Highlighting the Importance of Signaling Pathways and Immunohistochemistry Features in HCC: A Case Report and Literature Review
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Clinical Features
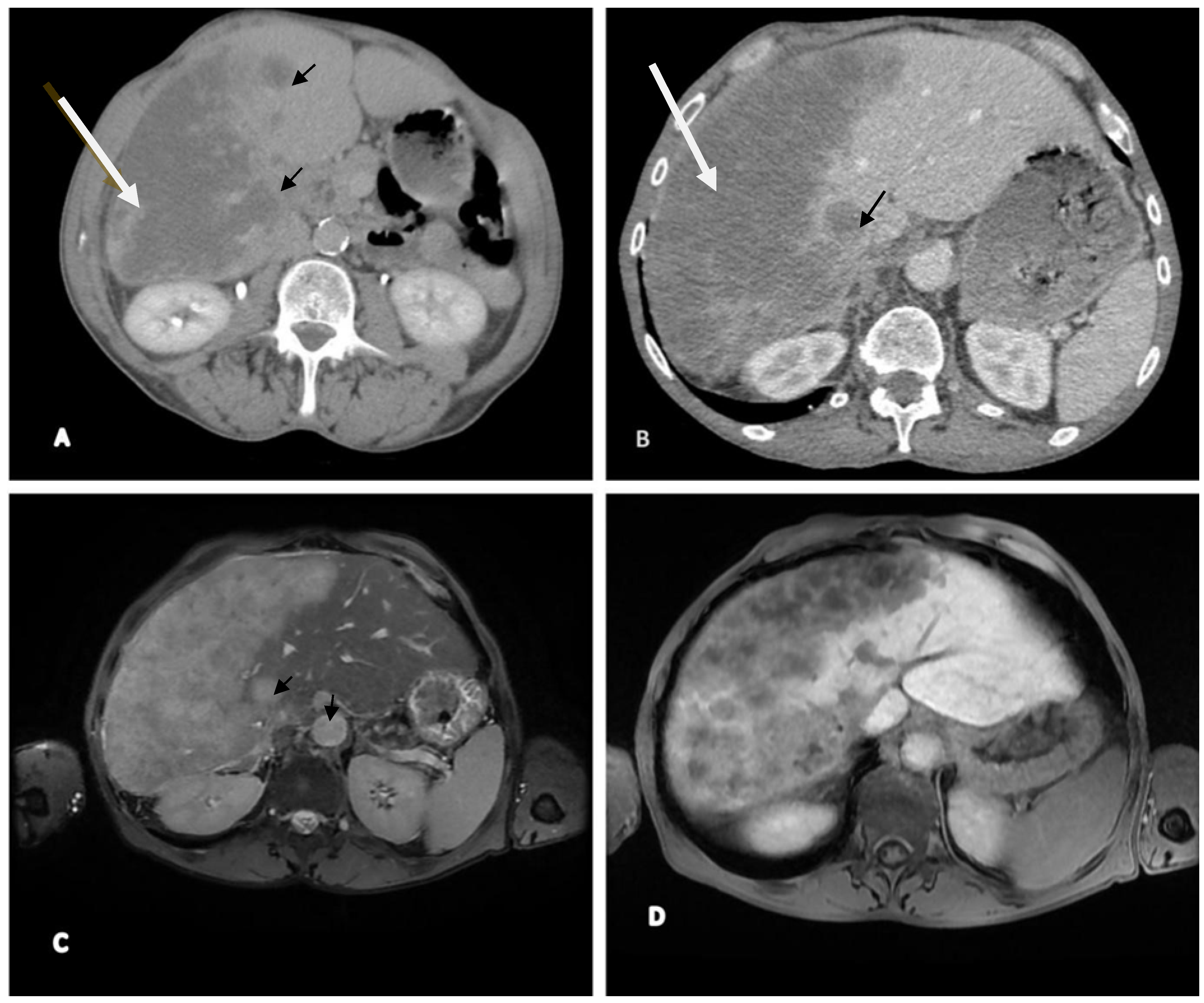
2.2. Imaging (CT and MRI)
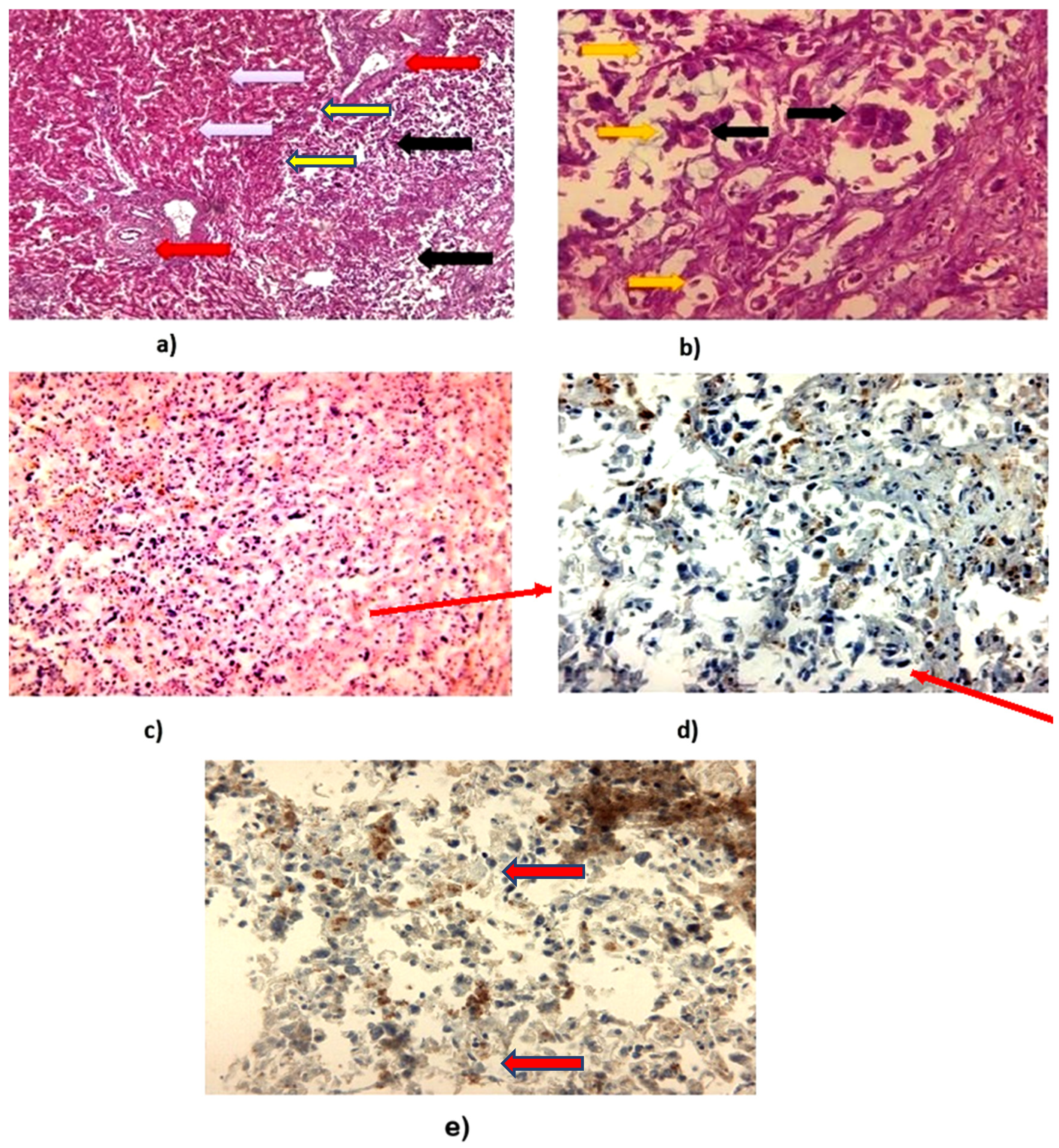
2.3. Histopathological and Immunohistochemistry Analysis
3. Discussion
3.1. PIK3CA Mutations and Their Role in Hepatocellular Carcinoma
3.2. PYGO2 Mutations
Oncogenic Effects of PI3K/AKT/mTOR Activation in Hepatocellular Carcinoma (HCC)
3.3. Challenges and Treatment Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Yoshioka, K.; Asanuma, K.; Okamoto, Y.; Takuwa, N.; Sasaki, T.; Takuwa, Y. Essential role of class II phosphatidylinositol-3-kinase-C2α in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells. J. Biol. Chem. 2013, 288, 2325–2339. [Google Scholar] [CrossRef]
- Städeli, R.; Basler, K. Dissecting nuclear Wingless signaling: Recruitment of the transcriptional co-activator Pygopus by a chain of adaptor proteins. Mech. Dev. 2005, 122, 1171–1182. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.E.; Saber, S.; Youssef, M.E.; Eissa, H.; El-Ahwany, E.; Amin, N.A.; Alqarni, M.; Batiha, G.E.; Obaidullah, A.J.; Kaddah, M.M.Y.; et al. AKT-AMPKα-mTOR-dependent HIF-1α Activation is a New Therapeutic Target for Cancer Treatment: A Novel Approach to Repositioning the Antidiabetic Drug Sitagliptin for the Management of Hepatocellular Carcinoma. Front. Pharmacol. 2022, 12, 720173. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Liu, L.J.; Xie, S.X.; Chen, Y.T.; Xue, J.L.; Zhang, C.J.; Zhu, F. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7486–7499. [Google Scholar] [CrossRef]
- Dimri, M.; Satyanarayana, A. Molecular Signaling Pathways and Therapeutic Targets in Hepatocellular Carcinoma. Cancers 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Mínguez, B.; Tovar, V.; Chiang, D.; Villanueva, A.; Llovet, J.M. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr. Opin. Gastroenterol. 2009, 25, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Liu, P.; Xu, J.; Zhao, W.; Xie, C.; Yin, Z.; Wang, X. Pygopus-2 promotes invasion and metastasis of hepatic carcinoma cell by decreasing E-cadherin expression. Oncotarget 2015, 6, 11074–11086. [Google Scholar] [CrossRef]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Buonaguro, L.; Tatangelo, F.; Botti, G.; Izzo, F.; Buonaguro, F.M. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics 2013, 102, 74–83. [Google Scholar] [CrossRef]
- He, G.; Karin, M. NF-κB and STAT3–key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef]
- Coulouarn, C.; Factor, V.M.; Thorgeirsson, S.S. Transforming growth factor-β gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology 2008, 47, 2059–2067. [Google Scholar] [CrossRef]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Aasland, R.; Gibson, T.J.; Stewart, A.F. The PHD finger: Implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 1995, 20, 56–59. [Google Scholar] [CrossRef]
