Unlocking Medical Breakthroughs: The Transformative Role of Case Reports in Clinical Discovery
1. Introduction
2. Human Retrovirus-Induced Diseases
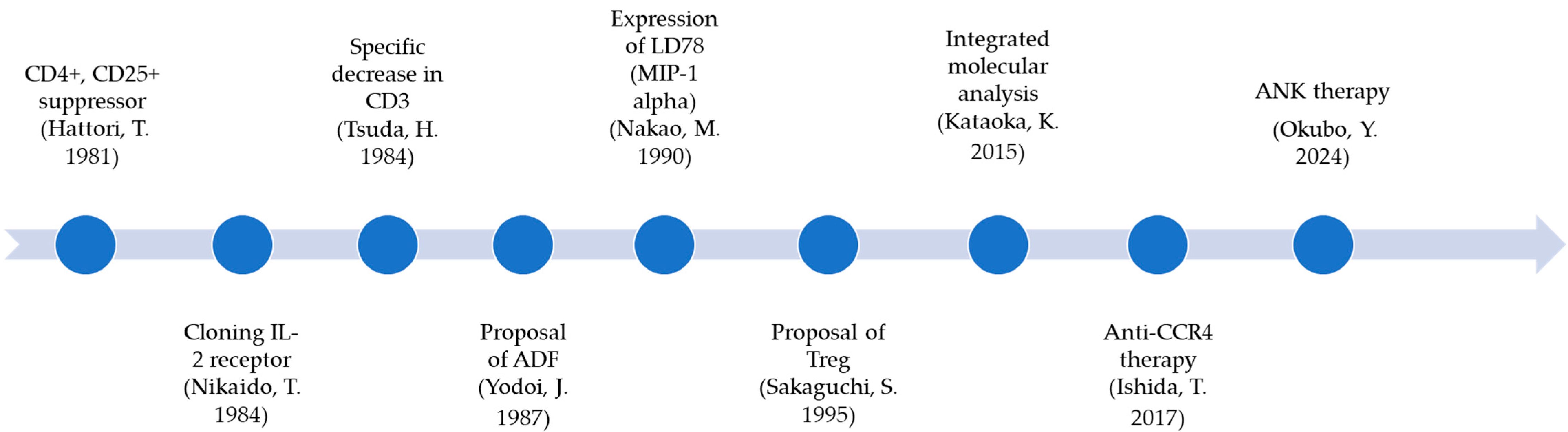
2.1. Development of ATL Biology
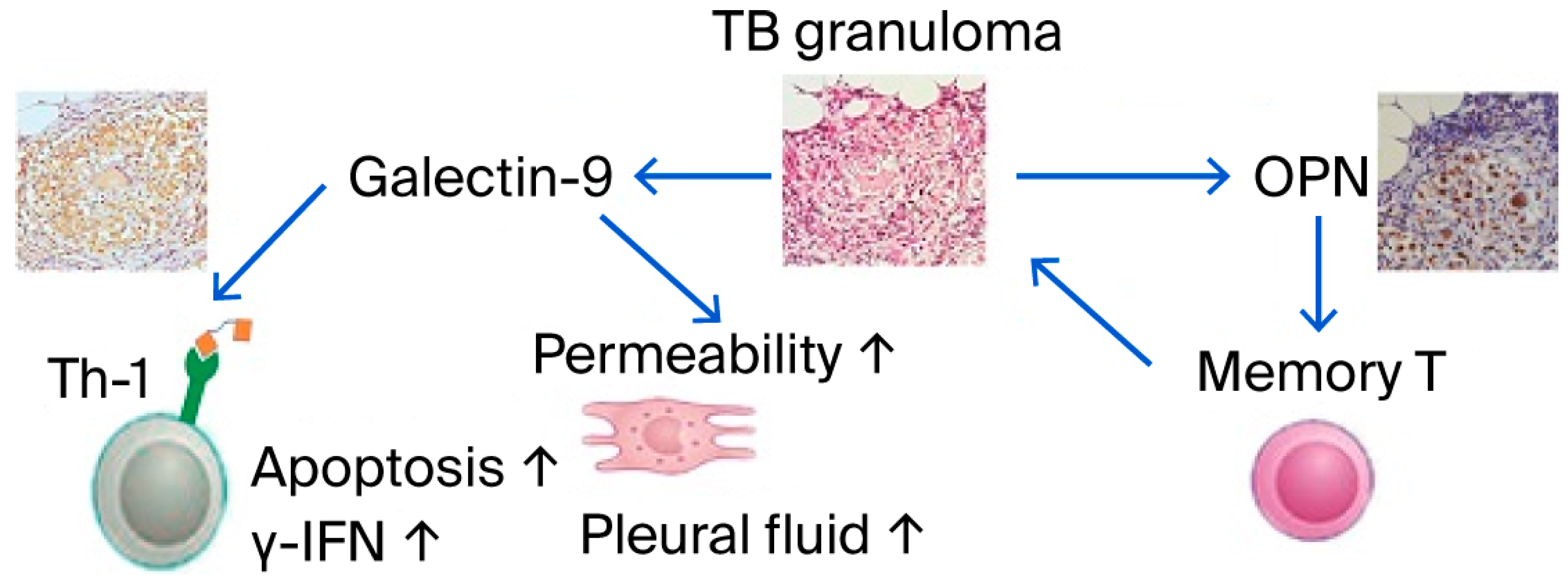
2.2. AIDS/TB Pathophysiology and Social Impact
3. Disaster/Tropical Infectious Diseases
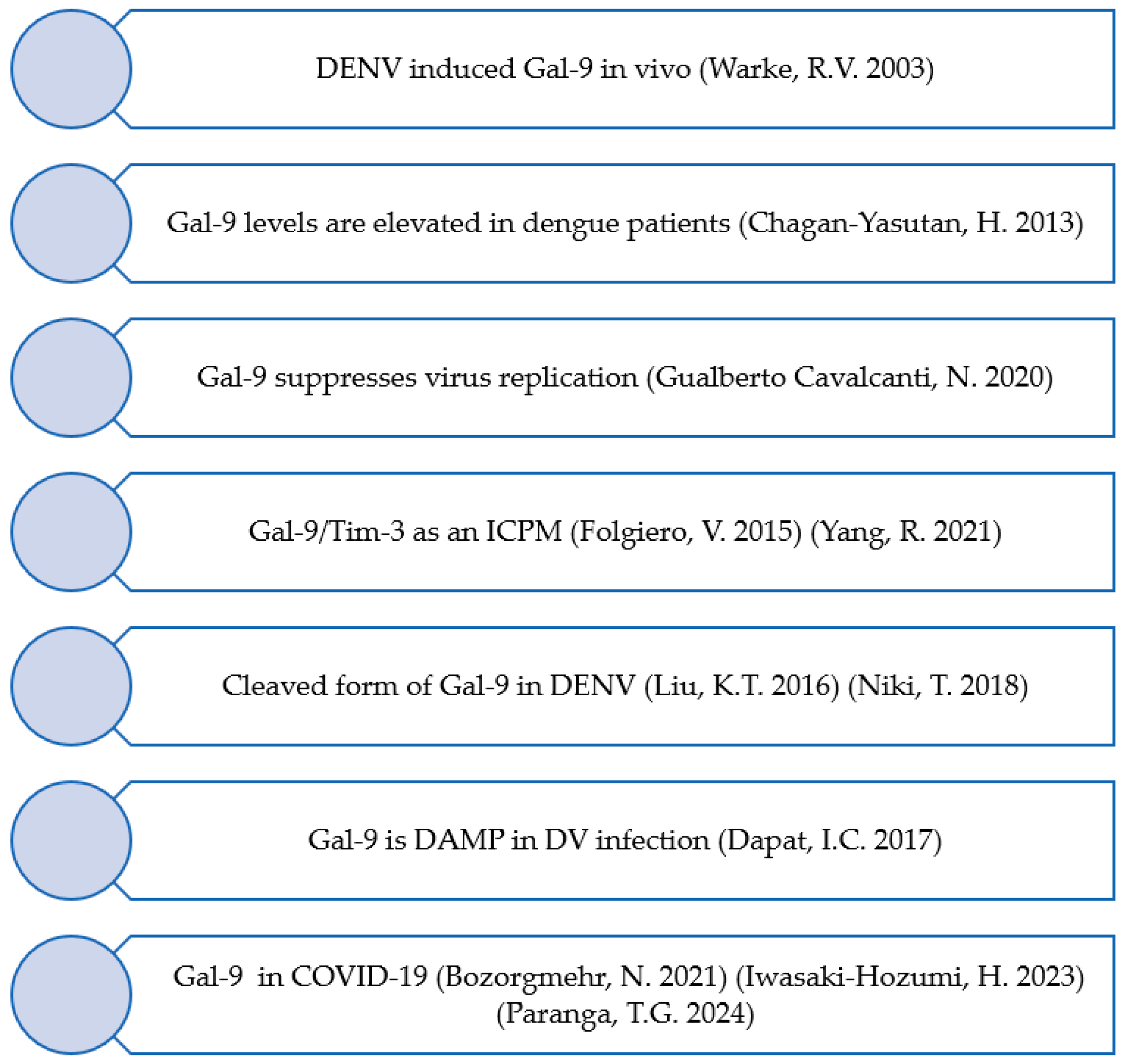
3.1. From Mosquito-Borne Diseases to COVID-19
COVID-19
4. Conclusions
Funding
Conflicts of Interest
References
- Miller, J.F. Immunological function of the thymus. Lancet 1961, 2, 748–749. [Google Scholar] [CrossRef]
- Yodoi, J.; Takatsuki, K.; Masuda, T. Letter: Two cases of T-cell chronic lymphocytic leukemia in Japan. N. Engl. J. Med. 1974, 290, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Reinherz, E.L.; Kung, P.C.; Goldstein, G.; Schlossman, S.F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 1979, 76, 4061–4065. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Uchiyama, T.; Toibana, T.; Takatsuki, K.; Uchino, H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood 1981, 58, 645–647. [Google Scholar] [CrossRef]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, T.; Shimizu, A.; Ishida, N.; Sabe, H.; Teshigawara, K.; Maeda, M.; Uchiyama, T.; Yodoi, J.; Honjo, T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature 1984, 311, 631–635. [Google Scholar] [CrossRef]
- Yodoi, J.; Tagaya, Y.; Okada, M.; Taniguchi, Y.; Hirata, M.; Naramura, M.; Maeda, M. Interleukin-2 receptor-inducing factor(s) in adult T cell leukemia. Acta Haematol. 1987, 78 (Suppl. S1), 56–63. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Nakao, M.; Nomiyama, H.; Shimada, K. Structures of human genes coding for cytokine LD78 and their expression. Mol. Cell. Biol. 1990, 10, 3646–3658. [Google Scholar] [CrossRef]
- Ishida, T.; Utsunomiya, A.; Jo, T.; Yamamoto, K.; Kato, K.; Yoshida, S.; Takemoto, S.; Suzushima, H.; Kobayashi, Y.; Imaizumi, Y.; et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017, 108, 2022–2029. [Google Scholar] [CrossRef]
