Soil Carbon Dioxide Respiration in Switchgrass Fields: Assessing Annual, Seasonal and Daily Flux Patterns
Abstract
:1. Introduction
2. Methods
2.1. Site Description
2.2. Soil Properties
2.3. Soil Respiration Measurements
3. Results and Discussion
3.1. Annual CO2 Flux
3.2. Seasonal CO2 Flux
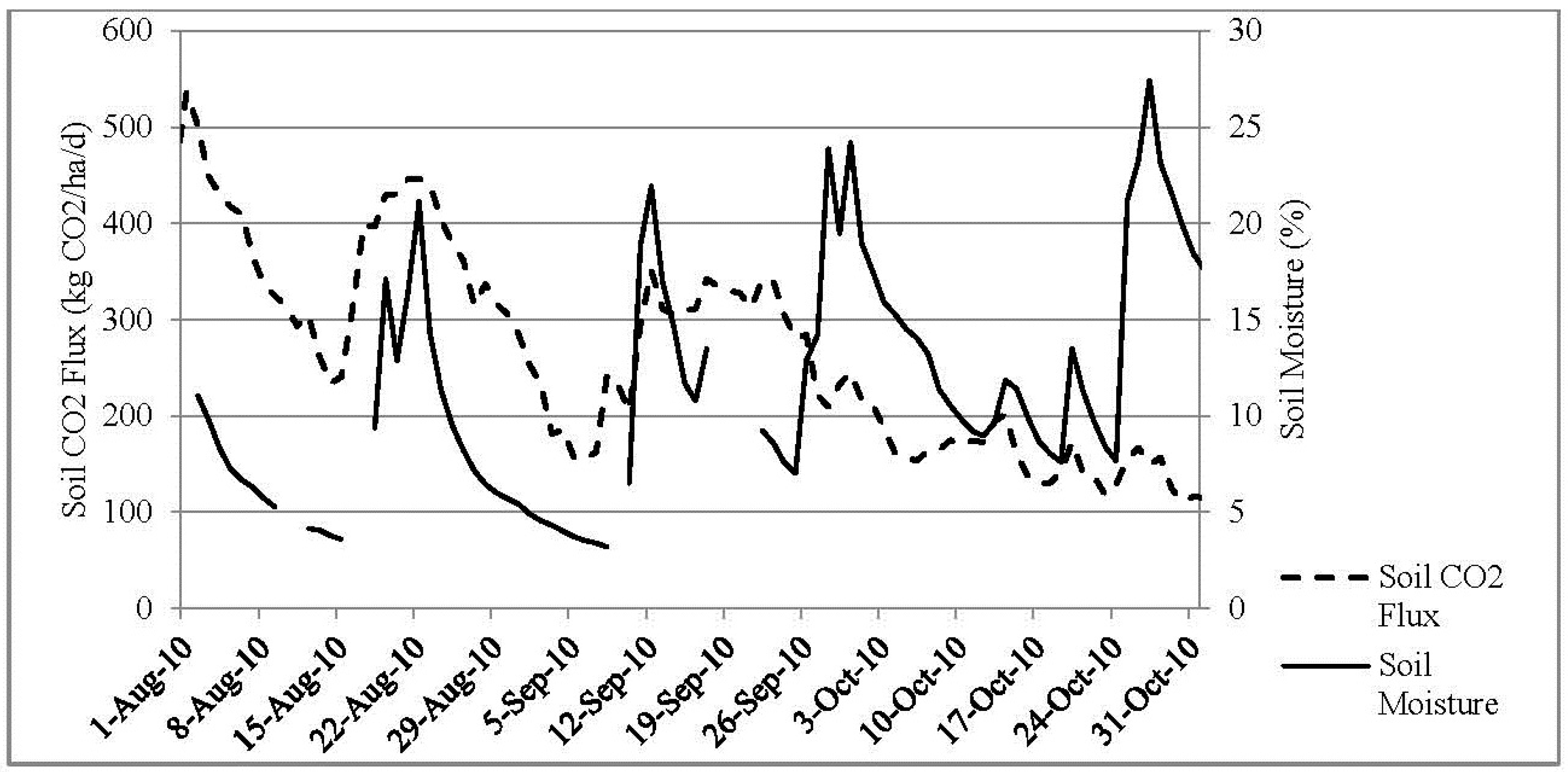
3.3. Daily CO2 Flux
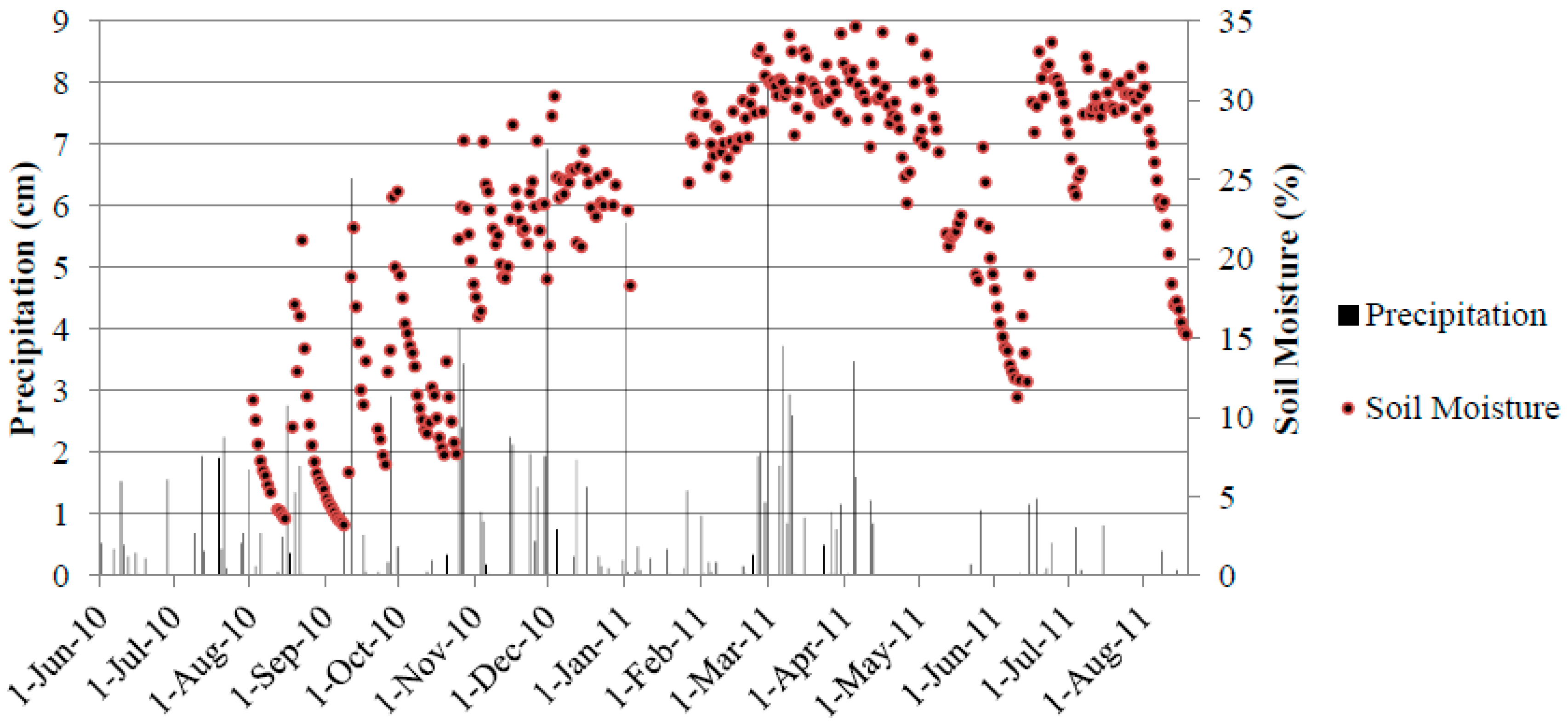
3.4. Soil Moisture and Soil Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dornbush, M.E.; Raich, J.W. Soil temperature, not aboveground plant productivity, best predicts intra-annual variations of soil respiration in central Iowa grasslands. Ecosystems 2006, 9, 909–920. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- McNally, S.; Laughlin, D.C.; Rutledge, S.; Dodd, M.B.; Six, J.; Schipper, L.A. Root carbon inputs under moderately diverse sward and conventional ryegrass-clover pasture: Implications for soil carbon sequestration. Plant Soil 2015, 392, 289–299. [Google Scholar] [CrossRef]
- Nickerson, C.; Ebel, R.; Borchers, A.; Carriazo, F. Major Uses of Land in the United States, 2007; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2011; Volume 89.
- US EPA. Global Greenhouse Gas Emissions Data. 2004. Available online: http://www.epa.gov/climatechange/ghgemissions/global.html (accessed on 8 August 2013).