- Tanase, A.M.; Marchio, A.; Dumitrascu, T.; Dima, S.; Herlea, V.; Oprisan, G.; Dejean, A.; Popescu, I.; Pineau, P. Mutation spectrum of hepatocellular carcinoma from eastern-European patients betrays the impact of a complex exposome. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 256–263. [Google Scholar] [CrossRef]
- Kondo, Y.; Kanai, Y.; Sakamoto, M.; Mizokami, M.; Ueda, R.; Hirohashi, S. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis--A comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology 2000, 32, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Xu, M.L.; Wang, S.Q.; Zhang, F.; Wang, L.; Wang, H.Q. Overexpression of Pygopus-2 is required for canonical Wnt activation in human lung cancer. Oncol. Lett. 2014, 7, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bienz, M.; Yan, X.X.; Xu, W. Structural basis of the interaction between BCL9-Pygo and LDB-SSBP complexes in assembling the Wnt enhanceosome. Nat. Commun. 2023, 14, 3702. [Google Scholar] [CrossRef] [PubMed]
- Carrera, I.; Janody, F.; Leeds, N.; Duveau, F.; Treisman, J.E. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proc. Natl. Acad. Sci. USA 2008, 105, 6644–6649. [Google Scholar] [CrossRef]
- Kessler, R.; Hausmann, G.; Basler, K. The PHD domain is required to link Drosophila Pygopus to Wnt target genes. Dev. Biol. 2009, 334, 93–103. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Q.; Yuan, Y.; Huang, X.; Cai, W.; Li, C.; Wei, T.; Zhang, L.; Yang, M.; Liu, Q.; et al. Pygo2 associates with MLL2 histone methyltransferase and GCN5 histone acetyltransferase complexes to augment Wnt target gene expression and breast cancer stem-like cell expansion. Mol. Cell. Biol. 2010, 30, 5621–5635. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Z.; Li, H.; Wang, Z.; Chen, Y.; Bao, J.; Chen, B.; Xu, S.; Xia, E.; Ye, D.; et al. Glycosylphosphatidylinositol anchor biosynthesis pathway and immune cell regulation. Front. Immunol. 2024, 15, 1392940. [Google Scholar] [CrossRef]
- Jessen, S.; Gu, B.; Dai, X. Pygopus and the Wnt signaling pathway: A diverse set of connections. Bioessays 2008, 30, 488–496. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Wu, Q.; Zheng, Y.; Kaweme, N.M.; Zhang, Z.; Cai, M.; Dong, Y. Pygo2 as a novel biomarker in gastric cancer for monitoring drug resistance by upregulating MDR1. J. Cancer 2021, 12, 2952–2959. [Google Scholar] [CrossRef]
- Kao, K.R.; Popadiuk, P.; Thoms, J.; Aoki, S.; Anwar, S.; Fitzgerald, E.; Andrews, P.; Voisey, K.; Gai, L.; Challa, S.; et al. PYGOPUS2 expression in prostatic adenocarcinoma is a potential risk stratification marker for PSA progression following radical prostatectomy. J. Clin. Pathol. 2018, 71, 402–411. [Google Scholar] [CrossRef]
