson, P.; Grell, M.N.; Lindquist, E.; et al. The ectomycorrhizal fungus Paxillus involutus converts organic matter in plant litter using a trimmed brown-rot mechanism involving Fenton chemistry. Environ. Microbiol. 2012, 14, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, R.L.; Gadgil, P.D. Mycorrhiza and Litter Decomposition. Nature 1971, 233, 133. [Google Scholar] [CrossRef] [PubMed]
- Averill, C.; Hawkes, C.V. Ectomycorrhizal fungi slow soil carbon cycling. Ecol. Lett. 2016, 19, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.W.; Kennedy, P.G. Revisiting the “Gadgil effect”: Do interguild fungal interactions control carbon cycling in forest soils? New Phytol. 2016, 209, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Santalahti, M.; Sun, H.; Jumpponen, A.; Pennanen, T.; Heinonsalo, J. Vertical and seasonal dynamics of fungal communities in boreal Scots pine forest soil. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Adamczyk, B.; Karonen, M.; Adamczyk, S.; Engström, M.T.; Laakso, T.; Saranpää, P.; Kitunen, V.; Smolander, A.; Simon, J. Tannins can slow-down but also speed-up soil enzymatic activity in boreal forest. Soil Biol. Biochem. 2017, 107, 60–67. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kitunen, V.; Smolander, A. Response of soil C and N transformations to condensed tannins and different organic N-condensed tannin complexes. Appl. Soil Ecol. 2013, 64, 163–170. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk, S.; Smolander, A.; Kitunen, V. Tannic acid and Norway spruce condensed tannins can precipitate various organic nitrogen compounds. Soil Biol. Biochem. 2011, 43, 628–637. [Google Scholar] [CrossRef]
- Adamczyk, S.; Adamczyk, B.; Kitunen, V.; Smolander, A. Influence of diterpenes (colophony and abietic acid) and a triterpene (beta-sitosterol) on net N mineralization, net nitrification, soil respiration, and microbial biomass in birch soil. Biol. Fertil. Soils 2011, 47, 715–720. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Halvorson, J.J.; Gonzalez, J.M.; Hagerman, A.E. Kinetics and binding capacity of six soils for structurally defined hydrolyzable and condensed tannins and related phenols. J. Soils Sediments 2012, 12, 366–375. [Google Scholar] [CrossRef]
- Harbone, J.B. Role of Phenolic Secondary Metaboltes in Plants and their degradation in Nature. In Driven by Nature. Plant Litter Quality and Decomposition; CAB International: Wallingford, UK, 1997; pp. 67–74. ISBN 0851991459. [Google Scholar]
- Agrawal, A.A.; Weber, M.G. On the study of plant defence and herbivory using comparative approaches: How important are secondary plant compounds. Ecol. Lett. 2015, 18, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products Secondary Metabolites. In Biochemistry Molecular Biology of Plants; Wiley: Hoboken, NJ, USA, 2000; Volume 7, pp. 1250–1318. ISBN 0943088399. [Google Scholar]
- Close, D.C.; McArthur, C. Rethinking the role of many plant phenolics—Protection from photodamage not herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Horwath, W. Chapter 12—Carbon Cycling: The Dynamics and Formation of Organic Matter. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 339–382. ISBN 978-0-12-415955-6. [Google Scholar]
- Tharayil, N.; Suseela, V.; Triebwasser, D.J.; Preston, C.M.; Gerard, P.D.; Dukes, J.S. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: Climatic stress-induced tannins are more reactive. New Phytol. 2011, 191, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, M.; Martz, F.; Kaarlejärvi, E.; Julkunen-Tiitto, R.; Stark, S. Phenolic responses of mountain crowberry (Empetrum nigrum ssp. hermaphroditum) to global climate change are compound specific and depend on grazing by reindeer (Rangifer tarandus). J. Chem. Ecol. 2013, 39, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Smolander, A.; Kanerva, S.; Adamczyk, B.; Kitunen, V. Nitrogen transformations in boreal forest soils—Does composition of plant secondary compounds give any explanations? Plant Soil 2012, 350, 1–26. [Google Scholar] [CrossRef]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2017, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wam, H.K.; Stolter, C.; Nybakken, L. Compositional Changes in Foliage Phenolics with Plant Age, a Natural Experiment in Boreal Forests. J. Chem. Ecol. 2017, 43, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Xia, M.; Talhelm, A.F.; Pregitzer, K.S. Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol. 2015, 208, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Ahvenainen, A.; Sietiö, O.-M.; Kanerva, S.; Kieloaho, A.-J.; Smolander, A.; Kitunen, V.; Saranpää, P.; Laakso, T.; Strakova, P.; et al. The contribution of ericoid plants to soil nitrogen chemistry and and organic matter composition in boreal forest soil. Soil Biol. Biochem. 2016, 103, 394–404. [Google Scholar] [CrossRef]
