Twice the Leak: Managing CSF Fistulas in a Recurrent Thoracic Arachnoid Cyst—A Case Report
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
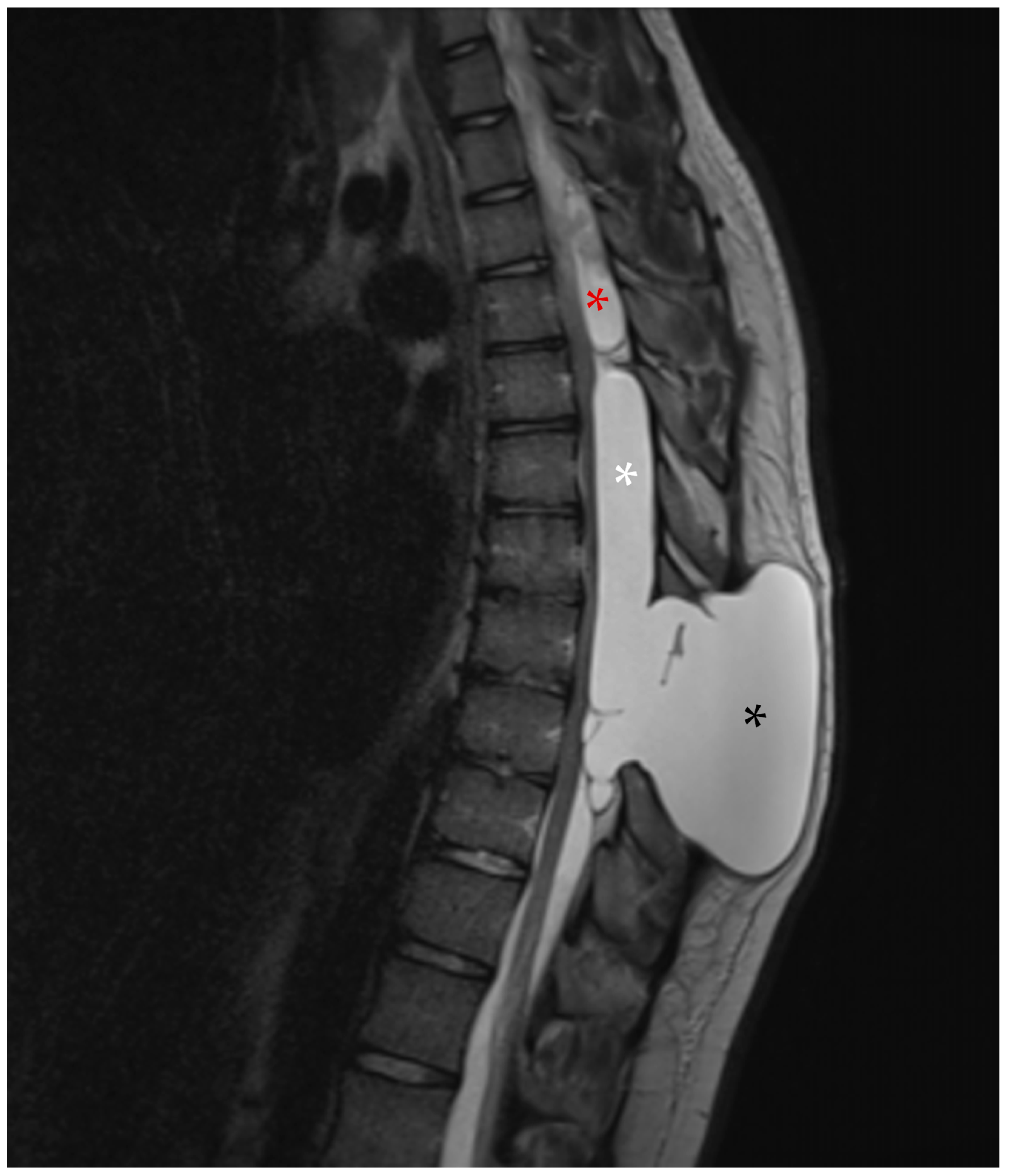
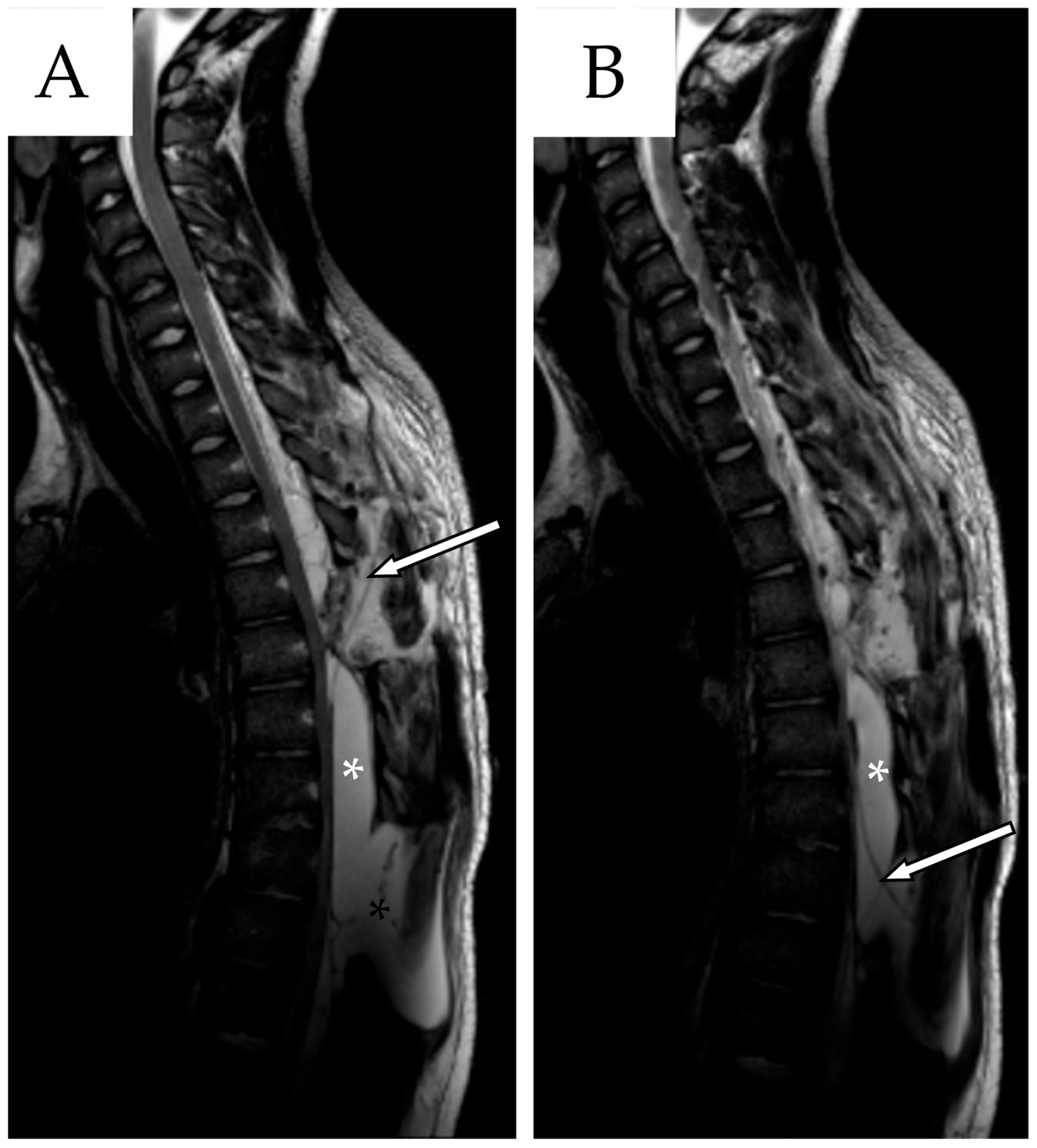
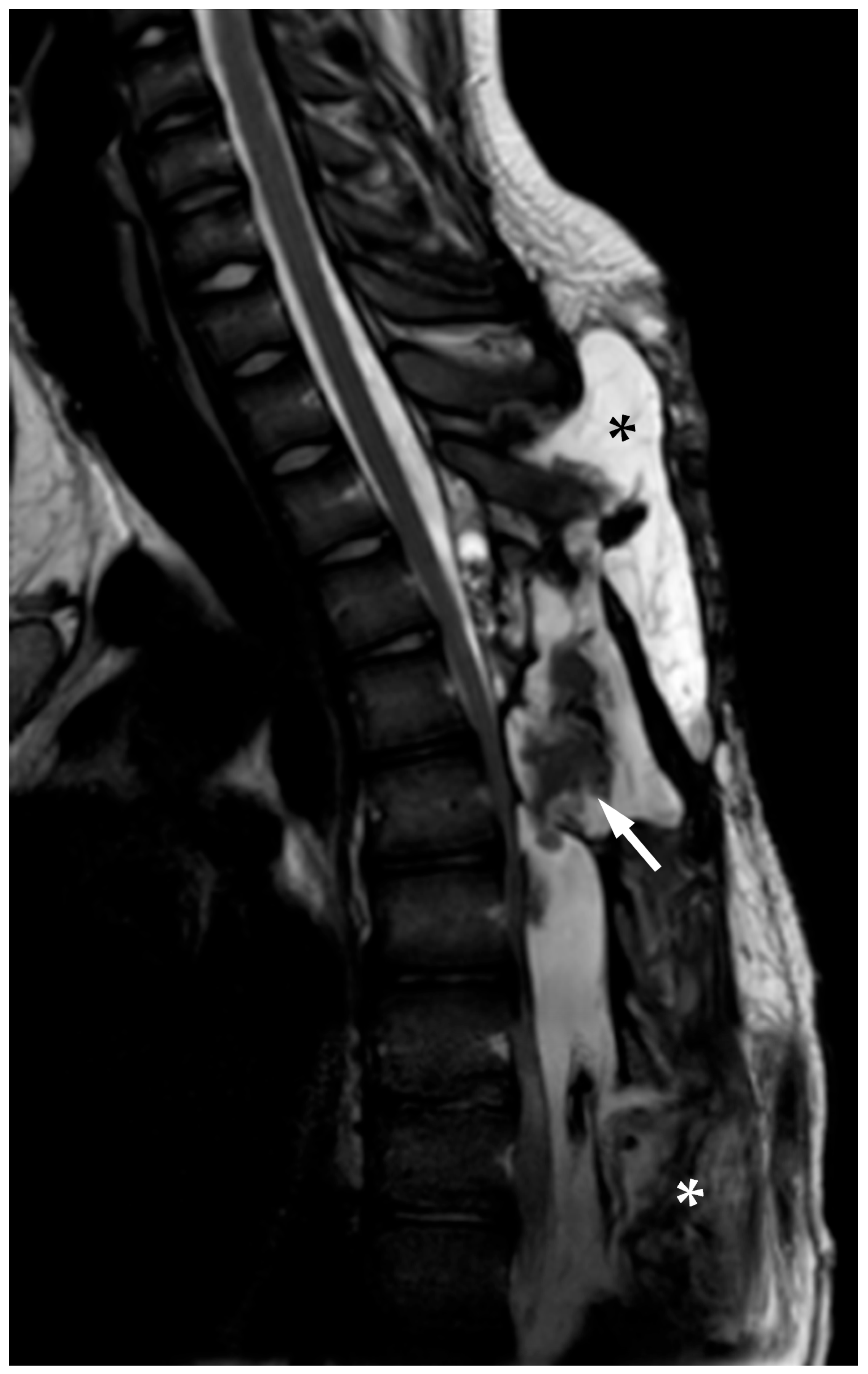
2.1. First Admission
2.2. First Follow-Up
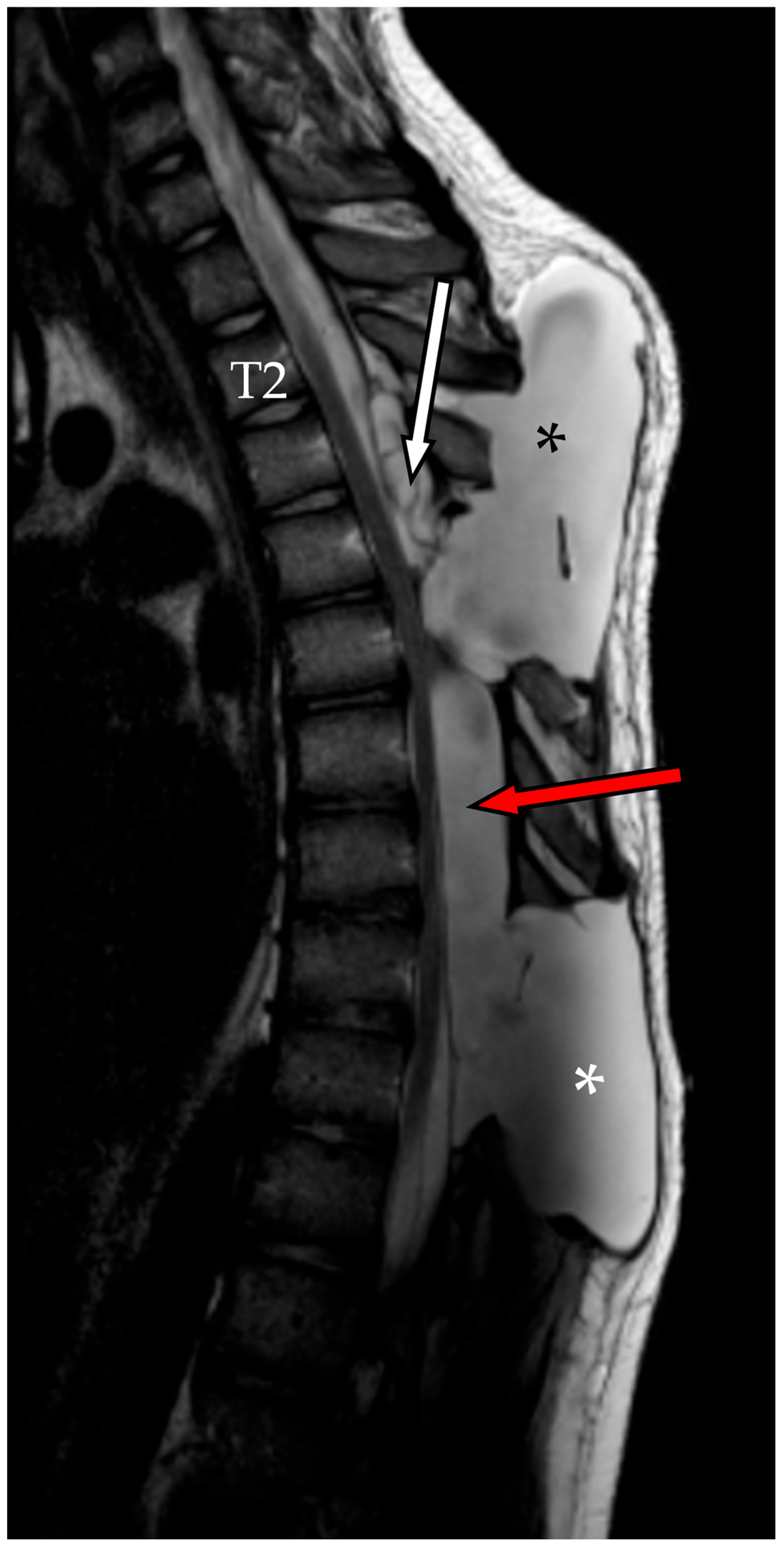
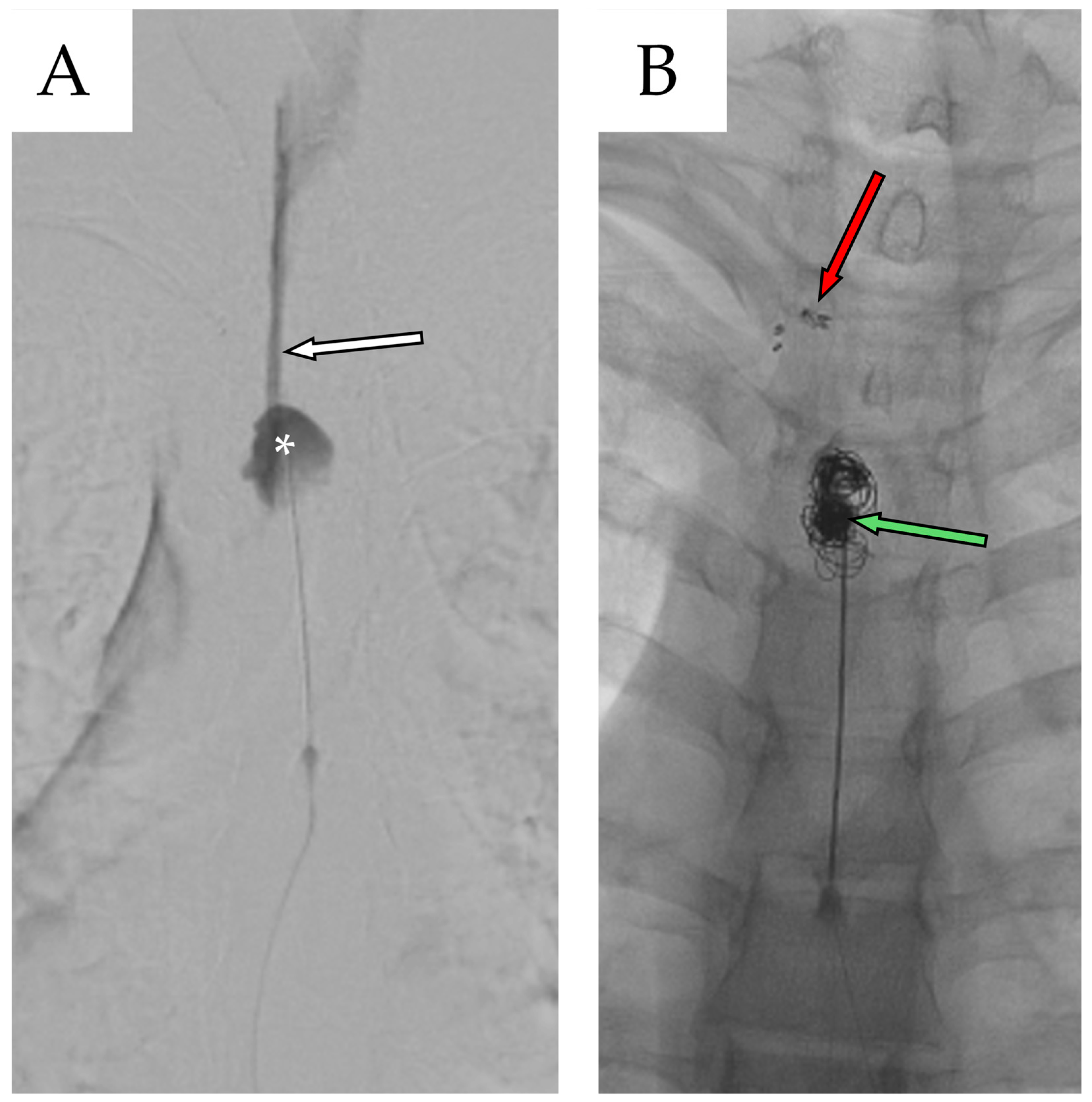
2.3. Second Admission
2.4. Second Follow-Up
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SACs | Spinal Arachnoid Cysts |
| CSF | Cerebrospinal Fluid |
| MRI | Magnetic Resonance Imaging |
| MRC | Medical Research Council |
| ceMRI | contrast-enhanced Magnetic Resonance Imaging |
| IONM | Intraoperative Neurophysiological Monitoring |
| TSE | Turbo Spin Echo |
| WI | Weighted Imaging |
| NA | Not Available |
| LBP | Low Back Pain |
| TLIF | Transforaminal Lumbar Interbody Fusion |
References
- Nabors, M.W.; Pait, T.G.; Byrd, E.B.; Karim, N.O.; Davis, D.O.; Kobrine, A.I.; Rizzoli, H.V. Updated assessment and current classification of spinal meningeal cysts. J. Neurosurg. 1988, 68, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Kriss, T.C.; Kriss, V.M. Symptomatic spinal intradural arachnoid cyst development after lumbar myelography: Case report and review of the literature. Spine 1997, 22, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Cossu, G.; Maduri, R.; Valerio, M.; Vamadevan, S.; Daniel, R.; Messerer, M. Endoscopic treatment of spinal arachnoid cysts. Heliyon 2021, 7, e06736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levin, T.L.; Zimmerman, R.D.; Lieberman, H. Intradural spinal arachnoid cyst: Case report. Clin. Imaging 1990, 14, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.B.; Bartram, J.; Vastani, A.; Gebreyohanes, A.; Al Banna, Q.; Lavrador, J.P.; Vasan, A.K.; Grahovac, G. Systematic review of surgical management of spinal intradural arachnoid cysts. World Neurosurg. 2022, 158, e298–e309. [Google Scholar] [CrossRef] [PubMed]