- Lemus, R.; Lal, R. Bioenergy crops and carbon sequestration. Crit. Rev. Plant Sci. 2005, 24, 1–21. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Parrish, D.J. Potential soil carbon sequestration and CO2 offset by dedicated energy crops in the USA. Crit. Rev. Plant Sci. 2006, 25, 441–472. [Google Scholar] [CrossRef]
- Parkin, T.B.; Kaspar, T.C. Temperature controls on diurnal carbon dioxide flux: Implications for estimating soil C loss. Soil Sci. Soc. Am. J. 2003, 67, 1763–1772. [Google Scholar] [CrossRef]
- Adler, P.R.; Del Grosso, S.J.; Parton, W.J. Life-cycle assessment of net greenhouse-gas flux for bioenergy cropping systems. Ecol. Appl. 2007, 17, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.; Brye, K.R.; Gbur, E.E.; Chen, P.; Korth, K. Long-term Residue Management Effects on Soil Respiration in a Wheat-Soybean Double-Crop System. Soil Sci. 2014, 179, 118–129. [Google Scholar] [CrossRef]
- Frank, A.B.; Berdahl, J.D.; Hanson, J.D.; Liebig, M.A.; Johnson, H.A. Biomass and carbon partitioning in switchgrass. Crop Sci. 2004, 44, 1391–1396. [Google Scholar] [CrossRef]
- Zeri, M.; Anderson-Teixeira, K.; Hickman, G.; Masters, M.; DeLucia, E.; Bernacchi, C.J. Carbon exchange by establishing biofuel crops in Central Illinois. Agric. Ecosyst. Environ. 2011, 144, 319–329. [Google Scholar] [CrossRef]
- Chang, X.; Zhu, X.; Wang, S.; Luo, C.; Zhang, Z.; Duan, J.; Bai, L.; Wang, W. Temperature and Moisture Effects on Soil Respiration in Alpine Grasslands. Soil Sci. 2012, 177, 554–560. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhua, B.; Gao, M.R.; Wang, Y.Q.; Zheng, X.H. Seasonal variations in soil respiration and temperature sensitivity under three land-use types in hilly areas of the Sichuan Basin. Aust. J. Soil Res. 2008, 46, 727–734. [Google Scholar] [CrossRef]
- Bauer, J.; Herbst, J.; Huisman, J.A.; Weihermüller, L.; Vereecken, H. Sensitivity of simulated soil heterotrophic respiration to temperature and moisture reduction functions. Geoderma 2008, 145, 17–27. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, G.; Brouder, S.M.; Smith, D.R.; Van Scoyoc, G.E. Greenhouse gas fluxes in an Eastern corn belt soil: Weather, nitrogen source, and rotation. J. Environ. Qual. 2009, 38, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Doolittle, J.J.; Owens, V.N. Soil carbon dioxide fluxes in established switchgrass land managed for biomass production. Soil Biol. Biochem. 2007, 39, 178–186. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S. Global patterns of carbon-dioxide emissions from soils. Glob. Biogeochem. Cycles 1995, 9, 23–36. [Google Scholar] [CrossRef]
- Sainju, U.M.; Stevens, W.B.; Caesar-TonThat, T.C.; Jabro, J.D. Land use and management practices impact on plant biomass carbon and soil carbon dioxide emission. Soil Sci. Soc. Am. J. 2010, 74, 1613–1622. [Google Scholar] [CrossRef]
- Savage, K.E.; Davidson, E.A. A comparison of manual and automated systems for soil CO2 flux measurements: Trade-offs between spatial and temporal resolution. J. Exp. Biol. 2003, 54, 891–899. [Google Scholar] [CrossRef]
- Miao, Y.; Song, C.; Wang, X.; Sun, X.; Meng, H.; Sun, L. Greenhouse gas emissions from different wetlands during the snow-covered season in Northeast China. Atmos. Environ. 2012, 62, 328–335. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, M. Separating the effects of moisture and temperature on soil CO2 efflux in a coniferous forest in the Sierra Nevada mountains. Plant Soil 2001, 237, 15–23. [Google Scholar] [CrossRef]
- Tufekcioglu, A.; Raich, J.W.; Isenhart, T.M.; Schultz, R.C. Soil respiration within riparian buffers and adjacent crop fields. Plant Soil 2001, 229, 117–124. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis Part 3—Chemical Methods SOIL Science Society of America; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Healy, R.W.; Striegl, R.G.; Russell, T.F.; Hutchinson, G.L.; Livingston, G.P. Numerical evaluation of static-chamber measurements of soil-atmosphere gas exchange: Identification of physical processes. Soil Sci. Soc. Am. J. 1996, 60, 740–747. [Google Scholar] [CrossRef]
