A Mucosal Change like Hypertrophic Gastritis Following Zolbetuximab-Based Therapy in a Conversion Surgery Case of Advanced Gastric Cancer
Abstract
1. Introduction and Clinical Significance
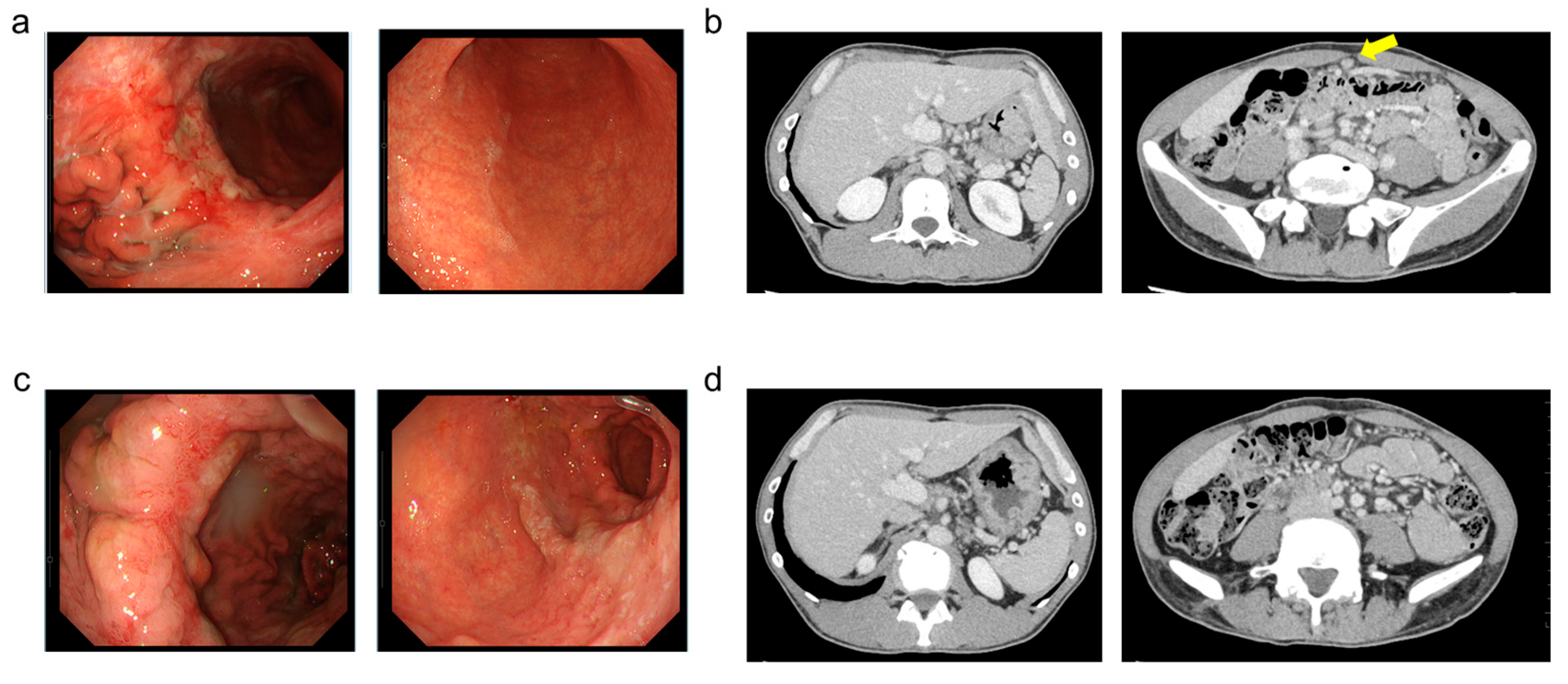
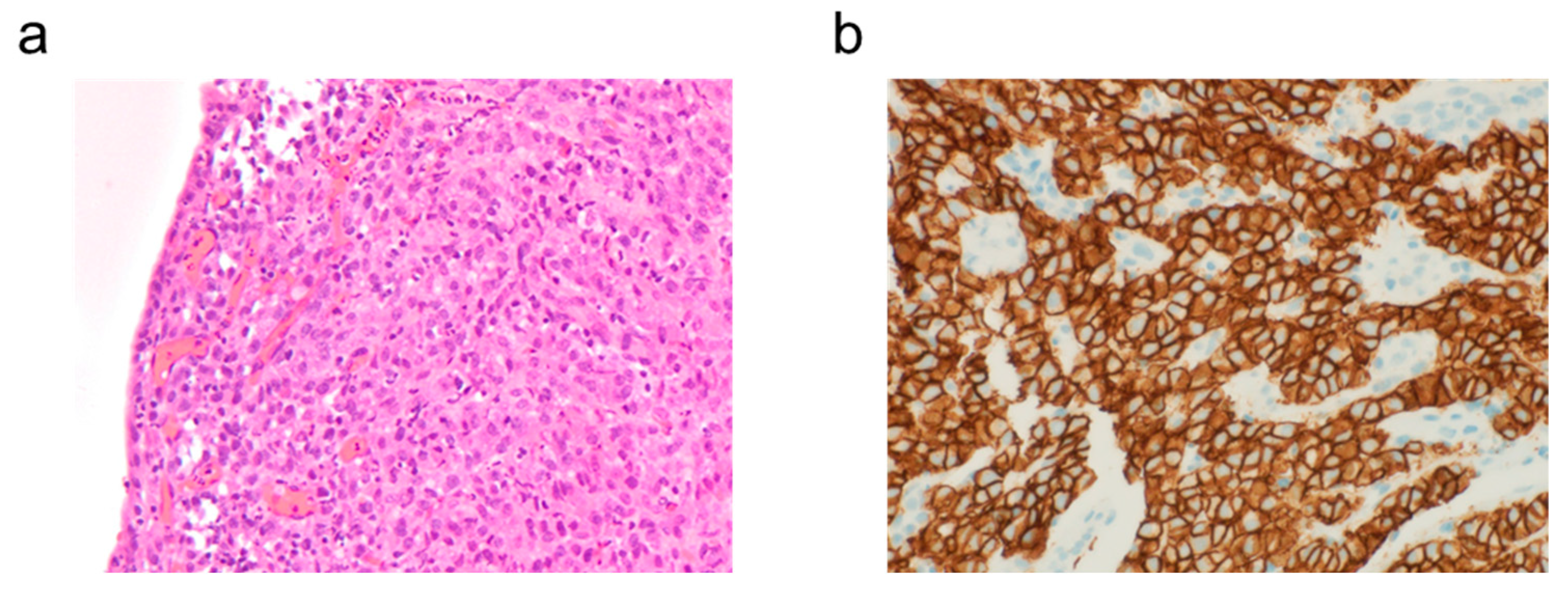
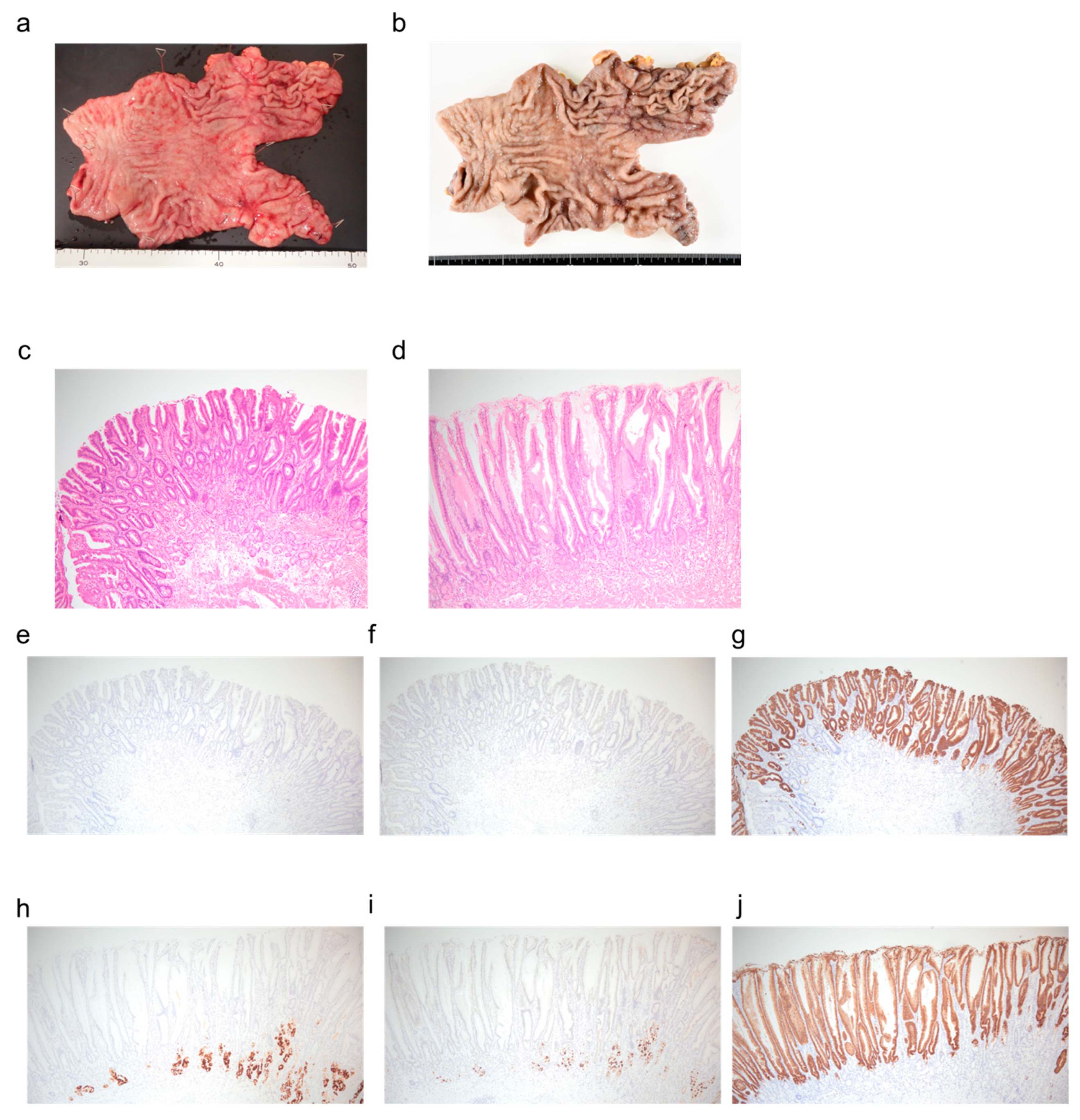
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLDN18.2 | Claudin 18.2 |
| HER2 | Human Epidermal Growth Factor Receptor Type 2 |
| PD-L1 | Program Death Ligand-1 |
| IHC | Immunohistochemistry |
| CTCAE | Common Terminology Criteria for Adverse Events |
References
- Japanese Gastric Cancer Association. Gastric Cancer Treatment Guidelines, 7th ed.; Japanese Gastric Cancer Association: Tokyo, Japan, 2025; ISBN 978-4-307-20487-3. [Google Scholar]
- National Cancer Center Japan, Cancer Registry and Statistics, Cancer Information Services. Hospital-Based Cancer Registry of Designated Cancer Care Hospitals, 2014–2015 5-Year Survival Rate Report. Available online: https://ganjoho.jp/public/qa_links/report/hosp_c/hosp_c_reg_surv/index.html (accessed on 20 March 2025).
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.Y.; Oh, D.Y.; Yañez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Pellino, A.; Brignola, S.; Riello, E.; Niero, M.; Murgioni, S.; Guido, M.; Nappo, F.; Businello, G.; Sbaraglia, M.; Bergamo, F.; et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J. Pers. Med. 