- Hussain, O.; Treffy, R.; Reecher, H.M.; DeGroot, A.L.; Palmer, P.; Bakhaidar, M.; Shabani, S. Management of a recurrent spinal arachnoid cyst presenting as arachnoiditis in the setting of spontaneous spinal subarachnoid hemorrhage: Illustrative case. J. Neurosurg. Case Lessons 2024, 7, CASE23660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelhameed, E.; Morsy, A.A. Surgical outcome of primary intradural spinal arachnoid cysts: A series of 10 cases. Egypt J. Neurol. Psychiatry Neurosurg. 2021, 57, 42. [Google Scholar] [CrossRef]
- Nath, P.C.; Mishra, S.S.; Deo, R.C.; Satapathy, M.C. Intradural spinal arachnoid cyst: A long-term postlaminectomy complication: Case report and review of the literature. World Neurosurg. 2016, 85, e1–e367. [Google Scholar] [CrossRef] [PubMed]
- Ramazanoglu, A.F.; Sarikaya, C.; Varol, E.; Aydin, S.O.; Etli, M.U.; Avci, F.; Naderi, S. Surgical treatment of spinal arachnoid cysts: Cyst excision or fenestration? Turk Neurosurg. 2022, 32, 1002–1006. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, D.H.; Deng, S.L. Symptomatic secondary spinal arachnoid cysts: A systematic review. Spine J. 2023, 23, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Theologou, M.; Natsis, K.; Kouskouras, K.; Chatzinikolaou, F.; Varoutis, P.; Skoulios, N.; Tsitouras, V.; Tsonidis, C. Cerebrospinal fluid homeostasis and hydrodynamics: A review of facts and theories. Eur. Neurol. 2022, 85, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, D.C.; Burchiel, K.J.; Gruber, D.P. Intraspinal extradural meningeal cyst demonstrating ball-valve mechanism of formation: Case report. J. Neurosurg. 