- Tsuda, H.; Takatsuki, K. Specific decrease in T3 antigen density in adult T-cell leukaemia cells: I. Flow microfluorometric analysis. Br. J. Cancer 1984, 50, 843–845. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Chambers, B.J.; Salcedo, M.; Ljunggren, H.G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity 1996, 5, 311–317. [Google Scholar] [CrossRef]
- Okubo, Y.; Nagai, S.; Katayama, Y.; Kitamura, K.; Hiwaki, K.; Teshigawara, K. Long-term survival of patients with adult T-cell leukemia/lymphoma treated with amplified natural killer cell therapy. Reports 2024, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morb. Mortal. Wkly Rep. 1981, 30, 250–252. [Google Scholar]
- Essex, M.; McLane, M.F.; Lee, T.H.; Falk, L.; Howe, C.W.; Mullins, J.I.; Cabradilla, C.; Francis, D.P. Antibodies to cell membrane antigens associated with human T-cell leukemia virus in patients with AIDS. Science 1983, 220, 859–862. [Google Scholar] [CrossRef]
- Montagnier, L. 25 years after HIV discovery: Prospects for cure and vaccine (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2009, 48, 5815–5826. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.A.; Schwander, S.; Torok, M.E.; Meintjes, G. Human immunodeficiency virus-associated tuberculosis. Clin. Dev. Immunol. 2011, 2011, 513967. [Google Scholar] [CrossRef]
- Aaron, L.; Saadoun, D.; Calatroni, I.; Launay, O.; Mémain, N.; Vincent, V.; Marchal, G.; Dupont, B.; Bouchaud, O.; Valeyre, D.; et al. Tuberculosis in HIV-infected patients: A comprehensive review. Clin. Microbiol. Infect. 2004, 10, 388–398. [Google Scholar] [CrossRef]
- Moyo, D.; Ncube, R.; Kavenga, F.; Chikwava, L.; Mapuranga, T.; Chiboyiwa, N.; Chimunhu, C.; Mudzingwa, F.; Muzvidziwa, O.; Ncube, P.; et al. The Triple Burden of Tuberculosis, Human Immunodeficiency Virus and Silicosis among Artisanal and Small-Scale Miners in Zimbabwe. Int. J. Environ. Res. Public Health 2022, 19, 13822. [Google Scholar] [CrossRef]