- Saxena, M.; Kalathur, R.K.R.; Rubinstein, N.; Vettiger, A.; Sugiyama, N.; Neutzner, M.; Coto-Llerena, M.; Kancherla, V.; Ercan, C.; Piscuoglio, S.; et al. A Pygopus 2-Histone Interaction Is Critical for Cancer Cell Dedifferentiation and Progression in Malignant Breast Cancer. Cancer Res. 2020, 80, 3631–3648. [Google Scholar] [CrossRef]
- Kramps, T.; Peter, O.; Brunner, E.; Nellen, D.; Froesch, B.; Chatterjee, S.; Murone, M.; Züllig, S.; Basler, K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 2002, 109, 47–60. [Google Scholar] [CrossRef]
- Sun, P.; Watanabe, K.; Fallahi, M.; Lee, B.; Afetian, M.E.; Rheaume, C.; Wu, D.; Horsley, V.; Dai, X. Pygo2 regulates β-catenin–induced activation of hair follicle stem/progenitor cells and skin hyperplasia. Proc. Natl. Acad. Sci. USA 2014, 111, 10215–10220. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian Shalchi, F.; Hosseini, N.; Nourmohammadi, F.; Ahmadian Shalchi, M.A.; Forghanifard, M.M. PYGO2 regulates IL10 and plays immunosuppressive role through ESCC progression. BMC Mol. Cell Biol. 2025, 29, 14. [Google Scholar] [CrossRef]
- Soleymani, S.; Khales, S.A.; Jafarian, A.H.; Kalat, H.R.; Forghanifard, M.M. PYGO2 as an independent diagnostic marker expressed in a majority of colorectal cancers. J. Histotechnol. 2019, 42, 98–103. [Google Scholar] [CrossRef]
- Qian, J.; Lei, X.; Sun, Y.; Zheng, L.; Li, J.; Zhang, S.; Zhang, L.; Li, W.; Shi, J.; Jia, W.; et al. Long non-coding RNA SNHG8 enhances triple-negative breast cancer cell proliferation and migration by regulating the miR-335-5p/PYGO2 axis. Biol. Direct 2021, 16, 13. [Google Scholar] [CrossRef]
- Zhang, Z.; Westover, D.; Tang, Z.; Liu, Y.; Sun, J.; Sun, Y.; Zhang, R.; Wang, X.; Zhou, S.; Hesilaiti, N.; et al. Wnt/β-catenin signaling in the development and therapeutic resistance of non-small cell lung cancer. J. Transl. Med. 2024, 22, 565. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: Signaling pathways and therapeutic advances. Sig. Transduct. Target Ther. 2025, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.; Lam, H.Y.; Yap, K.C.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
| Test | Results | UM | Normal Values |
|---|---|---|---|
| White blood cells (WBC) | 23.13 | 103/μL | 4.0–10.0 |
| Neutrophils (NEU) | 9.63 | 103/μL | 2.4–6.5 |
| Lymphocytes (LYM) | 5.62 | 103/μL | 1.0–4.0 |
| Monocytes (Mono) | 0.58 | 103/μL | 0.3–1.0 |
| Red blood cells (RBC) | 6.87 | 106/μL | 3.8–5.1 |
| Hematocrit (HCT) | 30.89 | % | 35–47 |
| Platelets | 80 | 109/μL | 150–400 |
| Hemoglobin (HGB) | 7.6 | g/dL | 13.2–17.3 |
| MCV | 82 | FL | 80–100FL |
| MCHC | 40 | g/dL | 31–36 |
| MCH | 38 | Pg | 24–32 |
| pH | 7.23 | - | 7.35–7.45 |
| Serum creatinine | 1.5 | mg/dL | 0.10–1.2 |
| Glycemia | 102 | mg/dL | 65–115 |
| Glomerular filtration rate (GFR) | 60.26 | mL/min/1.73 m2 | >90 mL/min/1.73 m2 |
| Uric acid | 5.5 | mg/dL | 3.5–7.2 |
| Aspartate aminotransferase (AST/GOT) | 125 | U/L | 5–34 |
| Alanine aminotransferase (AST/GOT) | 180 | U/L | 0–55 |
| Bilirubin | 3.2 | mg/dL | 0.2–1.2 |
| Cholesterol (CHOL) | 189 | mg/dL | 0–199 |