1993, 78, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Bhaisora, K.S.; Singh, S.; Sardhara, J.; Das, K.K.; Attri, G.; Mehrotra, A.; Srivastava, A.K.; Jasiwal, A.K.; Behari, S. Symptomatic extradural spinal arachnoid cyst: More than a simple herniated sac. J. Craniovertebr. Junction Spine 2019, 10, 64–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulkarni, A.G.; Goel, A.; Thiruppathy, S.P.; Desai, K. Extradural arachnoid cysts: A study of seven cases. Br. J. Neurosurg. 2004, 18, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Barth, A.; Arnold, M.; Seiler, R.W. Multiple spinal extradural meningeal cysts presenting as acute paraplegia: Case report and review of the literature. J. Neurosurg. Spine 2007, 6, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Panigrahi, M. Strategies to improve surgical outcome of spinal arachnoid cyst—A single center, single surgeon experience from a tertiary care center. J. Spinal Surg. 2025, 12, 3–7. [Google Scholar]
- Chatain, G.P.; Shrestha, K.; Kortz, M.W.; Serva, S.; Hosokawa, P.; Ward, R.C.; Sethi, A.; Finn, M. Impact of surgical timing on neurological outcomes for spinal arachnoid cyst: A single institution series. Neurospine 2022, 19, 453–462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Hajj, V.G.; Singh, A.; Pham, K.; Edström, E.; Elmi-Terander, A.; Fletcher-Sandersjöö, A. Long-term outcomes following surgical treatment of spinal arachnoid cysts: A population-based consecutive cohort study. Spine J. 2023, 23, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, H.; Sun, Z.; Guo, Y.; Wang, G.; Wang, J.J. Ultrafine flexible endoscope visualization to assist in the removal of a huge spinal extradural arachnoid cyst: Case report and literature review. World Neurosurg. 