- Mahairas, G.G.; Sabo, P.J.; Hickey, M.J.; Singh, D.C.; Stover, C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996, 178, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chandra, E.; Mrigpuri, P. Navigating the dual burden of diabetes mellitus and tuberculosis: A comprehensive review of clinical and public health strategies. Indian J. Tuberc. 2025, 72, 253–258. [Google Scholar] [CrossRef]
- Mandalakas, A.M.; Hesseling, A.C.; Chegou, N.N.; Kirchner, H.L.; Zhu, X.; Marais, B.J.; Black, G.F.; Beyers, N.; Walzl, G. High level of discordant IGRA results in HIV-infected adults and children. Int. J. Tuberc. Lung Dis. 2008, 12, 417–423. [Google Scholar]
- Sester, M.; Altet-Gomez, N.; Andersen, A.B.; Arias-Guillen, M.; Avsar, K.; Bakken Kran, A.M.; Bothamley, G.; Nordholm Breschel, A.C.; Brown, J.; Chesov, D.; et al. Diagnostic accuracy and predictive value of the QuantiFERON-TB gold plus assay for tuberculosis in immunocompromised individuals: A prospective TBnet study. Lancet Reg. Health Eur. 2025, 57, 101416. [Google Scholar] [CrossRef]
- Koguchi, Y.; Kawakami, K.; Uezu, K.; Fukushima, K.; Kon, S.; Maeda, M.; Nakamoto, A.; Owan, I.; Kuba, M.; Kudeken, N.; et al. High plasma osteopontin level and its relationship with interleukin-12-mediated type 1 T helper cell response in tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 1355–1359. [Google Scholar] [CrossRef]
- Inomata, S.; Shijubo, N.; Kon, S.; Maeda, M.; Yamada, G.; Sato, N.; Abe, S.; Uede, T. Circulating interleukin-18 and osteopontin are useful to evaluate disease activity in patients with tuberculosis. Cytokine 2005, 30, 203–211. [Google Scholar] [CrossRef]
- Zhao, J.; Shiratori, B.; Chagan-Yasutan, H.; Matsumoto, M.; Niki, T.; Tanaka, M.; Takahashi, Y.; Usami, O.; Ashino, Y.; Hattori, T. Secretion of IFN-gamma Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis. Int. J. Mol. Sci. 2017, 18, 1382. [Google Scholar] [CrossRef]
