A Rare Case Presentation of Intraoral Palatal Myoepithelioma
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Clicical History
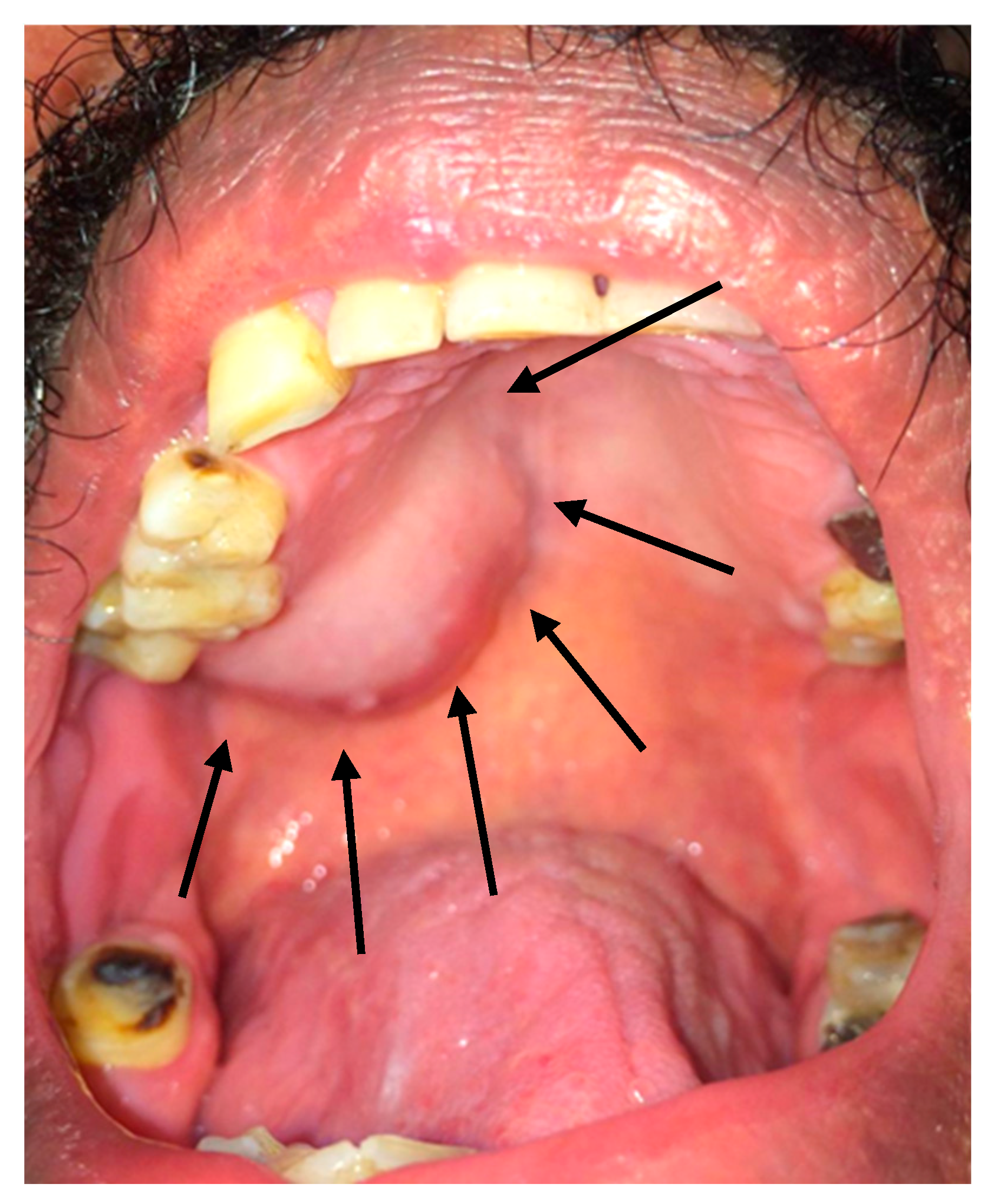
2.2. Clinical Findings
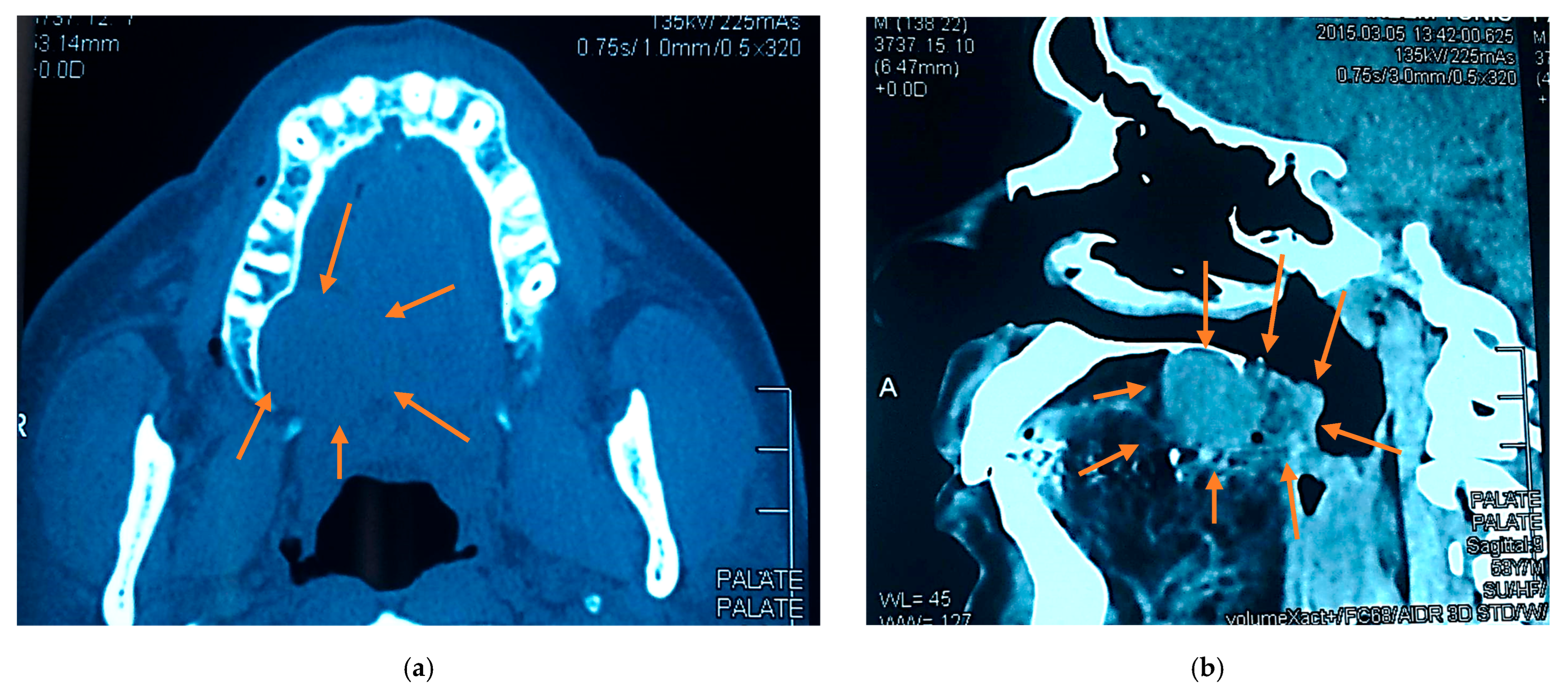
2.3. Radiographic Examination
2.4. Differential Diagnosis
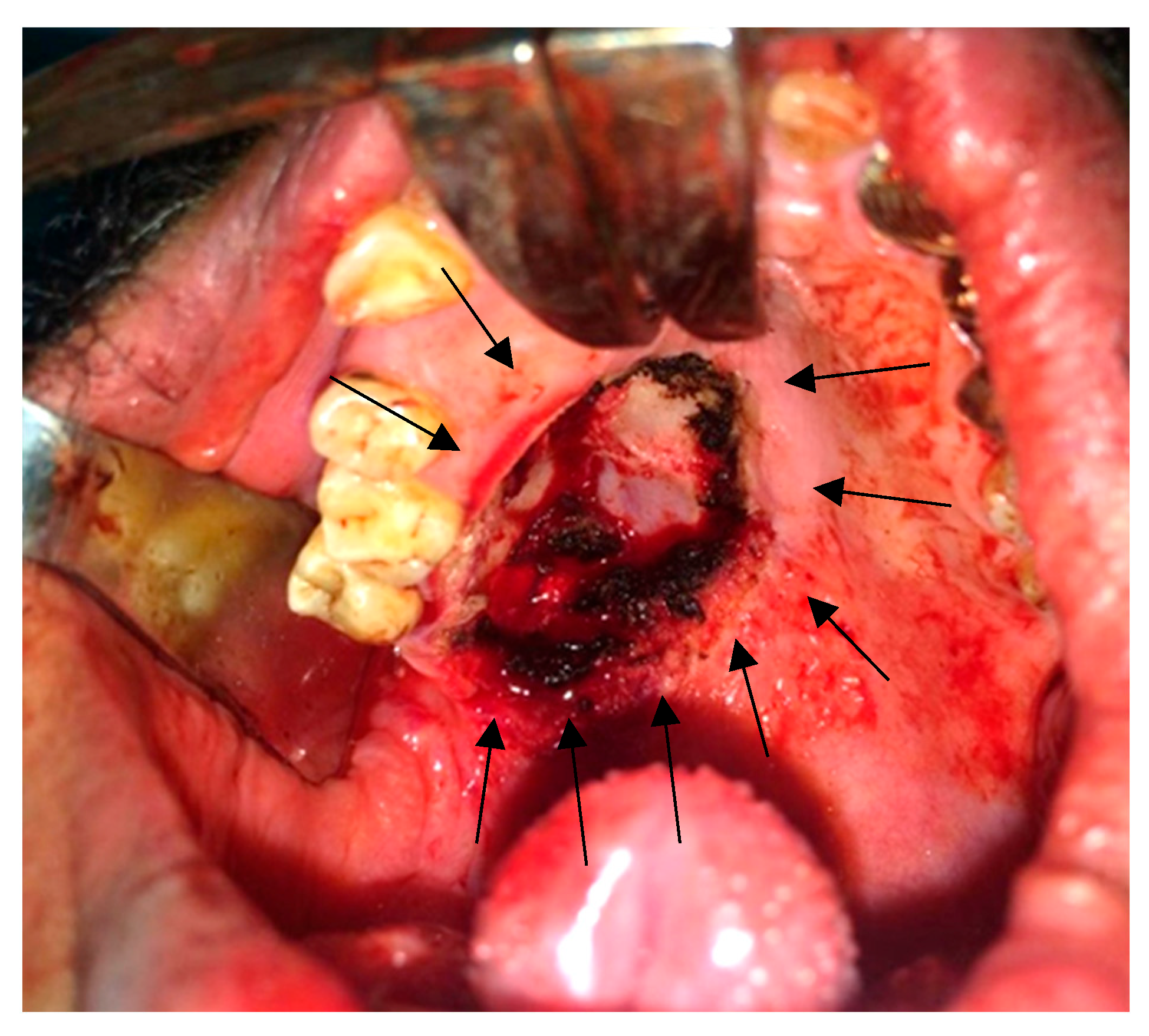
2.5. Surgical Findings
2.6. Histopathology
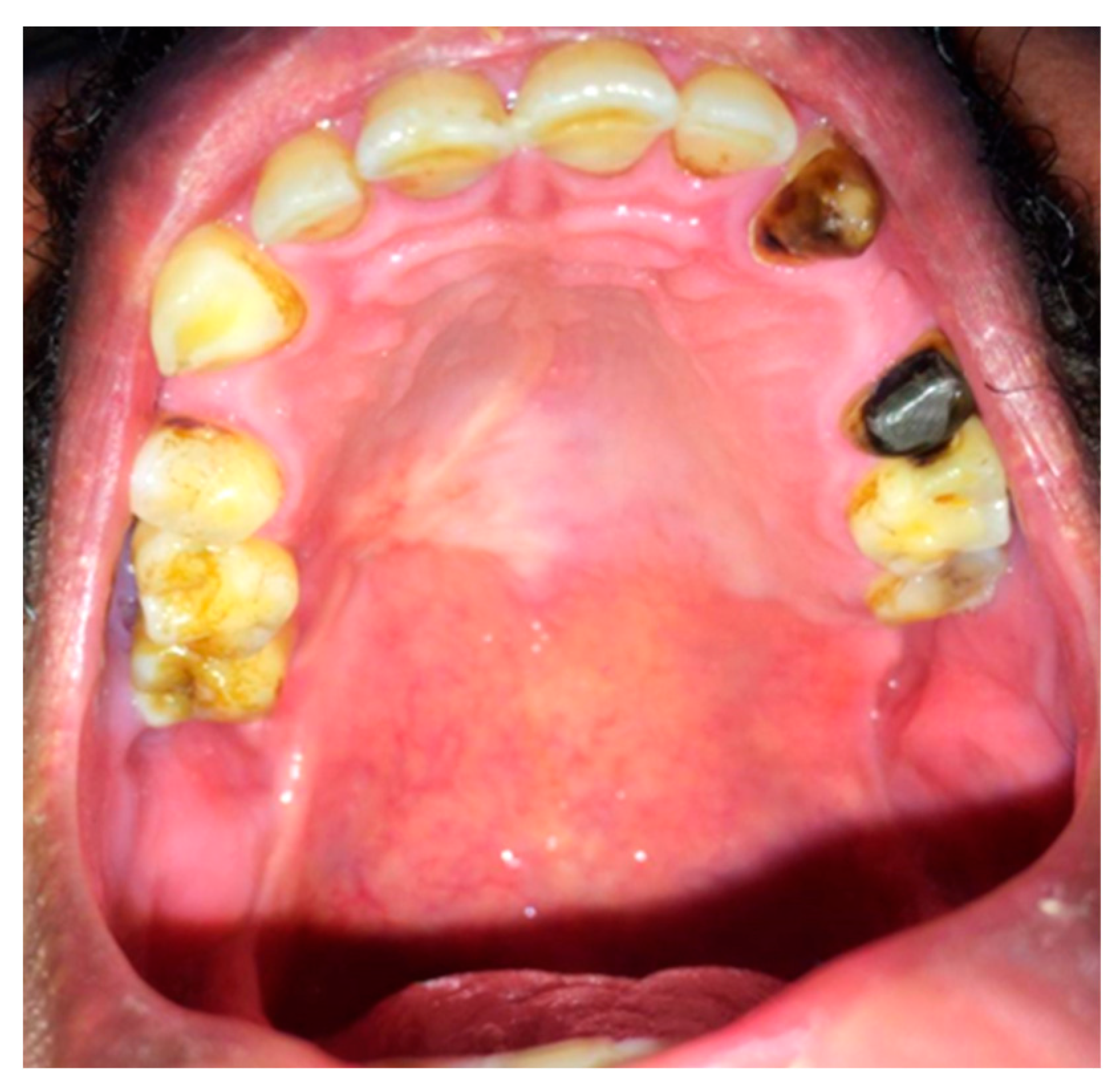
2.7. Postoperative Course
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barca, I.; Novembre, D.; Cordaro, R.; Faro, C.L.; Colangeli, W.; Boschetti, C.E.; Giudice, A.; Cristofaro, M.G. Myoepithelioma of the parotid gland: A case report with review of the literature. Oral. Maxillofac. Surg. Cases 2020, 6, 100131. [Google Scholar] [CrossRef]
- Cuadra Zelaya, F.; Quezada Rivera, D.; Tapia Vazquez, J.L.; Paez Valencia, C.; Gaitán Cepeda, L.A. Plasmacytoid myoepithelioma of the palate. Report of one case and review of the literature. Med. Oral. Patol. Oral. Cir. Bucal 2007, 12, E552–E555. [Google Scholar]
- Yadav, A.K.; Nadarajah, J.; Chandrashekhara, S.H.; Tambade, V.D.; Acharya, S. Myoepithelioma of the Soft Palate: A Case Report. Case Rep. Otolaryngol. 2013, 2013, 642806. [Google Scholar] [CrossRef]
- Vardag, A.B.S.; Danish, M.H.; Ikram, M.; Awan, M.O.; Kayani, N. Epithelioid myoepithelioma of the parotid gland: A case report with a brief literature overview. Egypt. J. Otolaryngol. 2022, 38, 60. [Google Scholar] [CrossRef]