2022, 159, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, S.; Seki, T.; Yamazaki, K.; Sasamori, T.; Houkin, K. Intraoperative visualization of a spinal arachnoid cyst using pyoktanin blue. World Neurosurg. 2018, 109, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, M.; Uei, H.; Tokuhashi, Y. Recurrence of a symptomatic spinal intradural arachnoid cyst 29 years after fenestration. J. Int. Med. Res. 2019, 47, 4530–4536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeGroot, A.L.; Treffy, R.W.; Bakhaidar, M.; Palmer, P.; Rahman, M.; Shabani, S. Minimally invasive management of a spinal arachnoid cyst with ultrasound-assisted catheter placement: Illustrative case. J. Neurosurg. Case Lessons 2024, 8, CASE24461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neo, M.; Koyama, T.; Sakamoto, T.; Fujibayashi, S.; Nakamura, T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine 2004, 29, E426–E430. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Miyashita, K.; Tanaka, S.; Oikawa, N.; Oishi, M.; Nambu, I.; Kinoshita, M.; Nakada, M. Recurrent spinal intramedullary arachnoid cyst: Case report and literature review. World Neurosurg. 2020, 138, 68–72. [Google Scholar] [CrossRef]
- Novak, L.; Dobai, J.; Nemeth, T.; Fekete, M.; Prinzinger, A.; Csecsei, G.I. Spinal extradural arachnoid cyst causing cord compression in a 15-year-old girl: A case report. Zentralbl. Neurochir. 2005, 66, 43–46. [Google Scholar] [CrossRef]
- Lee, H.G.; Kang, M.S.; Na, Y.C.; Jin, B.H. Spinal intradural arachnoid cyst as a complication of insertion of an interspinous device. Br. J. Neurosurg. 2023, 37, 811–815. [Google Scholar] [CrossRef]
- Krstačić, A.; Krstačić, G.; Butković Soldo, S. Atypical cause of radiculopathy—Intradural spinal arachnoid cyst. Acta Clin. Belg. 2016, 71, 267–268. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, D.Y. Symptomatic spinal intradural arachnoid cysts in the pediatric age group: Description of three new cases and review of the literature. Pediatr. Neurosurg. 2001, 35, 181–187. [Google Scholar] [CrossRef]