| HDL CHOL | 55 | mg/dL | 40–60 |
| LDL CHOL | 98 | mg/dL | <100 |
| INR | 3.01 | - | 0.8–1.2 |
| PT | 20 | Seconds | 10–13 |
| APTT | 52 | Seconds | 25–36 |
| Fibrinogen | 220 | mg/dL | 130–330 |
| HBsAg | Negative | Negative | |
| Anti-HBc IgM + IgG | Negative | - | Negative |
| Anti-HBs | 14 | mUi/mL | >10 mUi/mL |
| PCR for HBV-DNA | Negative | Copies/mL | Negative |
| Anti-HCV | Negative | - | Negative |
| HIV (Ab + Ag p24) | Negative | - | Negative |
| Serum albumin | 2.2 | g/dL | 3.4–5.4 |
| Lactate dehydrogenase | 360 | U/L | 140–280 |
| Alkaline Phosphatase | 289 | IU/L | 44–147 |
| Alpha 1 antitrypsin | 378 | mg/dL | 80–220 |
| Alpha fetoprotein | 420 | ng/dL | 0–40 |
| CRP | 52 | mg/dL | 0–5.0 |
| ESR | 50 | mm/h | 2–30.0 |
| Serum iron | 48 | Mcg/dL | 60–160 |
| Carcinoembryonic antigen (CEA) | 3.1 | Ng/mL | 0–2.9 |
| Carbohydrate antigen 19-9 (CA 19-9) | 58 | u/mL | 0–37 u/mL |
| Mutation Type | Description | Mutation Location | Mechanism of Action | Effect on PI3K Signaling | Functional Consequence | Cancer Types Associated | Prevalence in Cancer | Therapeutic Implications | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Hotspot Mutations (Gain-of-function) | Frequent mutations leading to enhanced kinase activity. | Exon 9 (E545K), Exon 20 (H1047R) | Alterations in the helicoidal (Exon 9) and kinase (Exon 20) domains, causing abnormal activation. | Constitutive activation of PI3K, driving tumorigenesis | Promotes cell survival, growth, and proliferation; resistance to apoptosis | Breast, Colon, Endometrial, Ovarian, Glioblastoma, and others | High in breast cancer, colon cancer, and more | Target for PI3K inhibitors, potential biomarker for therapy | [18] |
| Point Mutations (Missense) | Single base substitutions that lead to amino acid changes, often at hotspot sites. | Exon 9 (E542K, E545K), Exon 20 (H1047R, H1047L) | Missense mutations in critical regions of the gene, particularly affecting kinase and helical domains. | Increased PI3K kinase activity, activating downstream signaling | Altered protein function, leading to dysregulated growth and survival | Breast, Colon, Ovarian, Endometrial, and others | Common in breast, colon, and other epithelial cancers | Targetable with PI3K-specific inhibitors or combination therapies | [19] |
| In-frame Insertions and Deletions (Indels) | Small insertions or deletions (indels) within critical regions, especially in the helical or kinase domains. | Helical and kinase domains (Exons 9–20) | In-frame alterations in the sequence, leading to disruptions in protein folding and function. | Can lead to hyperactivation or dysfunction depending on the site and nature of the change | Leads to continuous activation of PI3K pathway or disrupts normal regulation | Breast, Endometrial, Gastrointestinal, and others | Less frequent than point mutations, but significant in some cancers | May be sensitive to PI3K inhibitors, depending on the mutation | [20] |