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. Claudin-18, a Novel Downstream Target Gene for the T/EBP/NKX2.1 Homeodomain Transcription Factor, Encodes Lung- and Stomach-Specific Isoforms through Alternative Splicing. Mol. Cell. Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive Clinical and Molecular Characterization of Claudin 18.2 Expression in Advanced Gastric or Gastroesophageal Junction Cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, C.; Peng, Z.; Zhang, Y.; Wei, J.; Qiu, W.; Zhang, X.; Pan, H.; Niu, Z.; Qiu, M.; et al. Claudin-18 Isoform 2-Specific CAR T-Cell Therapy (SATRI-CEL) versus Treatment of Physician’s Choice for Previously Treated Advanced Gastric or Gastro-oesophageal Junction Cancer (CT041-ST-01): A Randomised. Lancet 2025, 405, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Xu, R.H.; Ajani, J.A.; Moran, D.; Guerrero, A.; Li, R.; Pavese, J.; Matsangou, M.; Bhattacharya, P.; Ueno, Y.; et al. Global Prevalence of Claudin 18 Isoform 2 in Tumors of Patients with Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma. Gastric Cancer 2024, 27, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, O. Claudin-18 Splice Variant 2 Is a Pan-Cancer Target Suitable for Therapeutic Antibody Development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in Patients with CLDN18.2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma: The Randomized, Phase 3 GLOW Trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Kim, T.Y.; Nam, S.K.; Hwang, H.J.; Han, D.; Oh, H.J.; Kong, S.H.; Park, D.J.; Oh, D.Y.; Lee, H.J.; et al. Clinicopathologic and Molecular Characterization of Stages II–IV Gastric Cancer with Claudin 18.2 Expression. Oncologist 2025, 30. [Google Scholar] [CrossRef]
- Yakuwa, E.; Shoji, Y.; Oizumi, T.; Kobayashi, Y.; Motoishi, T.; Katagiri, T.; Suzuki, S. Safety and Feasibility of Outpatient Zolbetuximab Administration in Community Cancer Care: A Mixed-Methods Analysis. In Vivo 2025, 39, 951–960. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Yamamoto, K.; Hara, K.; Masuike, Y.; Ushimaru, Y.; Kitamura, M.; Honma, K.; Matsuura, N.; Sugase, T.; Kanemura, T.; et al. Two Cases of Protein-Losing Enteropathy Induced by Zolbetuximab in Patients with Unresectable Advanced Gastric Cancer. Jpn. J. Clin. Oncol. 2025, 55, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Shah, M.A.; Lordick, F.; Van Cutsem, E.; Ilson, D.H.; Klempner, S.J.; Kang, Y.K.; Lonardi, S.; Hung, Y.P.; Yamaguchi, K.; et al. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N. Engl. J. Med. 2024, 391, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Langer, J.C.; Zutter, M.; Swanson, P.; Kraus, M.D.; Bartlett, N.; Shackelford, G.D.; Longtine, J.A.; Perlmutter, D.H. Primary gastric plasmacytoma: A rare cause of hypertrophic gastritis in an adolescent. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Kim, D.H.; Ahn, J.Y.; Choi, K.D.; Kim, H.J.; Na, H.K.; Lee, J.H.; Jung, K.W.; Song, H.J.; Lee, G.H.; et al. Differential Diagnosis of Thickened Gastric Wall between Hypertrophic Gastritis and Borrmann Type 4 Advanced Gastric Cancer. Gut Liver 2024, 18, 961–969. [Google Scholar] [CrossRef] [PubMed]
| Biochemistry | Immunology | ||||
|---|---|---|---|---|---|
| TP | 6.9 | g/dL | CRP | 0.02 | mg/dL |
| Alb | 4.3 | g/dL | |||
| AST | 15 | U/L | Hematology | ||
| ALT | 17 | U/L | WBC | 9920 | /uL |
| Total Bil | 0.69 | mg/dL | Hb | 14.8 | g/dL |
| LDH | 187 | U/L | Plt | 259 | 103/uL |
| ALP | 66 | U/L | |||
| Crea | 0.77 | mg/dL | Tumor Markers | ||
| UN | 13.6 | mg/dL | CEA | 4.46 | ng/mL |
| Na | 141 | mmol/L | CA19-9 | 6.61 | U/mL |
| K | 4.3 | mmol/L | |||
| Cl | 104 | mmol/L | |||
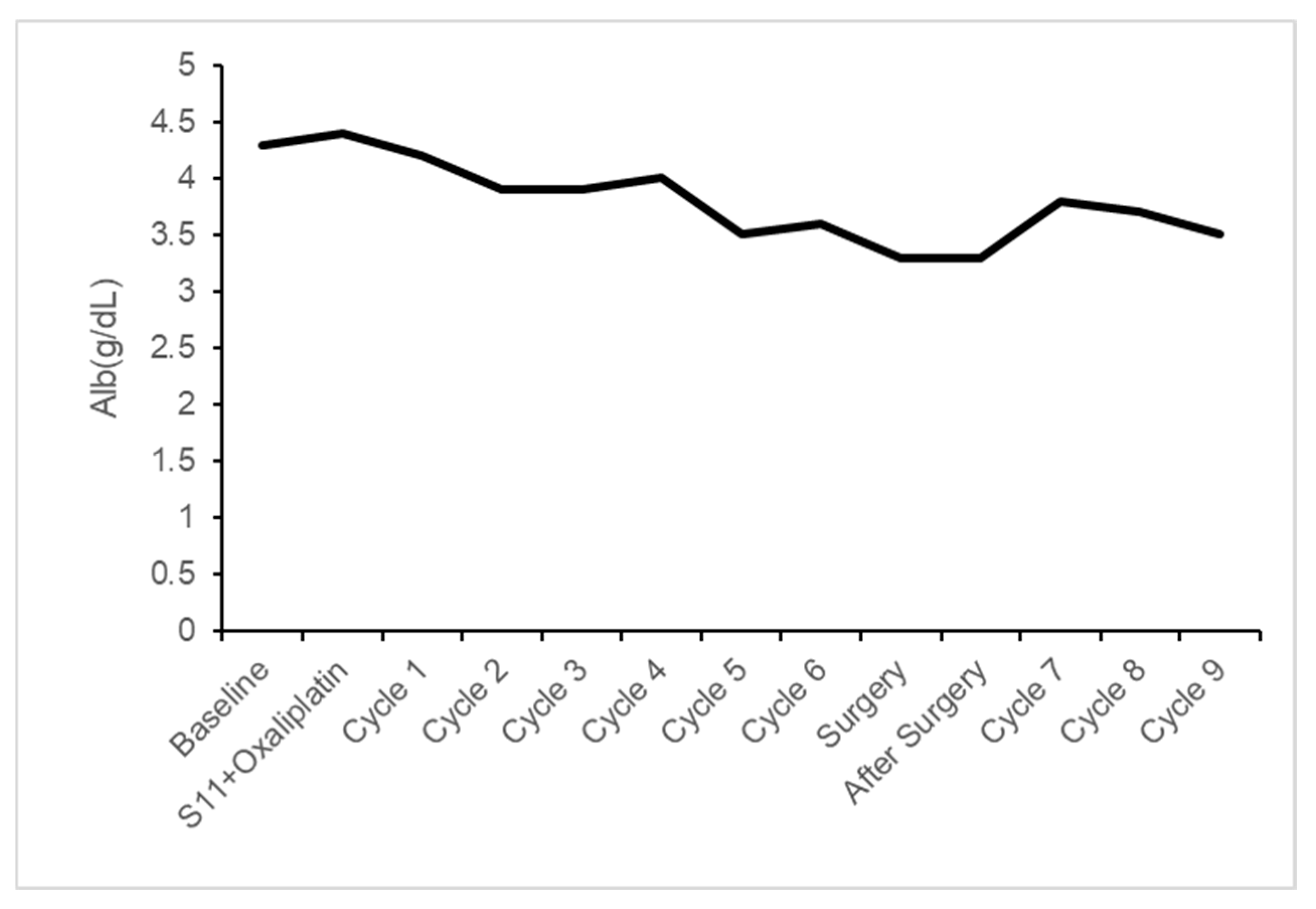
| At Time | Alb (g/dL) |
|---|---|
| Baseline | 4.3 |
| S11 + Oxaliplatin | 4.4 |
| Cycle 1 | 4.2 |
| Cycle 2 | 3.9 |
| Cycle 3 | 3.9 |