- Bond, A.E.; Zada, G.; Bowen, I.; McComb, J.G.; Krieger, M.D. Spinal arachnoid cysts in the pediatric population: Report of 31 cases and a review of the literature. J. Neurosurg. Pediatr. 2012, 9, 432–441. [Google Scholar] [CrossRef]
- Liu, J.K.; Cole, C.D.; Sherr, G.T.; Kestle, J.R.W.; Walker, M.L. Noncommunicating spinal extradural arachnoid cyst causing spinal cord compression in a child. J. Neurosurg. 2005, 103 (Suppl. 3), 266–269. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj, V.G.; Edström, E.; Elmi-Terander, A.; Fletcher-Sandersjöö, A. An unusual cause of chronic neuropathic pain: Report of a case of multiple intradural spinal arachnoid cysts and review of the literature. Acta Neurochir. 2023, 165, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, M.; Kavak, R.P.; Gülgönül, N. Spinal extradural arachnoid cyst in cervicothoracic junction. Spinal Cord. Ser. Cases 2019, 5, 45. [Google Scholar] [CrossRef]
- Ebot, J.; Domingo, R.; Ruiz Garcia, H.; Chen, S. Intradural thoracic arachnoid cyst fenestration for spinal cord compression: A case illustration and video demonstration. Cureus 2020, 12, e6572. [Google Scholar] [CrossRef]
- Alugolu, R.; Arradi, V.; Sahu, B.P. Intramedullary arachnoid cyst in an adult: Case report and review. Asian J. Neurosurg. 2016, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Chaudhuri, A.; Sadique, S.; Attry, S. Multiple spinal extradural arachnoidal cysts: An uncommon cause of thoracic cord compression. Asian J. Neurosurg. 2017, 12, 321–323. [Google Scholar] [CrossRef]
- Sharif, S.; Afsar, A.; Qadeer, M. Conus medullaris arachnoid cyst presenting as cauda equina syndrome. Asian J. Neurosurg. 2017, 12, 707–709. [Google Scholar] [CrossRef]
