Cowden Syndrome in Childhood: Gastrointestinal Involvement in a Multisystem Genetic Disorder—A Case Report
Abstract
1. Introduction and Clinical Significance
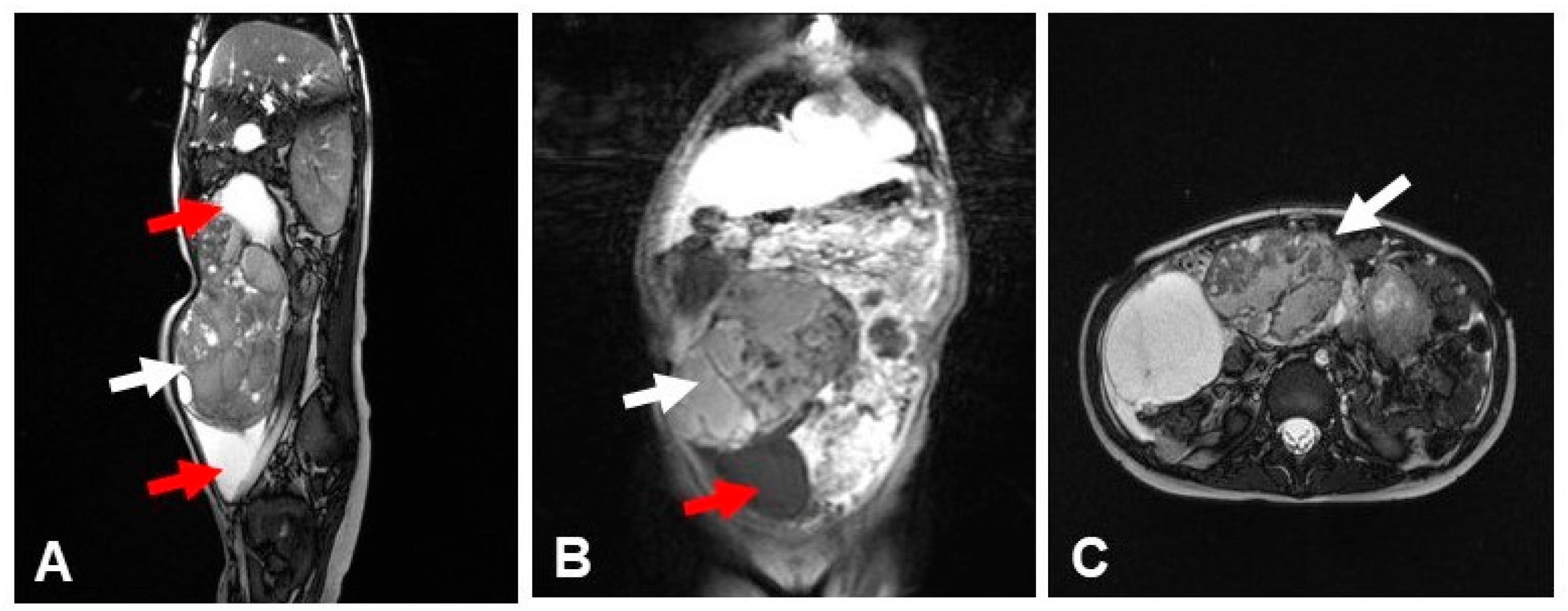
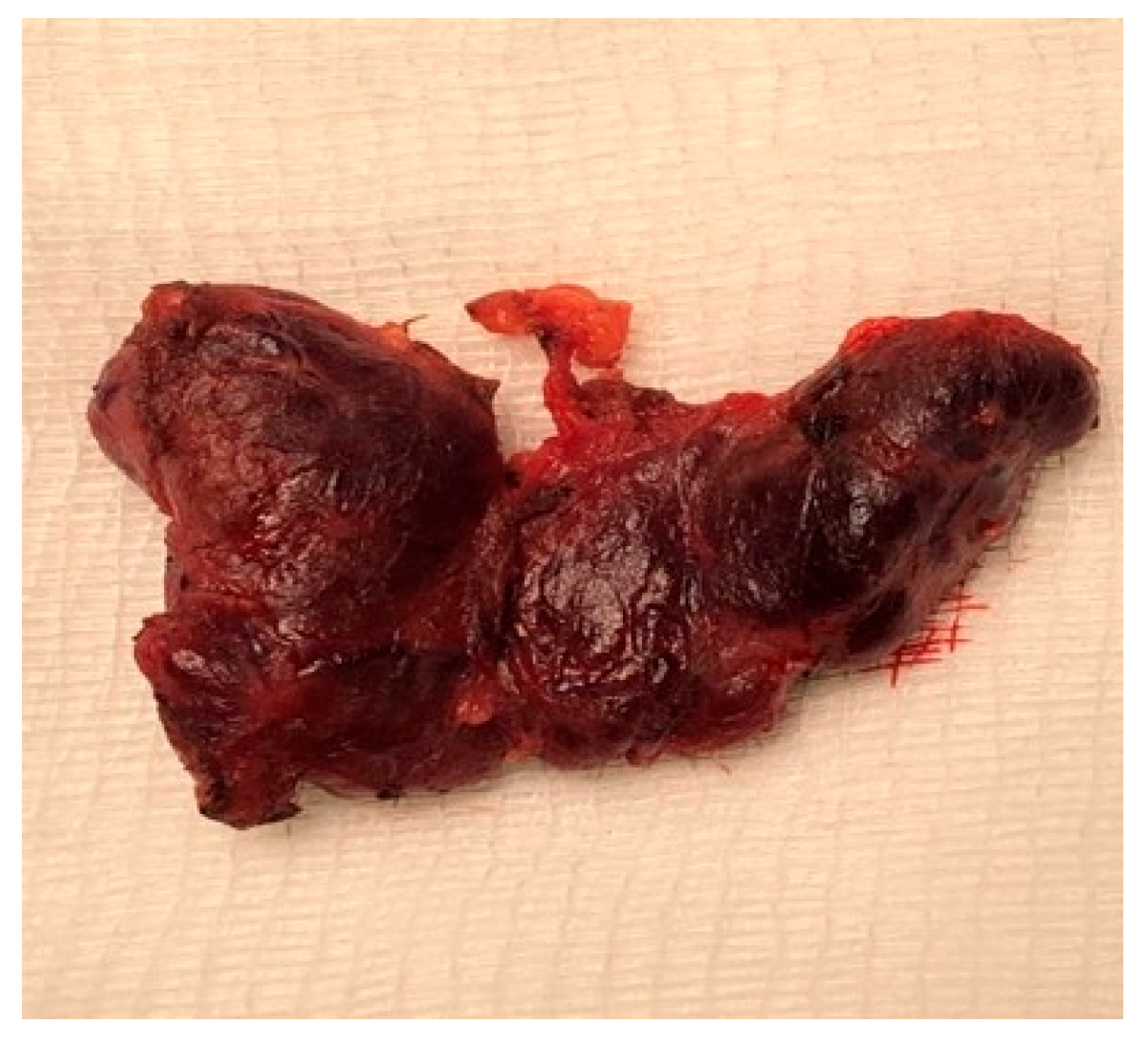
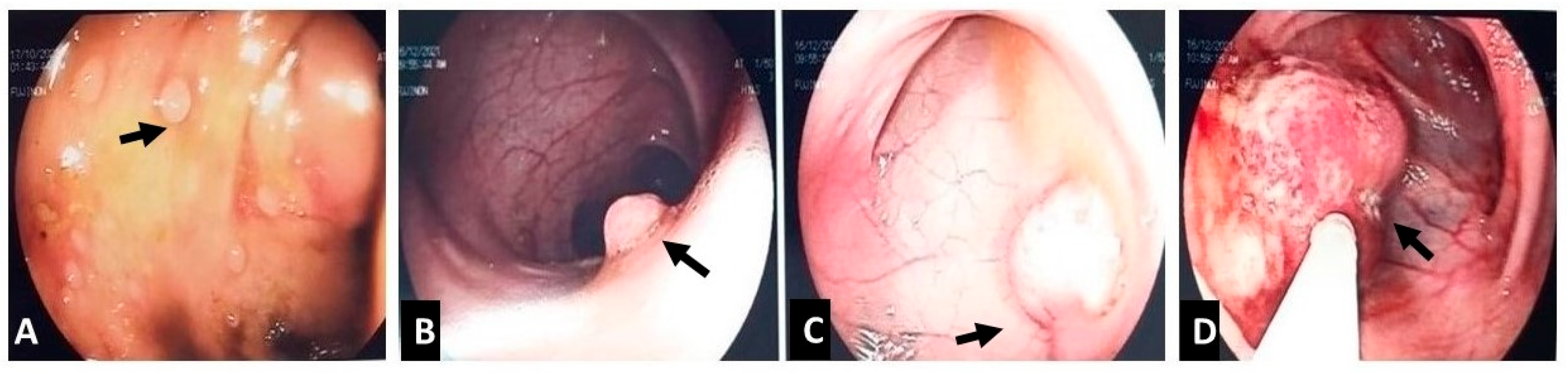
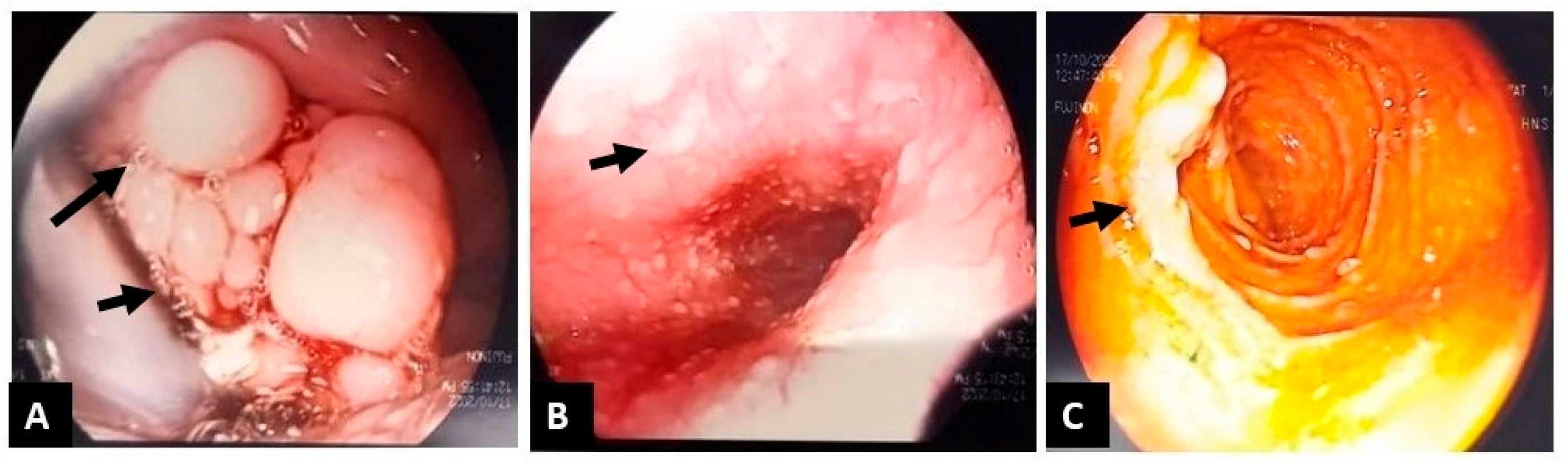