| Copy Number Variations (CNVs) | Gene amplifications or deletions, often leading to altered expression levels of PIK3CA. | Entire PIK3CA gene or flanking regions | Amplification of the PIK3CA gene leads to overexpression, causing constitutive activation. | Overexpression of PIK3CA, leading to sustained PI3K signaling | Increased cell proliferation and survival due to overexpression | Breast, Ovarian, Endometrial, and other cancers | Gene amplification detected in ~30% of breast cancers | Can be targeted by drugs that reduce gene expression or inhibit downstream signaling | [21] |
| Complex Mutations and Fusions | Chromosomal rearrangements or translocations affecting PIK3CA or regulatory regions. | Chromosomal translocations, structural variants | Gene rearrangements that result in altered expression or function of PIK3CA protein. | May lead to aberrant PI3K pathway activation or altered protein interactions | Can result in dysregulated cell growth or resistance to apoptosis | Rare, but can be seen in some aggressive cancers | Rare but aggressive cancer subtypes | Potential for targeted therapies based on the specific fusion partners | [22] |
| Loss-of-function Mutations (Rare) | Mutations leading to loss of function, such as nonsense, frameshift, or splice-site mutations. | Various regions of PIK3CA gene | Frameshift or nonsense mutations that create truncated, nonfunctional proteins. | Loss of PI3K signaling, possibly inhibiting cancer cell growth or promoting apoptosis | May have tumor-suppressive effects; reduced activation of the PI3K pathway | Rare, seen in some contexts like neurodevelopmental disorders or specific cancers | Very low prevalence in cancer, mostly in benign conditions | Potential for targeting with PI3K agonists to restore function | [23] |
| Mutation Type | Description | Molecular Mechanism | Functional Consequences | Cancer Pathology Implications | Examples of Related Cancer Types | Ref. |
|---|---|---|---|---|---|---|
| Point Mutations | Single nucleotide changes, including missense, nonsense, and silent mutations. | Missense: A single nucleotide change results in a different amino acid. Nonsense: Introduction of a premature stop codon, truncating the protein. Silent: A change in the codon, but no change in the amino acid. | Missense: Changes protein conformation, potentially impairing function or altering protein–protein interactions, destabilizing the protein. Nonsense: Leads to a truncated protein that lacks functional domains, disrupting signaling pathways. Silent: Often neutral, but may still affect mRNA splicing or stability. | Missensemutations could lead to Wnt/β-catenin pathway activation, promoting cell proliferation and survival. Nonsense mutations can result in the loss of tumor suppressor function by generating truncated, nonfunctional proteins, thereby facilitating uncontrolled cellular proliferation. Meanwhile, silent mutations, although not altering the amino acid