- Lin, L.C.; Jason, R. A rare case of spinal extradural arachnoid cyst with cord compression. Asian J. Neurosurg. 2018, 13, 468–470. [Google Scholar] [CrossRef]
- Shaaban, A.; Abdelrahman, A.; Jarir, R.; Al-Bozom, I.; Raza, A. Thoracic spinal intramedullary arachnoid cyst presented with myelopathy with marked postoperative improvement: A case report and review of literature. Asian J. Neurosurg. 2019, 14, 981–984. [Google Scholar] [CrossRef]
- Raes, K.; Oostra, K.M. Correlation of spinal cord injury with development of spinal arachnoid cysts: Two case reports. J. Rehabil. Med. Clin. Commun. 2021, 4, 1000066. [Google Scholar] [CrossRef]
- Taccone, M.S.; Theriault, P.G.; Roffey, D.M.; AlShumrani, M.; Alkherayf, F.; Wai, E.K. Intradural hematoma and arachnoid cyst following lumbar spinal surgery: A case report. Can. J. Neurol. Sci. 2018, 45, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Turel, M.K.; Kerolus, M.G.; Deutsch, H. Intradural spinal arachnoid cyst—A complication of lumbar epidural steroid injection. Neurol. India 2017, 65, 863–864. [Google Scholar] [CrossRef]
- Kong, W.K.; Cho, K.T.; Hong, S.K. Spinal extradural arachnoid cyst: A case report. Korean J. Spine 2013, 10, 32–34. [Google Scholar] [CrossRef]
- Fischer, K.M.; Madsen, P.J.; Tucker, A.M.; Long, C.J. Thoracic spinal arachnoid cyst in a pediatric patient presenting with isolated bladder and bowel incontinence. Urol. Case Rep. 2022, 44, 102129. [Google Scholar] [CrossRef]
- Sharma, R.; Kumarasamy, S.; Tiwary, S.K.; Kedia, S.; Sawarkar, D.; Doddamani, R.; Laythalling, R.K. Multiple spinal extradural arachnoid cysts presenting as compressive myelopathy in a teenager. Childs Nerv. Syst. 2024, 40, 729–747. [Google Scholar] [CrossRef]