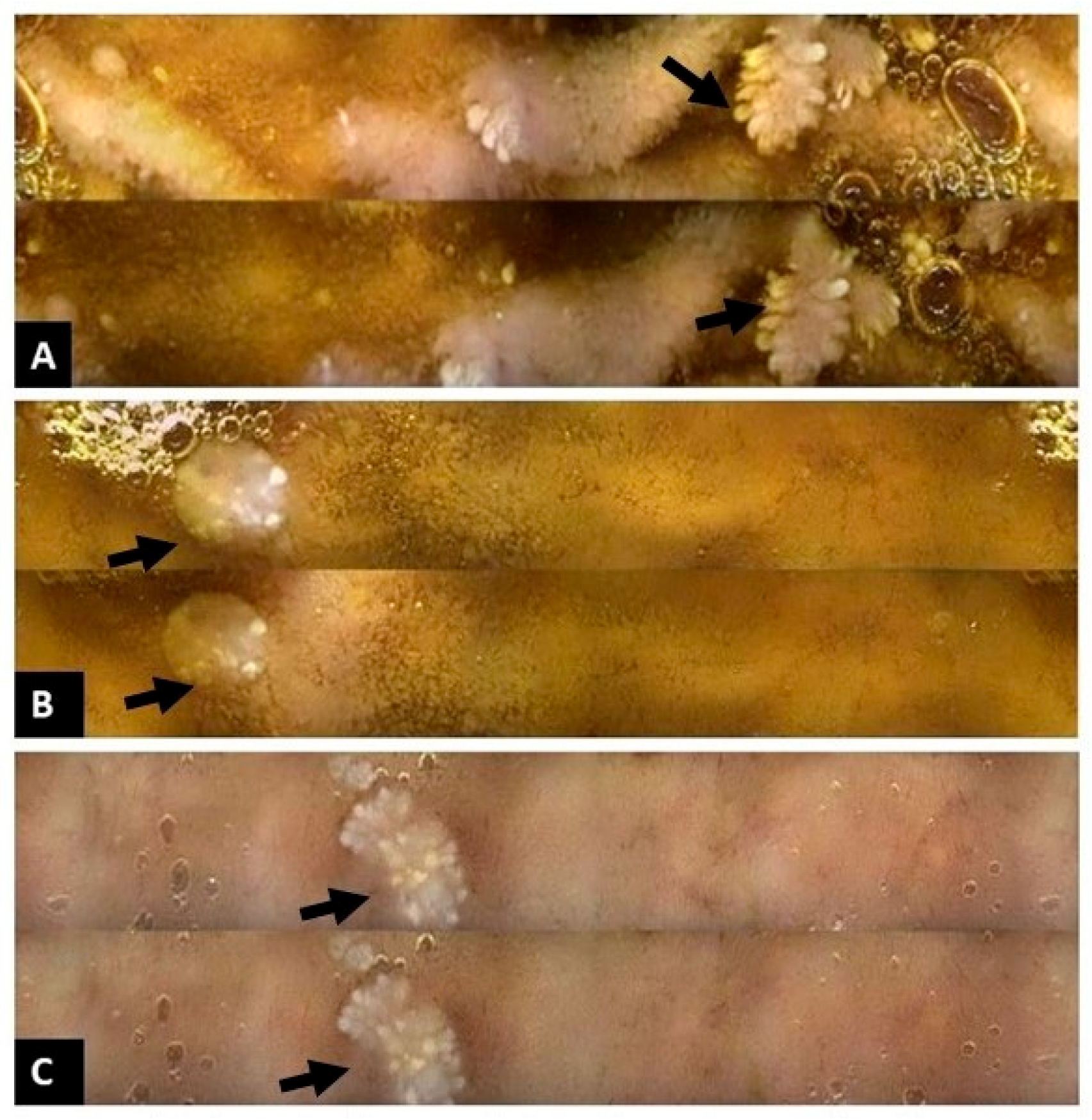
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTEN | Phosphatase and TENsin homolog |
| CS | Cowden Syndrome |
| PHTS | PTEN Hamartoma Tumor Syndrome |
| GI | Gastrointestinal |
| MRI | Magnetic Resonance Imaging |
| BRRS | Bannayan–Riley–Ruvalcaba Syndrome |
| PTEN PS | PTEN-related Proteus Syndrome |
| FNA | Fine-Needle Aspiration |
| AUS | Atypia of Undetermined Significance |
| FLUS | Follicular Lesion of Undetermined Significance |
References
- Yehia, L.; Plitt, G.; Tushar, A.M.; Liu, D.; Joo, J.; Ni, Y.; Patil, S.; Eng, C. Extended spectrum of cancers in PTEN hamartoma tumor syndrome. NPJ Precis. Oncol. 2025, 9, 61. [Google Scholar] [CrossRef]
- Tan, M.H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing based on a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 1.2023. NCCN Clinical Practice Guidelines in Oncology. Available online: https://cliniclancette.ru/docs/genetics_bop-2023.pdf (accessed on 7 September 2022).