sequence, may influence mRNA splicing or gene expression regulation, potentially exerting subtle but significant effects on tumorigenesis. | Colorectal Cancer: Missense mutations in PYGO2 could activate Wnt signaling, contributing to early tumor formation. Breast Cancer: Nonsense mutations leading to loss of function may increase cellular resistance to apoptosis. Lung Cancer: Altered Wnt signaling through PYGO2 mutations can drive tumor progression and metastasis. | [30] |
| Insertions/Deletions (Indels) | Insertion or deletion of nucleotides, causing reading frame shifts or amino acid changes. | Frameshift: Insertion/deletion of nucleotides not in multiples of three. In-frame Indels: Insertion/deletion of nucleotides in multiples of three, altering the protein sequence. | Frameshift: Alters the reading frame, creating a dysfunctional protein downstream. This can affect key signaling pathways like Wnt. In-frame Indels: Affects key amino acids, potentially altering protein function or disrupting its interaction with cofactors or other proteins. | Frameshift mutationscan lead to loss of function in tumor suppressor pathways (like Wnt signaling) or cause the gain of function in oncogenes, promoting tumorigenesis. In-frame Indelscould disrupt binding sites for cofactors in Wnt signaling, leading to enhanced cell proliferation. | Gastric Cancer: Frameshift mutations in PYGO2 could contribute to cancer progression by disabling tumor suppressor functions. Lung Cancer: In-frame indels may enhance Wnt/β-catenin pathway activity, leading to uncontrolled growth. Endometrial Cancer: Indels causing structural changes in PYGO2protein affect cell differentiation and proliferation. | [31] |
| Splice Site Mutations | Mutations in splice donor/acceptor sites, leading to incorrect mRNA splicing. | Disrupts the precise splicing process, leading to the inclusion of introns or the exclusion of exons. | - Results in alternative splicing, producing truncated proteins or dysfunctional protein isoforms. Mis-spliced mRNA may be degraded or lead to a protein that lacks key functional domains necessary for Wnt signaling. | Aberrant splicingcan produce proteins that either act as oncogenes (if gain-of-function) or tumor suppressors (if loss-of-function), promoting tumorigenesis. Mutations in PYGO2 could generate spliced isoforms that activate Wnt/β-catenin, driving cell survival and division. | Prostate Cancer: Aberrant splicing of PYGO2could lead to the production of a dominant-negative protein, contributing to resistance to apoptosis. Lung Cancer: Splice variants of PYGO2 may facilitate the tumorigenic effects of Wnt pathway dysregulation. Breast Cancer: Incorrect splicing may lead to loss of cell cycle regulation, contributing to tumor progression. | [32] |