- Engelhardt, J.; Vignes, J.R. Anterior cervical intradural arachnoid cyst, a rare cause of spinal cord compression: A case report with video systematic literature review. Eur. Spine J. 2016, 25 (Suppl. 1), 19–26. [Google Scholar] [CrossRef]
- Zekaj, E.; Saleh, C.; Servello, D. Intramedullary cyst formation after removal of multiple intradural spinal arachnoid cysts: A case report. Surg. Neurol. Int. 2016, 7 (Suppl. 17), S473–S474. [Google Scholar] [CrossRef]
- Watanabe, A.; Nakanishi, K.; Kataoka, K. Intradural spinal arachnoid cyst contributing to sudden paraparesis. Surg. Neurol. Int. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Neto, J.F.; Silva-Neto, L.S.; Leal, P.R.L.; Moreira, C.H.S.; Ribeiro, E.M.L.; Cristino-Filho, G.; da Ponte, K.F. Multiple extradural spinal arachnoid cysts: A case report. Surg. Neurol. Int. 2021, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Marrone, S.; Kharbat, A.F.; Palmisciano, P.; Umana, G.E.; Haider, A.S.; Iacopino, D.G.; Nicoletti, G.F.; Scalia, G. Thoracic spinal extradural arachnoid cyst: A case report and literature review. Surg. Neurol. Int. 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, R.F.; Namer, T.S.; Almalki, A.; AlSufiani, F.; Arias, D.P. Idiopathic thoracolumbar spinal epidural arachnoid cysts: A case report and systematic review. Surg. Neurol. Int. 2022, 13, 599. [Google Scholar] [CrossRef]
- Sawaya, J.; Savla, P.; Minasian, T. Extradural spinal cyst in a pediatric patient: A case report. Surg. Neurol. Int. 2024, 15, 123. [Google Scholar] [CrossRef]
- Ayantayo, T.O.; Owagbemi, O.F.; Rasskazoff, S.; Sulaiman, O.A.R. Thoracic spinal intradural arachnoid cyst with a fulminant course. Ochsner. J. 2023, 23, 332–342. [Google Scholar] [CrossRef]