- Yu, G.; Ma, D.; Sun, K.; Li, T.; Zhang, Y. Myoepithelial carcinoma of the salivary glands: Behavior and management. Chin. Med. J. 2003, 116, 163–165. [Google Scholar] [PubMed]
- Kyrgias, G.; Kalogeridi, M.A.; Tzitzikas, J.; Kouloulias, V. Malignant Myoepithelioma of the Parotid Gland: Literature Review on the Occasion of Our Report of a Case. Int. J. Clin. Med. 2012, 3, 49–54. [Google Scholar] [CrossRef]
- Georgantopoulou, C.; Aird, I.A. Management of myoepithelioma of the vulva--case report and review of the literature. Eur. J. Gynaecol. Oncol. 2009, 30, 203–205. [Google Scholar]
- Bains, A.; Dennis, T.; Doumpiotis, D. A Palatal Myoepithelioma. Dent. Update 2019, 46, 684–685. [Google Scholar] [CrossRef]
- Gnepp, D.R. Mucinous Myoepithelioma, a Recently Described New Myoepithelioma Variant. Head. Neck Pathol. 2013, 7, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shome, S.; Sil, S.; Bhaumik, A. Epithelial-myoepithelial carcinoma of retro-molar trigone: Unveiling the mystery of rare diagnosis. J. Cancer Res. Ther. 2023, 19, S422–S425. [Google Scholar] [CrossRef]
- Ochiai, S.; Yamada, M.; Suga, K.; Nishikawa, M.; Asoda, S.; Ymada, M. Myoepithelioma Arising in the Buccal Mucosa: A Case Report and Review of the Literature. Cureus 2024, 16, e73263. [Google Scholar] [CrossRef]
- Bocchino, G.; Capece, G.; Pietramala, S.; Rovere, G.; Rocchi, L.; Farsetti, P.; Maccauro, G.; Fulchignoni, C. Myoepithelioma of the Hand: A Systematic Review. Appl. Sci. 2024, 14, 9149. [Google Scholar] [CrossRef]