- Takayama, T.; Muguruma, N.; Igarashi, M.; Ohsumi, S.; Oka, S.; Kakuta, F.; Kubo, Y.; Kumagai, H.; Sasaki, M.; Sugai, T.; et al. Clinical guidelines for diagnosis and management of Cowden syndrome/PTEN hamartoma tumor syndrome in children and adults (secondary publication). J. Anus Rectum Colon 2023, 7, 284–300. [Google Scholar] [CrossRef]
- Hansen-Kiss, E.; Beinkampen, S.; Adler, B.; Frazier, T.W.; Prior, T.W.; Erdman, S.H.; Eng, C.; Herman, G. A retrospective chart review of the features of PTEN hamartoma tumour syndrome in children. J. Med. Genet. 2017, 54, 471–478. [Google Scholar] [CrossRef]
- Lachlan, K.L.; Lucassen, A.M.; Bunyan, D.; Temple, I.K. Cowden syndrome and Bannayan–Riley–Ruvalcaba syndrome represent one condition with variable expression and age-related penetrance: Results of a clinical study of PTEN mutation carriers. J. Med. Genet. 2007, 44, 579–585. [Google Scholar] [CrossRef]
- Ilić, N.; Jelavić Mitrović, N.; Radeta, R.; Krasić, S.; Vukomanović, V.; Samardžija, G.; Vasic, M.; Vlahovic, A.; Sarajlija, A. Phenotypic variability of Cowden syndrome within a single family: Impact on diagnosis, management and genetic counselling. Balk. J. Med. Genet. 2024, 27, 95–100. [Google Scholar] [CrossRef]
- Martín-Valbuena, J.; Gestoso-Uzal, N.; Justel-Rodríguez, M.; Isidoro-García, M.; Marcos-Vadillo, E.; Lorenzo-Hernández, S.M.; Criado-Muriel, M.C.; Prieto-Matos, P. PTEN hamartoma tumor syndrome: Clinical and genetic characterization in pediatric patients. Child’s Nerv. Syst. 2024, 40, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Tsai, S.; Singleton, D.; Isaza, A.; Brodeur, G.; MacFarland, S.; Zelley, K.; Mamula, P.; Bauer, A.J. PTEN hamartoma tumor syndrome in pediatrics: Triggers for evaluation and the value of surveillance. J. Endocr. Soc. 2020, 4, OR22-02. [Google Scholar] [CrossRef]
- Graziani, V.; Dal Bo, S.; Giovannini, M.; Marchetti, F. Clinical presentation of PTEN mutations. Pediatr. Neonatol. 2018, 59, 425–426. [Google Scholar] [CrossRef]
- Yan, L.; Tian, L.; Zhang, Y.; Liu, Y.; Cao, J.; Li, D.; Zou, J.; Li, H. Analysis of clinical features and genetic variant in a child with Cowden syndrome 1. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2024, 41, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; MacFarland, S.P.; Yehia, L.; Duvall, M.M.; Mamula, P.; Kurowski, J.A.; Greene, C.S.; Radhakrishnan, K.; Eng, C. A bi-institutional study of gastrointestinal and hepatic manifestations in children with PTEN hamartoma tumor syndrome. Gastro Hep Adv. 2024, 3, 250–259. [Google Scholar] [CrossRef]
- Ruemmele, F.; Vannerom, P.; Canioni, D.; Olschwang, S.; Martelli, H.; Brousse, N.; Jan, D.; Goulet, O.; Lyonnet, S.; Mougenot, J. Early onset PTEN hamartomatous syndrome. J. Pediatr. Gastroenterol. Nutr. 2004, 39, S380. [Google Scholar] [CrossRef]
