Metabolism-Guided LATTICE Radiotherapy in an Elderly Patient with Locally Advanced Head and Neck Cancer Treated with Curative Aim: A Case Report
Abstract
1. Introduction and Clinical Significance
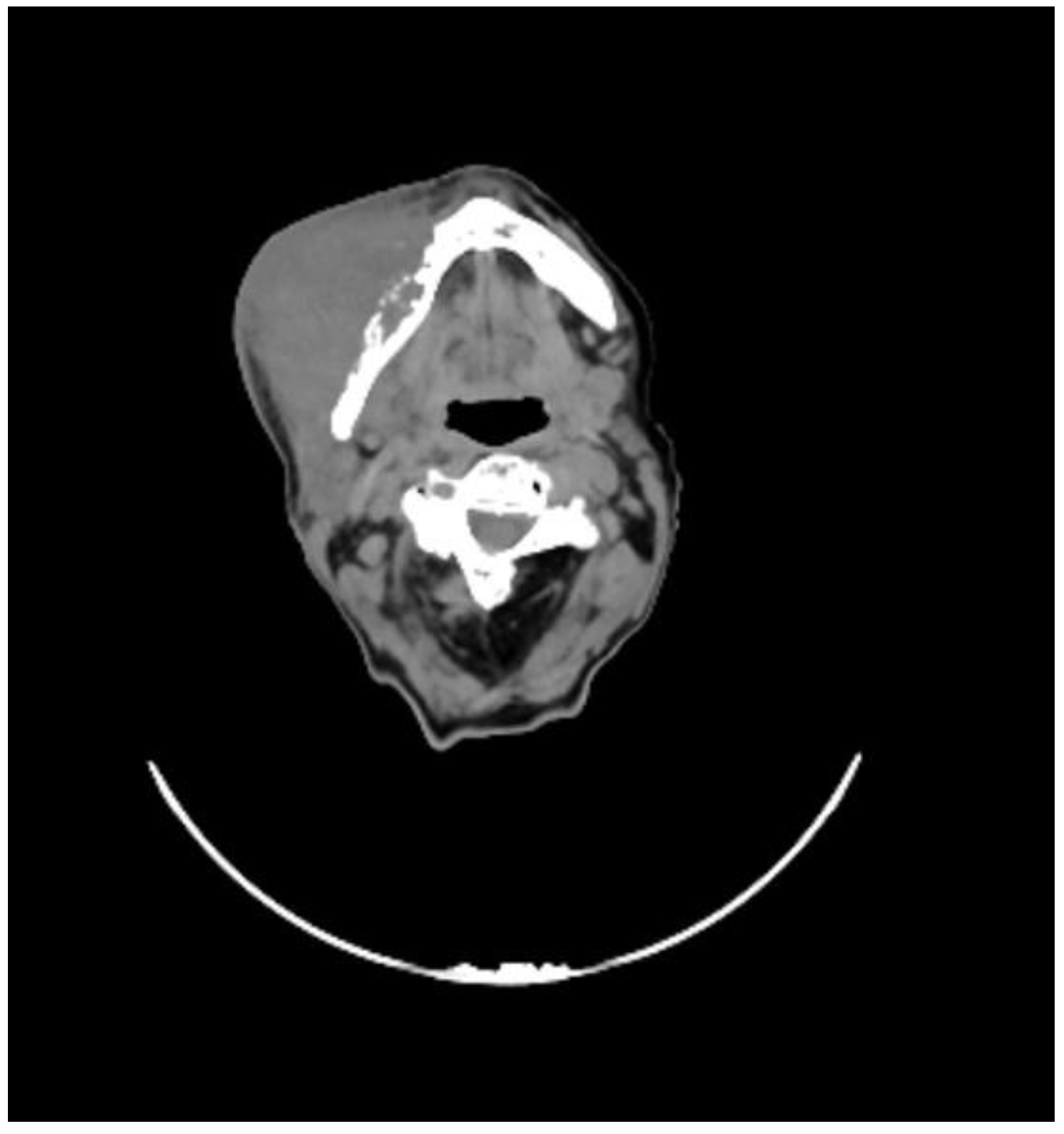
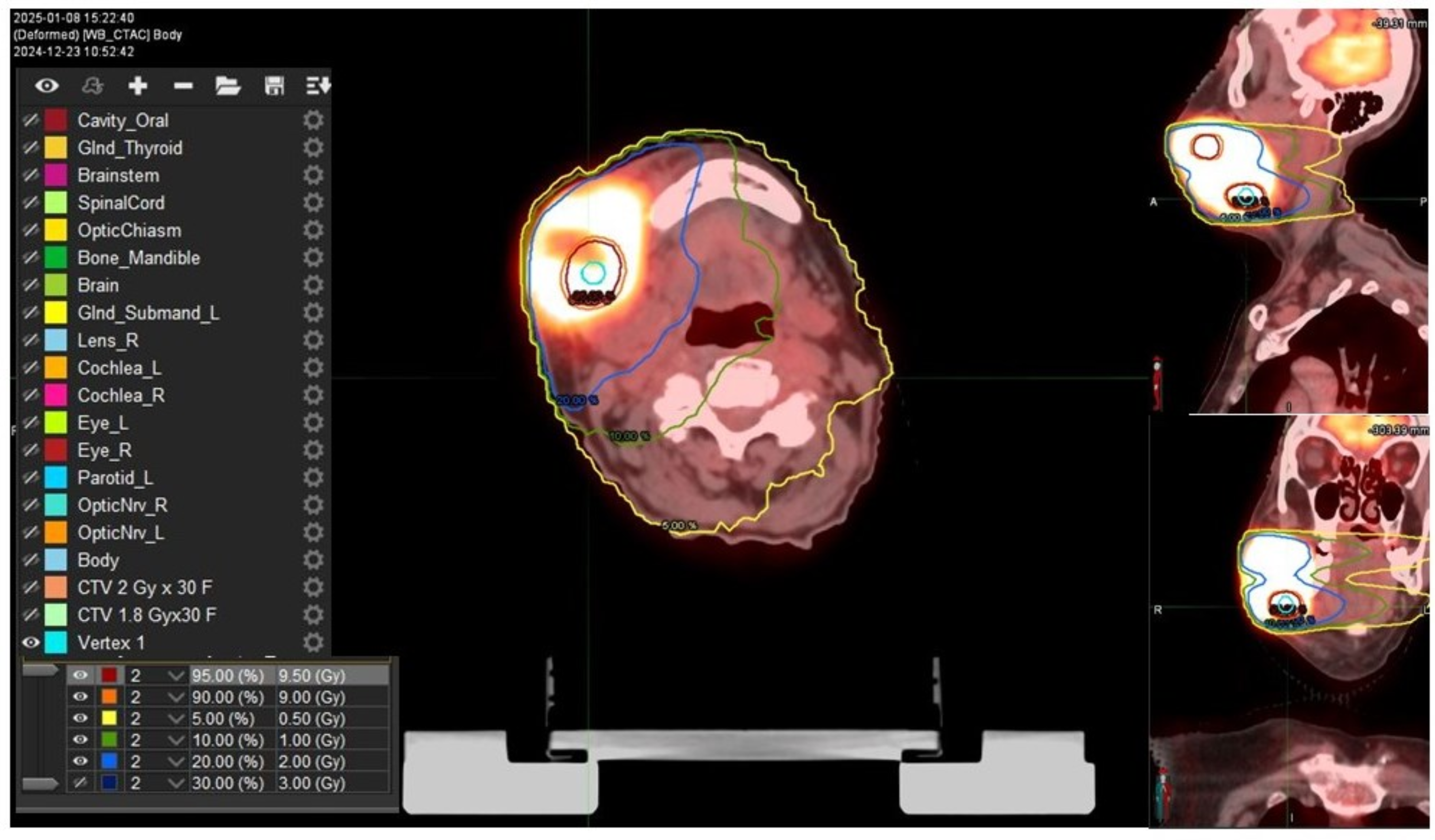
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HNSCC | Head and neck squamous cell carcinoma |
| RT | Radiotherapy |
| LA | Locally advanced |
| SFRT | Spatially fractionated radiotherapy |
| CT | Computed tomography |
| PET | Positron emission tomography |
| AJCC | American Joint Committee on Cancer |
| LRT | LATTICE radiotherapy |
| cVMAT | Conventional volumetric modulated arc radiotherapy |
| Vs | Vertices |
| SVMAT | Stereotactic volumetric modulated arc therapy |
| B-GTV | Bulky gross tumor volume |
| Vx | Percentage of volume receiving any dose |
| CTV | Clinical target volume |
| CNV | Clinical nodal volume |
| Dmax | Maximum of dose |
| Dmean | Mean dose |
| CRT | Chemo-radiotherapy |
| GRT | GRID radiotherapy |
| MRT | Microbeam radiation therapy |
| MBRT | Minibeam radiation therapy |
| APC | Antigen-presenting cells |
| TME | Tumor microenvironment |
References
- Zumsteg, Z.S.; Cook-Wiens, G.; Yoshida, E.; Shiao, S.L.; Lee, N.Y.; Mita, A.; Jeon, C.; Goodman, M.T.; Ho, S.A. Incidence of Oropharyngeal Cancer Among Elderly Patients in the United States. JAMA Oncol. 2016, 2, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haehl, E.; Rühle, A.; David, H.; Kalckreuth, T.; Sprave, T.; Stoian, R.; Becker, C.; Knopf, A.; Grosu, A.L.; Nicolay, N.H. Radiotherapy for geriatric head-and-neck cancer patients: What is the value of standard treatment in the elderly? Radiat. Oncol. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, W.; Khan, M.K.; Wu, X.; Simone, C.B., 2nd; Fan, J.; Gressen, E.; Zhang, X.; Limoli, C.L.; Bahig, H.; Tubin, S.; et al. Spatially fractionated radiation therapy: History, present and the future. Clin. Transl. Radiat. Oncol. 