- Northup, R.R.; Dahlgren, R.A.; McColl, J.G. Polyphenols as regulators of plant-litter-soil interactions in Northern California’s pygmy forest: A positive feedback? Biogeochemistry 1998, 42, 189–220. [Google Scholar] [CrossRef]
- Langenheim, J.H. Heigher plant terpenooids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, S.; Smolander, A. How do coniferous needle tannins influence C and N transformations in birch humus layer? Eur. J. Soil Biol. 2008, 44, 1–9. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kitunen, V.; Smolander, A. Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol. Biochem. 2009, 41, 2085–2093. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V.; Tamminen, P.; Kukkola, M. Removal of logging residue in Norway spruce thinning stands: Long-term changes in organic layer properties. Soil Biol. Biochem. 2010, 42, 1222–1228. [Google Scholar] [CrossRef]
- Smolander, A.; Loponen, J.; Suominen, K.; Kitunen, V. Organic matter characteristics and C and N transformations in the humus layer under two tree species, Betula pendula and Picea abies. Soil Biol. Biochem. 2005, 37, 1309–1318. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kitunen, V.; Smolander, A. Protein precipitation by tannins in soil organic horizon and vegetation in relation to tree species. Biol. Fertil. Soils 2008, 45. [Google Scholar] [CrossRef]
- Adamczyk, S.; Kitunen, V.; Lindroos, A.-J.; Adamczyk, B.; Smolander, A. Soil carbon and nitrogen cycling processes and composition of terpenes five years after clear-cutting a Norway spruce stand: Effects of logging residue. For. Ecol. Manag. 2016, 381, 318–326. [Google Scholar] [CrossRef]
- Stark, S.; Hilli, S.; Willför, S.; Smeds, A.I.; Reunanen, M.; Penttinen, M.; Hautajärvi, R. Composition of lipophilic compounds and carbohydrates in the accumulated plant litter and soil organic matter in boreal forests. Eur. J. Soil Sci. 2012, 63, 65–74. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kiikkilä, O.; Kitunen, V.; Smolander, A. Can we measure condensed tannins from tannin-protein complexes?—A case study with acid-butanol assay in boreal forest soil organic layer. Eur. J. Soil Biol. 2014, 64, 40–45. [Google Scholar] [CrossRef]
- Preston, C.M.; Bhatti, J.S.; Flanagan, L.B.; Norris, C. Stocks, chemistry, and sensitivity to climate change of dead organic matter along the Canadian boreal forest transect case study. Clim. Chang. 2006, 74, 233–251. [Google Scholar] [CrossRef]
- Schimel, J.P.; Van Cleve, K.; Cates, R.G.; Clausen, T.P.; Reichardt, P.B. Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: Implications for changes in N cycling during succession. Can. J. Bot. 1996, 74, 84–90. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Cates, R.G.; Zou, J. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol. Biochem. 2001, 33, 1827–1839. [Google Scholar] [CrossRef]
- Asensio, D.; Rapparini, F.; Peñuelas, J. AM fungi root colonization increases the production of essential isoprenoids vs. nonessential isoprenoids especially under drought stress conditions or after jasmonic acid application. Phytochemistry 2012, 77, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Bardon, C.; Piola, F.; Bellvert, F.; Haichar, F.Z.; Comte, G.; Meiffren, G.; Pommier, T.; Puijalon, S.; Tsafack, N.; Poly, F. Evidence for biological denitrification inhibition (BDI) by plant secondary metabolites Evidence for biological denitrification inhibition (BDI) by plant secondary metabolites. New Phytol. 2014, 204, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [PubMed]
- Northup, R.R.; Yu, Z.; Dahlgren, R.A.; Vogt, K.A. Polyphenol control of nitrogen release from pine litter. Nature 1995, 377, 227–229. [Google Scholar] [CrossRef]
- Schimel, J.P.; Cates, R.G.; Ruess, R. The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochemistry 1998, 42, 221–234. [Google Scholar] [CrossRef]