- Pilarski, R. PTEN hamartoma tumor syndrome: A clinical overview. Cancers 2019, 11, 844. [Google Scholar] [CrossRef]
- Lim, A.; Ngeow, J. The skin in Cowden syndrome. Front. Med. 2021, 8, 658842. [Google Scholar] [CrossRef]
- Borowsky, J.; Setia, N.; Rosty, C.; Conrad, R.; Susman, R.; Misdraji, J.; Hart, J.; Lauwers, G.Y.; Brown, I.S. Spectrum of gastrointestinal tract pathology in a multicenter cohort of Cowden syndrome patients. Mod. Pathol. 2019, 32, 1814–1822. [Google Scholar] [CrossRef]
- Cheung, K.M.; Lam, C.W.; Chan, Y.K.; Siu, W.K.; Yong, L. Atypical focal cortical dysplasia in a patient with Cowden syndrome. Hong Kong Med. J. 2014, 20, 165–167. [Google Scholar] [CrossRef]
- Garcia, M.; Petit, I.O.; Franchet, C.; Abbo, O.; Cartault, A.; Savagner, F. Atypical thyroid manifestation in Cowden disease: A case report and literature review. Front. Pediatr. 2025, 13, 1499664. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.Y.; Kim, H.S.; Eng, C.; Kim, D.S.; Kang, S.J.; Eom, M.; Yi, S.Y.; Bronner, M.P. Ovarian dysgerminoma in Cowden syndrome with germline PTEN mutation and PTEN-related loss of heterozygosity. Am. J. Surg. Pathol. 2008, 32, 1258–1264. [Google Scholar] [CrossRef]
- Smpokou, P.; Fox, V.L.; Tan, W.H. PTEN hamartoma tumour syndrome: Early tumour development in children. Arch. Dis. Child. 2015, 100, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Zelenova, E.; Belysheva, T.; Sharapova, E.; Barinova, I.; Fedorova, A.; Semenova, V.; Vishnevskaya, Y.; Kletskaya, I.; Mitrofanova, A.; Sofronov, D.; et al. Atypical manifestations of Cowden syndrome in pediatric patients. Diagnostics 2025, 15, 1456. [Google Scholar] [CrossRef] [PubMed]
- Tischkowitz, M.; Colas, C.; Pouwels, S.; Hoogerbrugge, N.; PHTS Guideline Development Group; European Reference Network for Genetic Tumour Risk Syndromes (ERN-GENTURIS). Cancer surveillance guideline for individuals with PTEN hamartoma tumour syndrome (PHTS). Eur. J. Hum. Genet. 2020, 28, 1387–1403. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; MacFarland, S.P.; Perrino, M.R.; Mitchell, S.G.; Kamihara, J.; Nelson, A.T.; Mallinger, P.H.; Brzezinski, J.J.; Maxwell, K.N.; Woodward, E.R.; et al. Update on pediatric surveillance recommendations for PTEN hamartoma tumor syndrome, DICER1-related tumor predisposition, and tuberous sclerosis complex. Clin. Cancer Res. 2025, 31, 234–244. [Google Scholar] [CrossRef]
- Dhawan, A.; Baitamouni, S.; Liu, D.; Yehia, L.; Anthony, K.; McCarther, A.; Tischkowitz, M.; MacFarland, S.P.; Ngeow, J.; Hoogerbrugge, N.; et al. Cancer and overgrowth manifestations of PTEN hamartoma tumor syndrome: Management recommendations from the International PHTS Consensus Guidelines Working Group. Clin. Cancer Res. 2025, 31, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
| Major Criteria | Minor Criteria |
|---|---|
| Breast cancer | Autism spectrum disorder |