- Kaur, R.; Melgandi, W.; Rathi, A.K.; Singh, K.; Rahman, F.; Mandal, S. Epithelial myoepithelial carcinoma: A case report. J. Cancer Res. Ther. 2025, 21, 182–185. [Google Scholar] [CrossRef]
- Mireles, M.G.; Julián, A.; Salgado-Chavarría, F.; González, G.M. Benign myoepithelioma of the soft palate: An unusual clinical entity. BMJ Case Rep. 2021, 14, e240384. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Giudice, F.S.; Pinto-Junior Ddos, S.; de Sousa, S.C. Myoepithelioma of the soft palate: A case report giving special attention to the differential diagnosis. J. Oral. Maxillofac. Res. 2011, 2, e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baral, R.; Ojha, B.; Bajracharya, D.; Singh, S. Myoepithlioma of Soft Palate: A case report. J. Coll. Med. Sci. 2018, 14, 225–227. [Google Scholar] [CrossRef]
- Kilpatrick, S.E.; Hitchcock, M.G.; Kraus, M.D.; Calonje, E.; Fletcher, C.D. Mixed Tumors and Myoepitheliomas of Soft Tissue. Am. J. Surg. Pathol. 1997, 21, 13–22. [Google Scholar] [CrossRef]
- Dardick, I.; Thomas, M.J.; van Nostrand, A.W.P. Myoepithelioma-New Concepts of Histology and Classification: A Light and Electron Microscopic Study. Ultrastruct. Pathol. 1989, 13, 187–224. [Google Scholar] [CrossRef]
- Chatterjee, S. Swellings of the Palate: A Review. Indian. J. Forensic Med. Toxicol. 2020, 14, 9040–9045. [Google Scholar] [CrossRef]
- Maffi-Berthier, L.; Le pelletier, F.; Ejeil, A. Benign myoepithelioma of the hard palate: A clinical and histological diagnostic challenge. Case report and literature review. J. Oral. Med. Oral. Surg. 2018, 24, 81–88. [Google Scholar] [CrossRef]