2019, 20, 30–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferini, G.; Parisi, S.; Lillo, S.; Viola, A.; Minutoli, F.; Critelli, P.; Valenti, V.; Illari, S.I.; Brogna, A.; Umana, G.E.; et al. Impressive Results after “Metabolism-Guided” Lattice Irradiation in Patients Submitted to Palliative Radiation Therapy: Preliminary Results of LATTICE_01 Multicenter Study. Cancers 2022, 14, 3909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bisello, S.; Cilla, S.; Benini, A.; Cardano, R.; Nguyen, N.P.; Deodato, F.; Macchia, G.; Buwenge, M.; Cammelli, S.; Wondemagegnehu, T.; et al. Dose-Volume Constraints fOr oRganS at risk In Radiotherapy (CORSAIR): An “All-in-One” Multicenter-Multidisciplinary Practical Summary. Curr. Oncol. 2022, 29, 7021–7050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maggiore, R.; Zumsteg, Z.S.; BrintzenhofeSzoc, K.; Trevino, K.M.; Gajra, A.; Korc-Grodzicki, B.; Epstein, J.B.; Bond, S.M.; Parker, I.; Kish, J.A.; et al. CARG-HNC Study Group. The Older Adult with Locoregionally Advanced Head and Neck Squamous Cell Carcinoma: Knowledge Gaps and Future Direction in Assessment and Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 868–883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pontoriero, A.; Iatì, G.; Aiello, D.; Pergolizzi, S. Stereotactic Radiotherapy in the Retreatment of Recurrent Cervical Cancers, Assessment of Toxicity, and Treatment Response: Initial Results and Literature Review. Technol. Cancer Res. Treat. 2016, 15, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Michal, S.A.; Adelstein, D.J.; Rybicki, L.A.; Rodriguez, C.P.; Saxton, J.P.; Wood, B.G.; Scharpf, J.; Ives, D.I. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head Neck 2012, 34, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Barzan, L.; Franchin, G.; Cinelli, R.; Balestreri, L.; Tirelli, U.; Vaccher, E. Treatment of head and neck cancer in elderly patients: State of the art and guidelines. Crit. Rev. Oncol. Hematol. 2005, 53, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Brockstein, B.E.; Haraf, D.J.; Stenson, K.M.; Mittal, B.B.; Kies, M.S.; Rosen, F.R.; Jovanovic, B.; Vokes, E.E. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin. Cancer. Res. 2004, 10, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X. Which Modality of SFRT Should be Considered First for Bulky Tumor Radiation Therapy, GRID or LATTICE? Semin. Radiat. Oncol. 2024, 34, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Fujita, M.; Regine, W.F.; Megooni, A.S.; Ibbott, G.S.; Ahmed, M.M. High-dose spatially fractionated radiation (GRID): A new paradigm in the management of advanced cancers. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Perez, N.C.; Zheng, Y.; Li, X.; Jiang, L.; Amendola, B.E.; Xu, B.; Mayr, N.A.; Lu, J.J.; Hatoum, G.F.; et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat. Res. 2020, 194, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Eley, J.; Schmid, T.E.; Combs, S.E.; Dendale, R.; Prezado, Y. Spatially fractionated proton minibeams. Br. J. Radiol. 2019, 92, 20180466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekker, R.A.; Obertopp, N.; Redler, G.; Penagaricano, J.; Caudell, J.J.; Yamoah, K.; Pilon-Thomas, S.; Moros, E.G.; Enderling, H. Spatially fractionated GRID radiation potentiates immune-mediated tumor control. Radiat. Oncol. 2024, 19, 121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMillan, M.T.; Khan, A.J.; Powell, S.N.; Humm, J.; Deasy, J.O.; Haimovitz-Friedman, A. Spatially Fractionated Radiotherapy in the Era of Immunotherapy. Semin. Radiat. Oncol. 2024, 34, 276–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carvalho, H.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018, 73 (Suppl. S1), e557s. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrera, F.G.; Ronet, C.; Ochoa de Olza, M.; Barras, D.; Crespo, I.; Andreatta, M.; Corria-Osorio, J.; Spill, A.; Benedetti, F.; Genolet, R.G.; et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022, 12, 108–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parisi, S.; Sciacca, M.; Critelli, P.; Ferrantelli, G.; Chillari, F.; Venuti, V.; Napoli, C.; Shteiwi, I.; Siragusa, C.; Brogna, A.; et al. Lattice radiotherapy in inflammatory breast cancer: Report of a first case treated with curative aim. Radiat. Oncol. J. 2024, 42, 160–165. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Zhou, J.; Yuan, K.; Wang, X.; Li, L.; Lang, J.; Lu, S. Spatially fractionated radiotherapy (Lattice SFRT) in the palliative treatment of locally advanced bulky unresectable head and neck cancer. Clin. Transl. Radiat. Oncol. 2024, 48, 100830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Studer, G.; Jeller, D.; Streller, T.; Huebner, D.; Glanzmann, C. Time-Related Outcome Following Palliative Spatially Fractionated Stereotactic Radiation Therapy (Lattice) of Large Tumors—A Case Series. Adv. Radiat. Oncol. 2024, 9, 101566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferini, G.; Valenti, V.; Tripoli, A.; Illari, S.I.; Molino, L.; Parisi, S.; Cacciola, A.; Lillo, S.; Giuffrida, D.; Pergolizzi, S. Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers 2021, 13, 3290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iati’, G.; Parisi, S.; Ferrantelli, G.; Pergolizzi, S. Metabolism-Guided LATTICE Radiotherapy in an Elderly Patient with Locally Advanced Head and Neck Cancer Treated with Curative Aim: A Case Report. Reports 2025, 8, 213. https://doi.org/10.3390/reports8040213
Iati’ G, Parisi S, Ferrantelli G, Pergolizzi S. Metabolism-Guided LATTICE Radiotherapy in an Elderly Patient with Locally Advanced Head and Neck Cancer Treated with Curative Aim: A Case Report. Reports. 2025; 8(4):213. https://doi.org/10.3390/reports8040213
Chicago/Turabian StyleIati’, Giuseppe, Silvana Parisi, Giacomo Ferrantelli, and Stefano Pergolizzi. 2025. "Metabolism-Guided LATTICE Radiotherapy in an Elderly Patient with Locally Advanced Head and Neck Cancer Treated with Curative Aim: A Case Report" Reports 8, no. 4: 213. https://doi.org/10.3390/reports8040213
APA StyleIati’, G., Parisi, S., Ferrantelli, G., & Pergolizzi, S. (2025). Metabolism-Guided LATTICE Radiotherapy in an Elderly Patient with Locally Advanced Head and Neck Cancer Treated with Curative Aim: A Case Report. Reports, 8(4), 213. https://doi.org/10.3390/reports8040213