| Endometrial cancer (epithelial) | Intellectual disability/developmental delay |
| Thyroid cancer (papillary or follicular) | Lipomas |
| Multiple GI hamartomas or ganglioneuromas (≥3) | Fibrocystic breast disease |
| Macrocephaly (≥97th percentile) | Uterine fibroids |
| Lhermitte-Duclos disease (cerebellar dysplastic gangliocytoma) | Vascular anomalies (AVMs, hemangiomas) |
| Mucocutaneous lesions: Trichilemmomas (facial) Acral keratoses Papillomatous papules (esp. oral mucosa) Mucosal lesions (oral papillomas) | Esophageal glycogenic acanthosis (≥3) |
| Colon adenomas | |
| Renal cell carcinoma | |
| Testicular lipomatosis |
| Feature | Description |
|---|---|
| Prevalence | ~28–50% of children with PTEN mutations have GI manifestations |
| Polyp burden | Variable: from few scattered to extensive polyposis across the GI tract |
| Polyp location | Colon, rectum, stomach, duodenum, esophagus, small intestine |
| Polyp types | Mixed histology: ▪ Juvenile-like hamartomas (most common) ▪ Inflammatory polyps ▪ Hyperplastic polyps ▪ Ganglioneuromas ▪ Adenomatous polyps (occasionally) |
| Other less common | Glycogenic acanthosis of esophagus |
| Other findings | Nonspecific inflammation: esophagitis, gastritis, duodenitis Lymphoid hyperplasia EGIDs (Eosinophilic Gastrointestinal disorders) |
| Symptoms | Often asymptomatic; when present: ▪ Constipation ▪ Feeding difficulties, food aversion, aspiration ▪ Gastroesophageal Reflux disease ▪ Rectal bleeding ▪ Abdominal pain ▪ Iron-deficiency anemia ▪ Diarrhea ▪ Failure to thrive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rogalidou, M.; Katzilakis, N.; Stefanaki, K.; Dimakou, K.; Margoni, D.; Pelagiadis, I.; Papadopoulou, A.; Stiakaki, E. Cowden Syndrome in Childhood: Gastrointestinal Involvement in a Multisystem Genetic Disorder—A Case Report. Reports 2026, 9, 21. https://doi.org/10.3390/reports9010021
Rogalidou M, Katzilakis N, Stefanaki K, Dimakou K, Margoni D, Pelagiadis I, Papadopoulou A, Stiakaki E. Cowden Syndrome in Childhood: Gastrointestinal Involvement in a Multisystem Genetic Disorder—A Case Report. Reports. 2026; 9(1):21. https://doi.org/10.3390/reports9010021
Chicago/Turabian StyleRogalidou, Maria, Nikolaos Katzilakis, Kalliopi Stefanaki, Konstantina Dimakou, Dafni Margoni, Iordanis Pelagiadis, Alexandra Papadopoulou, and Eftichia Stiakaki. 2026. "Cowden Syndrome in Childhood: Gastrointestinal Involvement in a Multisystem Genetic Disorder—A Case Report" Reports 9, no. 1: 21. https://doi.org/10.3390/reports9010021
APA StyleRogalidou, M., Katzilakis, N., Stefanaki, K., Dimakou, K., Margoni, D., Pelagiadis, I., Papadopoulou, A., & Stiakaki, E. (2026). Cowden Syndrome in Childhood: Gastrointestinal Involvement in a Multisystem Genetic Disorder—A Case Report. Reports, 9(1), 21. https://doi.org/10.3390/reports9010021