| Copy Number Variations (CNVs) | Alterations in the number of gene copies, such as duplications or deletions | Duplication: Multiple copies of PYGO2 gene lead to overexpression. Deletion: Loss of one or more copies of PYGO2 gene, reducing expression | Duplication: Overexpression of PYGO2 leads to excessive activation of Wnt signaling, promoting cell proliferation and resistance to cell death. Deletion: Insufficient expression of PYGO2 disrupts Wnt signaling, potentially impairing developmental processes and tissue homeostasis | Gene duplicationcan enhance PYGO2expression, amplifying Wnt/β-catenin signaling, which promotes tumor initiation, progression, and metastasis. Gene deletionin PYGO2 can impair tumor suppressor functions, leading to genome instability and increased metastatic potential | Breast Cancer: Overexpression of PYGO2through gene duplication has been linked to enhanced metastatic behavior. Glioblastoma: Loss of PYGO2 function due to deletion has been associated with increased tumorigenicity and poor prognosis. Ovarian Cancer: Deletions of PYGO2 can cause dysregulated cell differentiation and growth. | [33] |
| Regulatory Region Mutations | Mutations in promoter, enhancer, or silencer regions affecting PYGO2 expression. | Promoter Mutations: Alteration of transcription factor binding sites. Enhancer/Silencer Mutations: Disruption of cis-regulatory elements. | Overexpression: Increased PYGO2 expression may hyperactivate Wnt signaling, leading to tumor initiation and progression.Underexpression: Reduced expression of PYGO2 results in loss of function, potentially leading to unchecked cellular proliferation. | Overexpression of PYGO2has been shown to activate the Wnt/β-cateninsignaling pathway, which plays a critical role in promoting cell cycle progression, cellular invasion, and metastatic dissemination. Conversely, underexpression of PYGO2 may result in impaired regulation of cellular differentiation, thereby facilitating malignant transformation. | Colon Cancer: Overexpression of PYGO2 through promoter mutations activates Wnt signaling and accelerates tumor progression.Hepatocellular Carcinoma: Disruption of regulatory regions leads to PYGO2 silencing, impairing cell differentiation and promoting carcinogenesis.Leukemia: Deregulated PYGO2 expression contributes to leukemogenesis by promoting uncontrolled cell proliferation. | [34] |
| Large-Scale Structural Variants | Chromosomal rearrangements, such as inversions, translocations, and deletions, affecting PYGO2. | Inversion: Reversal of a chromosomal region containing PYGO2.Translocation: Movement of PYGO2 to a new chromosomal location. Deletion: Loss of the region containing PYGO2. | Inversion: May disrupt PYGO2’s expression or function by altering its regulatory landscape.Translocation: PYGO2 may be placed under the control of an oncogenic promoter, driving overexpression. Deletion: Complete loss of PYGO2function, resulting in defective signaling and loss of tumor suppressor functions. | Inversions and translocationscould place PYGO2 under the control of strong oncogenic promoters or fuse it with