- Shete, A.; Bhat, M.; Sawant, J.; Deshpande, S. Both N- and C-terminal domains of galectin-9 are capable of inducing HIV reactivation despite mediating differential immunomodulatory functionalities. Front. Immunol. 2022, 13, 994830. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tong, X.; Wang, L.; Zhang, S.; Huang, J.; Zhang, L.; Fan, H. The association between osteopontin and tuberculosis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0242702. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Bichare, S.; Pujari, V.; Virkar, R.; Thakar, M.; Ghate, M.; Patil, S.; Vyakarnam, A.; Gangakhedkar, R.; Bai, G.; et al. Elevated Levels of Galectin-9 but Not Osteopontin in HIV and Tuberculosis Infections Indicate Their Roles in Detecting MTB Infection in HIV Infected Individuals. Front. Microbiol. 2020, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Ghate, M.; Iwasaki-Hozumi, H.; Patil, S.; Shidhaye, P.; Matsuba, T.; Bai, G.; Pharande, P.; Hattori, T. Association of SARS-CoV-2 Seropositivity with Persistent Immune Activation in HIV/Tuberculosis Co-Infected Patients. Reports 2024, 7, 61. [Google Scholar] [CrossRef]
- Hellamand, M.; Moleman, T.E.; Post, A.P.; Mantel-Teeuwisse, A.K.; Suleman, F.; van den Ham, H.A. Time to inclusion of selected medicines for priority diseases in National Essential Medicines Lists compared with the WHO Model List. BMJ Glob. Health 2025, 10, e018550. [Google Scholar] [CrossRef]
- Shibahara, S. The 2011 Tohoku earthquake and devastating tsunami. Tohoku J. Exp. Med. 2011, 223, 305–307. [Google Scholar] [CrossRef]
- Hattori, T.; Chagan-Yasutan, H.; Koga, S.; Yanagihara, Y.; Tanaka, I. Seminar Lessons: Infectious diseases associated with and causing disaster. Reports 2022, 5, 7. [Google Scholar] [CrossRef]