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M.; Munson, A.D. Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: The case of Kalmia angustifolia. New Phytol. 2007, 175, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mutabaruka, R.; Hairiah, K.; Cadisch, G. Microbial degradation of hydrolysable and condensed tannin polyphenol-protein complexes in soils from different land-use histories. Soil Biol. Biochem. 2007, 39, 1479–1492. [Google Scholar] [CrossRef]
- Bending, G.D. Litter decomposition, ectomycorrhizal roots and the “Gadgil” effect. New Phytol. 2003, 158, 228–229. [Google Scholar] [CrossRef]
- Bennett, J.N.; Prescott, C.E. Organic and inorganic nitrogen nutrition of western red cedar, western hemlock and salal in mineral N-limited cedar-hemlock forests. Oecologia 2004, 141, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Wallander, H.; Nilsson, L.O.; Hagerberg, D.; Baath, E. Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol. 2001, 151, 753–760. [Google Scholar] [CrossRef]
- Adamczyk, S.; Kiikkilä, O.; Kitunen, V.; Smolander, A. Potential response of soil processes to diterpenes, triterpenes and tannins: Nitrification, growth of microorganisms and precipitation of proteins. Appl. Soil Ecol. 2013, 67, 47–52. [Google Scholar] [CrossRef]
- Kaal, J.; Nierop, K.G.J.; Verstraten, J.M. Retention of tannic acid and condensed tannin by Fe-oxide-coated quartz sand. J. Colloid Interface Sci. 2005, 287, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III. New cog in the nitrogen cycle. Nature 1995, 377, 199–200. [Google Scholar] [CrossRef]
- Adamczyk, B.; Salminen, J.P.; Smolander, A.; Kitunen, V. Precipitation of proteins by tannins: Effects of concentration, protein/tannin ratio and pH. Int. J. Food Sci. Technol. 2012, 47, 875–878. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Swain, T. Changes in tannins in ripening fruits. Phytochemistry 1963, 2, 371–383. [Google Scholar] [CrossRef]
- Hagerman, A.E. Fifty Years of Polyphenol-Protein Complexes. In Recent Advances in Polyphenol Research; WILEY: Hoboken, NJ, USA, 2012; Volume 3, pp. 71–97. ISBN 9781444337464. [Google Scholar]
- Juntheikki, M.-R.; Julkunen-Tiitto, R. Inhibition of β-glucosidase and esterase by tannins from Betula, Salix, and Pinus species. J. Chem. Ecol. 2000, 26, 1151–1165. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- White, C.S. Monoterpenes: Their effects on ecosystem nutrient cycling. J. Chem. Ecol. 1994, 20, 1381–1406. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.B.; Courtney, K.J.; Langenheim, J.H. Inhibition of nitrosomonas europaea by monoterpenes from coastal redwood (Sequoia sempervirens) in whole-cell studies. J. Chem. Ecol. 1997, 23, 2583–2598. [Google Scholar] [CrossRef]
- Adamczyk, S.; Adamczyk, B.; Kitunen, V.; Smolander, A. Monoterpenes and higher terpenes may inhibit enzyme activities in boreal forest soil. Soil Biol. Biochem. 2015, 87, 59–66. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. Summary for Policymakers; IPCC: Geneve, Switzerland, 2013; pp. 1–29. [Google Scholar]
- Stark, S.; Väisänen, M.; Ylänne, H.; Julkunen-Tiitto, R.; Martz, F. Decreased phenolic defence in dwarf birch (Betula nana) after warming in subarctic tundra. Polar Biol. 2015, 38, 1993–2005. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, B.; Adamczyk, S.; Smolander, A.; Kitunen, V.; Simon, J. Plant Secondary Metabolites—Missing Pieces in the Soil Organic Matter Puzzle of Boreal Forests. Soil Syst. 2018, 2, 2. https://doi.org/10.3390/soils2010002
Adamczyk B, Adamczyk S, Smolander A, Kitunen V, Simon J. Plant Secondary Metabolites—Missing Pieces in the Soil Organic Matter Puzzle of Boreal Forests. Soil Systems. 2018; 2(1):2. https://doi.org/10.3390/soils2010002
Chicago/Turabian StyleAdamczyk, Bartosz, Sylwia Adamczyk, Aino Smolander, Veikko Kitunen, and Judy Simon. 2018. "Plant Secondary Metabolites—Missing Pieces in the Soil Organic Matter Puzzle of Boreal Forests" Soil Systems 2, no. 1: 2. https://doi.org/10.3390/soils2010002
APA StyleAdamczyk, B., Adamczyk, S., Smolander, A., Kitunen, V., & Simon, J. (2018). Plant Secondary Metabolites—Missing Pieces in the Soil Organic Matter Puzzle of Boreal Forests. Soil Systems, 2(1), 2. https://doi.org/10.3390/soils2010002