other genes, driving tumorigenesis. Gene deletions result in the loss of Wnt signaling, increasing susceptibility to cancer progression and metastasis | Leukemia: Chromosomal translocations involving PYGO2 lead to fusion genes, driving leukemogenesis. Breast Cancer: Rearrangement or deletions of PYGO2 are linked to poor prognosis due to loss of cell cycle regulation. Ovarian Cancer: Structural variants of PYGO2 contribute to dysregulated cellular pathways, facilitating tumor growth and spread. | [35] |
| Epigenetic Modifications | DNA methylation, histone modification, and chromatin remodeling that regulate PYGO2 expression. | DNA Methylation: Addition of methyl groups to cytosine residues in the promoter region, silencing gene expression. Histone Modifications: Acetylation or methylation of histones that impact chromatin accessibility and gene expression. | Gene silencing: Methylation of PYGO2’s promoter can lead to loss of expression, disrupting the control of cell proliferation.Gene activation: Loss of methylation or histone acetylation can increase PYGO2 expression, pushing cells into unregulated proliferation. | DNA methylation silencingof PYGO2 may contribute to tumorigenesis by silencing a key tumor suppressor. Increased expressiondue to loss of silencing or aberrant histone modification enhances tumor cell survival and metastasis. | Colon Cancer: DNA methylation of PYGO2is linked to silencing in tumor cells, allowing cancer progression. Lung Cancer: Epigenetic silencing of PYGO2disrupts normal cellular homeostasis and promotes carcinogenesis. Hepatocellular Carcinoma: Histone modifications leading to overexpression of PYGO2 contribute to aggressive tumor behavior. | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, A.M.; Paul, I.L.; Cavalu, S.; Pop, O.L.; Paduraru, L.; Magyar, I.; Chirila, M.D. Highlighting the Importance of Signaling Pathways and Immunohistochemistry Features in HCC: A Case Report and Literature Review. Reports 2025, 8, 197. https://doi.org/10.3390/reports8040197
Hasan AM, Paul IL, Cavalu S, Pop OL, Paduraru L, Magyar I, Chirila MD. Highlighting the Importance of Signaling Pathways and Immunohistochemistry Features in HCC: A Case Report and Literature Review. Reports. 2025; 8(4):197. https://doi.org/10.3390/reports8040197
Chicago/Turabian StyleHasan, Alexandru Madalin, Ioana Larisa Paul, Simona Cavalu, Ovidiu Laurean Pop, Lorena Paduraru, Ioan Magyar, and Mihaela Doina Chirila. 2025. "Highlighting the Importance of Signaling Pathways and Immunohistochemistry Features in HCC: A Case Report and Literature Review" Reports 8, no. 4: 197. https://doi.org/10.3390/reports8040197
APA StyleHasan, A. M., Paul, I. L., Cavalu, S., Pop, O. L., Paduraru, L., Magyar, I., & Chirila, M. D. (2025). Highlighting the Importance of Signaling Pathways and Immunohistochemistry Features in HCC: A Case Report and Literature Review. Reports, 8(4), 197. https://doi.org/10.3390/reports8040197