- Arce, N., Jr.; Boonnak, K.; Bernasor, L.T.; Salas, C.J.; Putri, A.; Aung, P.L.; Imad, H.A.; Chierakul, W.; Luvira, V.; Phonrat, B.; et al. Clinical and Epidemiological Characteristics of Chikungunya and Dengue Infections in Provincial Hospitals of Davao de Oro, Philippines. Reports 2024, 7, 112. [Google Scholar] [CrossRef]
- Imad, H.A.; Putri, A.; Charoenwisedsil, R.; Charoensakulchai, S.; Caumes, E. Febrile Rash: An Early Diagnostic Clue to Infectious Illness in Travelers Returning from Thailand. Reports 2024, 7, 45. [Google Scholar] [CrossRef]
- Warke, R.V.; Xhaja, K.; Martin, K.J.; Fournier, M.F.; Shaw, S.K.; Brizuela, N.; de Bosch, N.; Lapointe, D.; Ennis, F.A.; Rothman, A.L.; et al. Dengue virus induces novel changes in gene expression of human umbilical vein endothelial cells. J. Virol. 2003, 77, 11822–11832. [Google Scholar] [CrossRef]
- Chagan-Yasutan, H.; Ndhlovu, L.C.; Lacuesta, T.L.; Kubo, T.; Leano, P.S.; Niki, T.; Oguma, S.; Morita, K.; Chew, G.M.; Barbour, J.D.; et al. Galectin-9 plasma levels reflect adverse hematological and immunological features in acute dengue virus infection. J. Clin. Virol. 2013, 58, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.T.; Liu, Y.H.; Chen, Y.H.; Lin, C.Y.; Huang, C.H.; Yen, M.C.; Kuo, P.L. Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection. Int. J. Mol. Sci. 2016, 17, 832. [Google Scholar] [CrossRef] [PubMed]
- Gualberto Cavalcanti, N.; Melo Vilar, K.; Branco Pinto Duarte, A.L.; Barreto de Melo Rego, M.J.; Pereira, M.C.; da Rocha Pitta, I.; Diniz Lopes Marques, C.; Galdino da Rocha Pitta, M. Increased serum levels of galectin-9 in patients with chikungunya fever. Virus Res. 2020, 286, 198062. [Google Scholar] [CrossRef]
- Niki, T.; Fujita, K.; Rosen, H.; Hirashima, M.; Masaki, T.; Hattori, T.; Hoshino, K. Plasma Galectin-9 Concentrations in Normal and Diseased Condition. Cell Physiol. Biochem. 