- Riedell, W.E.; Osborne, S.L.; Schumacher, T.E.; Pikul, J.L., Jr. Grassland canopy management and native trallgrass species composition effects on C and N in grass canopies and soil. Plant Soil 2010, 338, 51–61. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT 9.1 User’s Guide the Reg Procedure: (Book Excerpt); SAS Institute: Cary, NC, USA, 2008 November 24. [Google Scholar]
- Maljanen, M.; Martikainen, P.J.; Walden, J.; Silvola, J. CO2 exchange in an organic field growing barley or grass in eastern Finland. Glob. Chang. Biol. 2001, 7, 679–692. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Lu, F.; Pang, J.; Feng, Z.; Liu, W.; Ouyang, Z.; Wang, X. Seasonal dynamics of soil CO2 efflux in a conventional tilled wheat field of the Loess Plateau, China. Ecol. Res. 2011, 26, 735–743. [Google Scholar] [CrossRef]
- Blazier, M.A.; Clason, T.R.; Vance, E.D.; Leggett, Z.; Sucre, E.B. Loblolly pine age and density affects switchgrass growth and soil carbon in an agroforestry system. For. Sci. 2012, 58, 485–496. [Google Scholar] [CrossRef]
- Garten, C.T.; Brice, D.J.; Castro, H.F.; Graham, R.L.; Mayes, M.A.; Phillips, J.R.; Post, W.M.; Schadt, C.W.; Wullschleger, S.D.; Tyler, D.D.; et al. Response of “Alamo” switchgrass tissue chemistry and biomass to nitrogen fertilization in West Tennessee, USA. Agric. Ecosyst. Environ. 2011, 140, 289–297. [Google Scholar] [CrossRef]
- Song, W.; Chen, S.; Wu, B.; Zhu, Y.; Zhou, Y.; Li, Y.; Caro, Y.; Lu, Q.; Lin, G. Vegetation cover and rain timing co-regulate the responses of soil CO2 efflux to rain increase in an arid desert ecosystem. Soil Biol. Biogeochem. 2012, 49, 114–123. [Google Scholar] [CrossRef]
- Thomson, B.C.; Ostle, N.; McNamara, N.; Bailey, M.J.; Whiteley, A.S.; Griffiths, R.I. Vegetation affects the relative abundances of dominant soil bacterial taxa and soil respiration rates in an upland grassland soil. Microb. Ecol. 2010, 59, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Grahammer, K.; Jawson, M.D.; Skopp, J. Day and night soil respiration from a grassland. Soil Biol. Biochem. 1991, 23, 77–81. [Google Scholar] [CrossRef]
- Trevors, J.T. Effect of temperature on selected microbial activities in aerobic and anaerobically incubated sediment. Hydrobiologia 1985, 126, 189–192. [Google Scholar] [CrossRef]





| UT PSES 1 | UT HDF 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Depth | 0–5 cm | 5–10 cm | 10–15 cm | 15–30 cm | 0–5 cm | 5–10 cm | 10–15 cm | 15–30 cm |
| Soil Classification | Shady Loam (fine-loamy, mixed, subactive, thermic Typic Hapludults) | Whitwell sandy clay (fine-loamy, siliceous, semiactive, thermic Aquic Hapludults) | ||||||
| pH | 5.61 | 5.46 | 5.52 | 5.02 | 5.54 | 5.72 | 6.15 | 6.38 |
| CEC (meq/100 g) | 10.20 | 9.36 | 8.96 | 8.95 | 7.85 | 7.57 | 8.85 | 8.75 |
| % Organic Carbon | 1.45 | 1.21 | 1.03 | 0.75 | 1.19 | 0.81 | 0.53 | 0.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; McKnight, J.; Skinner, L.S.; Sherfy, A.; Tyler, D.; English, B. Soil Carbon Dioxide Respiration in Switchgrass Fields: Assessing Annual, Seasonal and Daily Flux Patterns. Soil Syst. 2018, 2, 13. https://doi.org/10.3390/soilsystems2010013
Lee J, McKnight J, Skinner LS, Sherfy A, Tyler D, English B. Soil Carbon Dioxide Respiration in Switchgrass Fields: Assessing Annual, Seasonal and Daily Flux Patterns. Soil Systems. 2018; 2(1):13. https://doi.org/10.3390/soilsystems2010013
Chicago/Turabian StyleLee, Jaehoon, Julie McKnight, Leah S. Skinner, Andrew Sherfy, Donald Tyler, and Burton English. 2018. "Soil Carbon Dioxide Respiration in Switchgrass Fields: Assessing Annual, Seasonal and Daily Flux Patterns" Soil Systems 2, no. 1: 13. https://doi.org/10.3390/soilsystems2010013
APA StyleLee, J., McKnight, J., Skinner, L. S., Sherfy, A., Tyler, D., & English, B. (2018). Soil Carbon Dioxide Respiration in Switchgrass Fields: Assessing Annual, Seasonal and Daily Flux Patterns. Soil Systems, 2(1), 13. https://doi.org/10.3390/soilsystems2010013