- Giammarinaro, E.; Cosola, S.; Oldoini, G.; Gulia, F.; Peñarrocha-Oltra, D.; Marconcini, S.; Genovesi, A.M. Local Formula with Mucoadhesive Property: A Randomized Clinical Trial of a Therapeutic Agent for the Treatment of Oral Aphthous Ulcers. J. Contemp. Dent. Pract. 2019, 20, 1249–1253. [Google Scholar] [CrossRef]
- Oldoini, G.; Frabattista, G.R.; Saragoni, M.; Cosola, S.; Giammarinaro, E.; Genovesi, A.M.; Marconcini, S. Ozone Therapy for Oral Palatal Ulcer in a Leukaemic Patient. Eur. J. Case Rep. Intern. Med. 2020, 7, 001406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samar, M.; Avila, R.; Furnes, M.; Fonseca, I.; Juri, H.; Olmedo, L.; Anderson, W. Epithelioid Myoepithelioma of the Parotid Gland: A Histopathological and Immunohistochemical Study. Int. J. Med. Surg. Sci. 2018, 1, 177–183. [Google Scholar] [CrossRef]
- Alzahrani, S.; Wazzan, T.; Almaghrabi, A.; Alkhudran, A.; Aljereb, H.; Elsayed, S.; Alolayan, A.B. The Prevalence and Diagnostic Patterns of Oral and Maxillofacial Lesions: A Seven-Year, Retrospective, Single-Center Cone Beam Computed Tomography and Histopathology Study in Saudi Arabia. J. Clin. Med. 2024, 13, 7774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viswanathan, V.; Londhe, M.; Gurwale, S.; Buch, A. Clear cell myoepithelioma of palate: A rare case report with brief review of literature. J. Oral. Maxillofac. Pathol. 