2018, 50, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Dapat, I.C.; Pascapurnama, D.N.; Iwasaki, H.; Labayo, H.K.; Chagan-Yasutan, H.; Egawa, S.; Hattori, T. Secretion of Galectin-9 as a DAMP during Dengue Virus Infection in THP-1 Cells. Int. J. Mol. Sci. 2017, 18, 1644. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Wang, M.Y.; Ho, L.J.; Huang, C.Y.; Lai, J.H. Up-regulation of galectin-9 induces cell migration in human dendritic cells infected with dengue virus. J. Cell. Mol. Med. 2015, 19, 1065–1076. [Google Scholar] [CrossRef]
- Merani, S.; Chen, W.; Elahi, S. The bitter side of sweet: The role of Galectin-9 in immunopathogenesis of viral infections. Rev. Med. Virol. 2015, 25, 175–186. [Google Scholar] [CrossRef]
- Folgiero, V.; Cifaldi, L.; Li Pira, G.; Goffredo, B.M.; Vinti, L.; Locatelli, F. TIM-3/Gal-9 interaction induces IFNgamma-dependent IDO1 expression in acute myeloid leukemia blast cells. J. Hematol. Oncol. 2015, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Asgarian-Omran, H.; Valadan, R.; Dindarloo, M.M.; Najafi, A.; Kahrizi, A.; Poursheikhani, A.; Karami, H.; Naderi, M.; Sabeti, S.; et al. Expression of Galectin-9-related immune checkpoint receptors in B-cell acute lymphoblastic leukemia. Iran. J. Basic Med. Sci. 2023, 26, 1468–1474. [Google Scholar] [CrossRef]
- Lam, B.; Miller, J.; Kung, Y.J.; Wu, T.C.; Hung, C.F.; Roden, R.B.S.; Best, S.R. Profiling of VEGF Receptors and Immune Checkpoints in Recurrent Respiratory Papillomatosis. Laryngoscope 2024, 134, 2819–2825. [Google Scholar] [CrossRef]
- Hattori, T.; Ashino, Y. (Eds.) Novel Aspects of COVID-19 After a Two-Year Pandemic; MDPI: Basel, Switzerland, 2024. [Google Scholar]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Paranga, T.G.; Pavel-Tanasa, M.; Constantinescu, D.; Iftimi, E.; Plesca, C.E.; Miftode, I.L.; Cianga, P.; Miftode, E. Distinct soluble immune checkpoint profiles characterize COVID-19 severity, mortality and SARS-CoV-2 variant infections. Front. Immunol. 2024, 15, 1464480. [Google Scholar] [CrossRef]