| Cycle 4 | 4.0 |
| Cycle 5 | 3.5 |
| Cycle 6 | 3.6 |
| Surgery | 3.3 |
| After Surgery | 3.3 |
| Cycle 7 | 3.8 |
| Cycle 8 | 3.7 |
| Cycle 9 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, S.; Suzuki, S.; Kabasawa, T.; Kanauchi, T.; Akiba, S. A Mucosal Change like Hypertrophic Gastritis Following Zolbetuximab-Based Therapy in a Conversion Surgery Case of Advanced Gastric Cancer. Reports 2025, 8, 235. https://doi.org/10.3390/reports8040235
Oyama S, Suzuki S, Kabasawa T, Kanauchi T, Akiba S. A Mucosal Change like Hypertrophic Gastritis Following Zolbetuximab-Based Therapy in a Conversion Surgery Case of Advanced Gastric Cancer. Reports. 2025; 8(4):235. https://doi.org/10.3390/reports8040235
Chicago/Turabian StyleOyama, Soshi, Shuhei Suzuki, Takanobu Kabasawa, Takumi Kanauchi, and Shotaro Akiba. 2025. "A Mucosal Change like Hypertrophic Gastritis Following Zolbetuximab-Based Therapy in a Conversion Surgery Case of Advanced Gastric Cancer" Reports 8, no. 4: 235. https://doi.org/10.3390/reports8040235
APA StyleOyama, S., Suzuki, S., Kabasawa, T., Kanauchi, T., & Akiba, S. (2025). A Mucosal Change like Hypertrophic Gastritis Following Zolbetuximab-Based Therapy in a Conversion Surgery Case of Advanced Gastric Cancer. Reports, 8(4), 235. https://doi.org/10.3390/reports8040235