2024, 28, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Leon Barnes, E.; Hunt, J.L. Epithelial-Myoepithelial Carcinoma: A Review of the Clinicopathologic Spectrum and Immunophenotypic Characteristics in 61 Tumors of the Salivary Glands and Upper Aerodigestive Tract. Am. J. Surg. Pathol. 2007, 31, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Rekhi, B. Myoepithelial tumor of soft tissue and bone: A current perspective. Histol. Histopathol. 2017, 32, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Mohamed, R.N.; Al-Thomali, Y.; Al Shamrani, A.S. The Prevalence of Oral Cancer in Saudi Arabia—A Systematic Review. Ann. Med. Health Sci. Res. 2019, 9, 553–557. [Google Scholar]
- Zhang, X.; Hu, J.; Lu, J.J.; Gao, J.; Guan, X.; Kong, L. Incidence patterns for myoepithelial carcinoma: A Surveillance, Epidemiology, and End Results (SEER) study. Transl. Cancer Res. 2017, 6, 441–449. [Google Scholar] [CrossRef]
- De Ru, J.A.; Plantinga, R.F.; Majoor, M.H.; van Benthem, P.P.; Slootweg, P.J.; Peeters, P.H.; Hordijk, G.J. Warthin’s tumour and smoking. B-ENT. 2005, 1, 63–66. [Google Scholar] [PubMed]
- Vories, A.A.; Ramirez, S.G. Warthin’s tumor and cigarette smoking. South. Med. J. 1997, 90, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, X.; Li, Z. Smoking and the development of Warthin tumor of the parotid gland. Zhonghua Kou Qiang Yi Xue Za Zhi 1995, 30, 195–197. (In Chinese) [Google Scholar] [PubMed]
- Barbosa, A.; Rodrigues, Á.; Corrales, T.; Viegas, S. Myoepithelioma of Minor Salivary Gland: A Case Report. Eur. J. Dent. Oral. Health 2022, 3, 4–5. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Krishnan, M.; Kumar, M.P.S. Myoepithelioma of the Palatal Minor Salivary Gland: A Case Report. Cureus 2024, 16, e56305. [Google Scholar] [CrossRef]