- Bozorgmehr, N.; Mashhouri, S.; Perez Rosero, E.; Xu, L.; Shahbaz, S.; Sligl, W.; Osman, M.; Kutsogiannis, D.J.; MacIntyre, E.; O’Neil, C.R.; et al. Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection. mBio 2021, 12, e00384-21. [Google Scholar] [CrossRef]
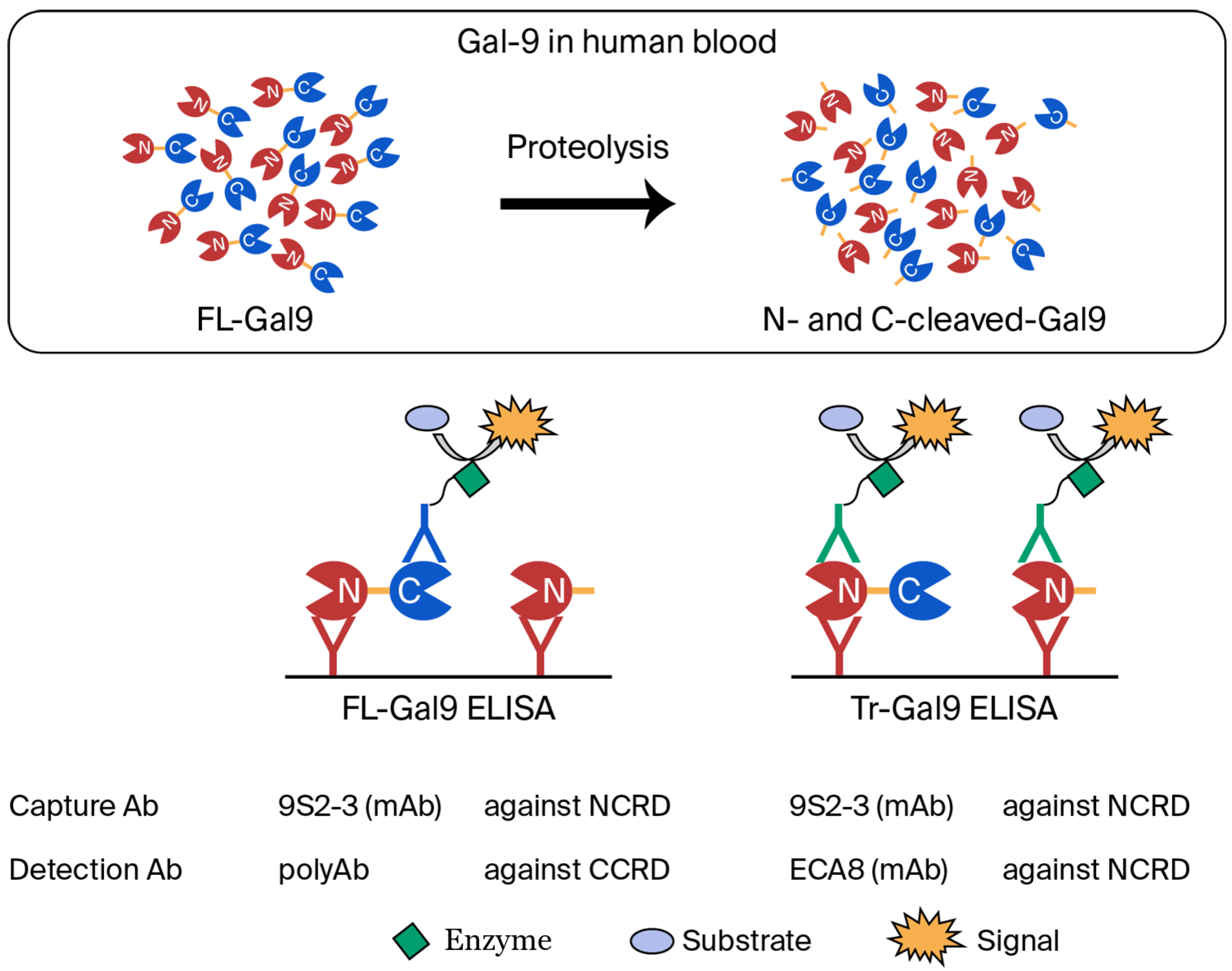
- Iwasaki-Hozumi, H.; Maeda, Y.; Niki, T.; Chagan-Yasutan, H.; Bai, G.; Matsuba, T.; Furushima, D.; Ashino, Y.; Hattori, T. Plasma N-Cleaved Galectin-9 Is a Surrogate Marker for Determining the Severity of COVID-19 and Monitoring the Therapeutic Effects of Tocilizumab. Int. J. Mol. Sci. 2023, 24, 3591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, X.; Sun, J.; Shao, Y.; Song, W.; Qi, T.; Wang, Z.; Tang, Y.; Sun, J.; Xun, J.; et al. Plasma proteomic profiling of hospitalized patients co-infected with HIV and SARS-CoV-2. Front. Immunol. 2025, 16, 1601672. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Mpofu, E.; Brock, K.; Ingman, S. COVID-19 hospitalization place of live discharge outcomes for long-term care facility residents with dementia: Mediation by comorbidities index scores and moderation by health insurance status. Geriatr. Nurs. 2025, 64, 103356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, T. Unlocking Medical Breakthroughs: The Transformative Role of Case Reports in Clinical Discovery. Reports 2025, 8, 216. https://doi.org/10.3390/reports8040216
Hattori T. Unlocking Medical Breakthroughs: The Transformative Role of Case Reports in Clinical Discovery. Reports. 2025; 8(4):216. https://doi.org/10.3390/reports8040216
Chicago/Turabian StyleHattori, Toshio. 2025. "Unlocking Medical Breakthroughs: The Transformative Role of Case Reports in Clinical Discovery" Reports 8, no. 4: 216. https://doi.org/10.3390/reports8040216
APA StyleHattori, T. (2025). Unlocking Medical Breakthroughs: The Transformative Role of Case Reports in Clinical Discovery. Reports, 8(4), 216. https://doi.org/10.3390/reports8040216