- Patankar, S.; Sharma, R.; Patankar, A.; Kulkarni, V. Rare case of giant myoepithelioma in minor salivary glands of palate in a 9-year-old child. Natl. J. Maxillofac. Surg. 2021, 12, 284–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elsayed, S.A.; Abdullah, A.A.B.; Dar-Odeh, N.; Altaweel, A.A. Intraoral Wound Dehiscence After Open Reduction Internal Fixation of Mandibular Fractures: A Retrospective Cohort Study. Wounds 2021, 33, 60–64. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeidi, A.; Alolayan, A.; Zaki, H.; Essa, E.; Alzahrani, S.; Fareed, W.; Elsayed, S. A Rare Case Presentation of Intraoral Palatal Myoepithelioma. Reports 2025, 8, 196. https://doi.org/10.3390/reports8040196
Saeidi A, Alolayan A, Zaki H, Essa E, Alzahrani S, Fareed W, Elsayed S. A Rare Case Presentation of Intraoral Palatal Myoepithelioma. Reports. 2025; 8(4):196. https://doi.org/10.3390/reports8040196
Chicago/Turabian StyleSaeidi, Abdullah, Albraa Alolayan, Hattan Zaki, Emad Essa, Shadi Alzahrani, Wamiq Fareed, and Shadia Elsayed. 2025. "A Rare Case Presentation of Intraoral Palatal Myoepithelioma" Reports 8, no. 4: 196. https://doi.org/10.3390/reports8040196
APA StyleSaeidi, A., Alolayan, A., Zaki, H., Essa, E., Alzahrani, S., Fareed, W., & Elsayed, S. (2025). A Rare Case Presentation of Intraoral Palatal Myoepithelioma. Reports, 8(4), 196. https://doi.org/10.3390/reports8040196