- Werner, C.; Mathkour, M.; Scullen, T.; Dallapiazza, R.F.; Dumont, A.S.; Maulucci, C.M. Recurrent arachnoid cysts secondary to spinal adhesive arachnoiditis successfully treated with a ventriculoperitoneal shunt. Clin. Neurol. Neurosurg. 2020, 194, 105835. [Google Scholar] [CrossRef]
- Pillai, M.K. Dorsal cervical spinal arachnoid cyst (Type III) presenting with dorsal column dysfunction: A case report. J. Spinal Cord. Med. 2017, 40, 250–252. [Google Scholar] [CrossRef][Green Version]
- French, H.; Somasundaram, A.; Biggs, M.; Parkinson, J.; Allan, R.; Ball, J.; Little, N. Idiopathic intradural dorsal thoracic arachnoid cysts: A case series and review of the literature. J. Clin. Neurosci. 2017, 40, 147–152. [Google Scholar] [CrossRef]
- Shi, L.; Su, Y.; Yan, T.; Wang, H.; Wang, K.; Liu, L. Early microsurgery on thoracolumbar spinal extradural arachnoid cysts: Analysis of a series of 41 patients. J. Clin. Neurosci. 2021, 94, 257–265. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gezer, B.; Şen, H.E.; Gündüz, B.; Etuş, V.; Karabagli, H.; Karabagli, P. Intradural spinal arachnoid cysts in children: A collective experience of 2 centers. World Neurosurg. 2023, 177, e637–e643. [Google Scholar] [CrossRef]
- Tokmak, M.; Ozek, E.; Iplikcioglu, A.C. Spinal extradural arachnoid cysts: A series of 10 cases. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2015, 76, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.H.; Hitchon, P.W.; Dlouhy, B.J. Symptomatic spinal extradural arachnoid cyst with cord compression in a family: Case report. J. Neurosurg. Spine 2017, 27, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, P.; Hejrati, N.; Charalampidis, A.; Wu, P.H.; Schneider, M.; Wilson, J.R.; Gao, A.F.; Massicotte, E.M.; Fehlings, M.G. Spinal arachnoid cysts: A case series & systematic review of the literature. Brain Spine 2022, 2, 100904. [Google Scholar] [CrossRef] [PubMed]
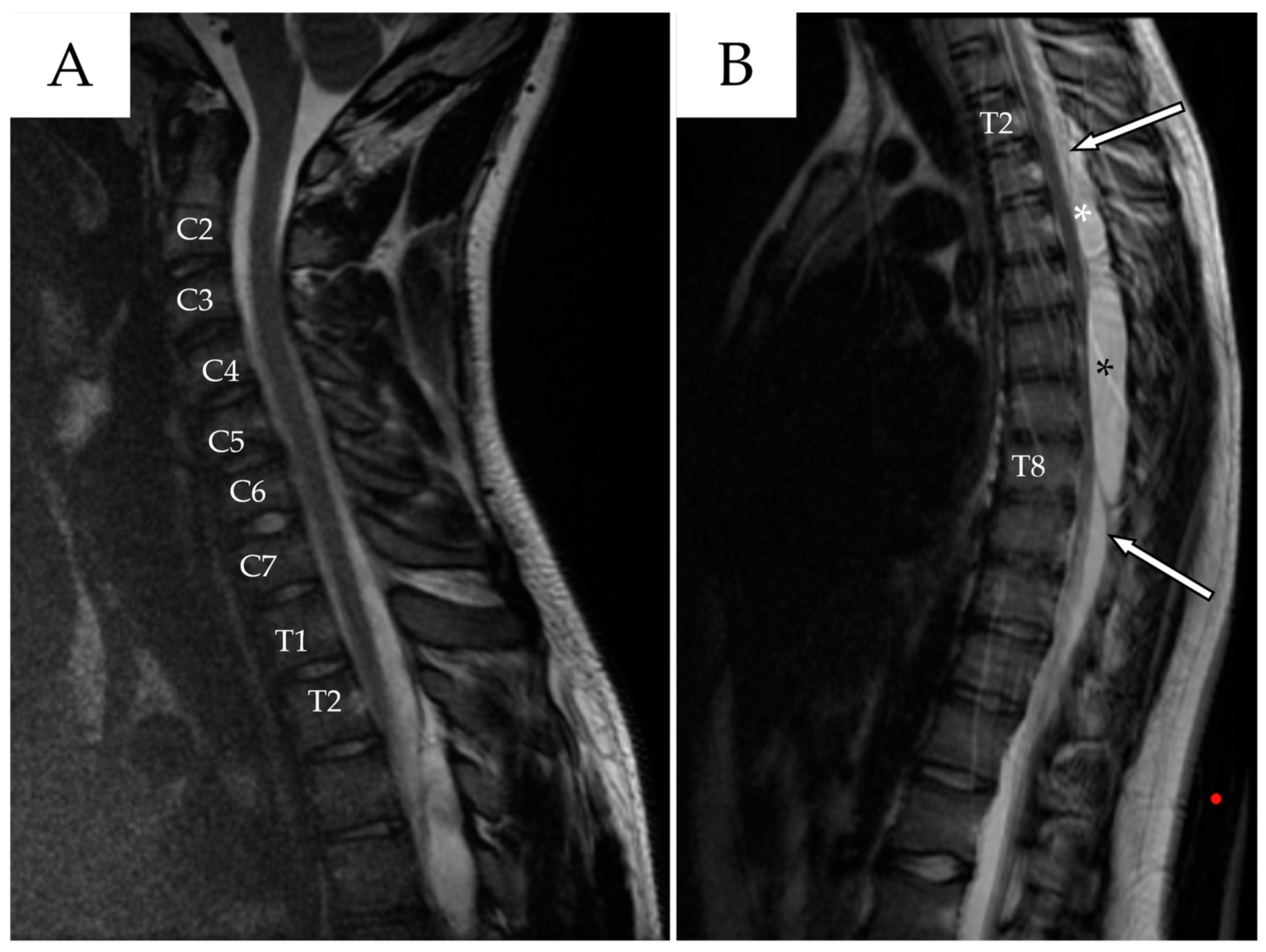
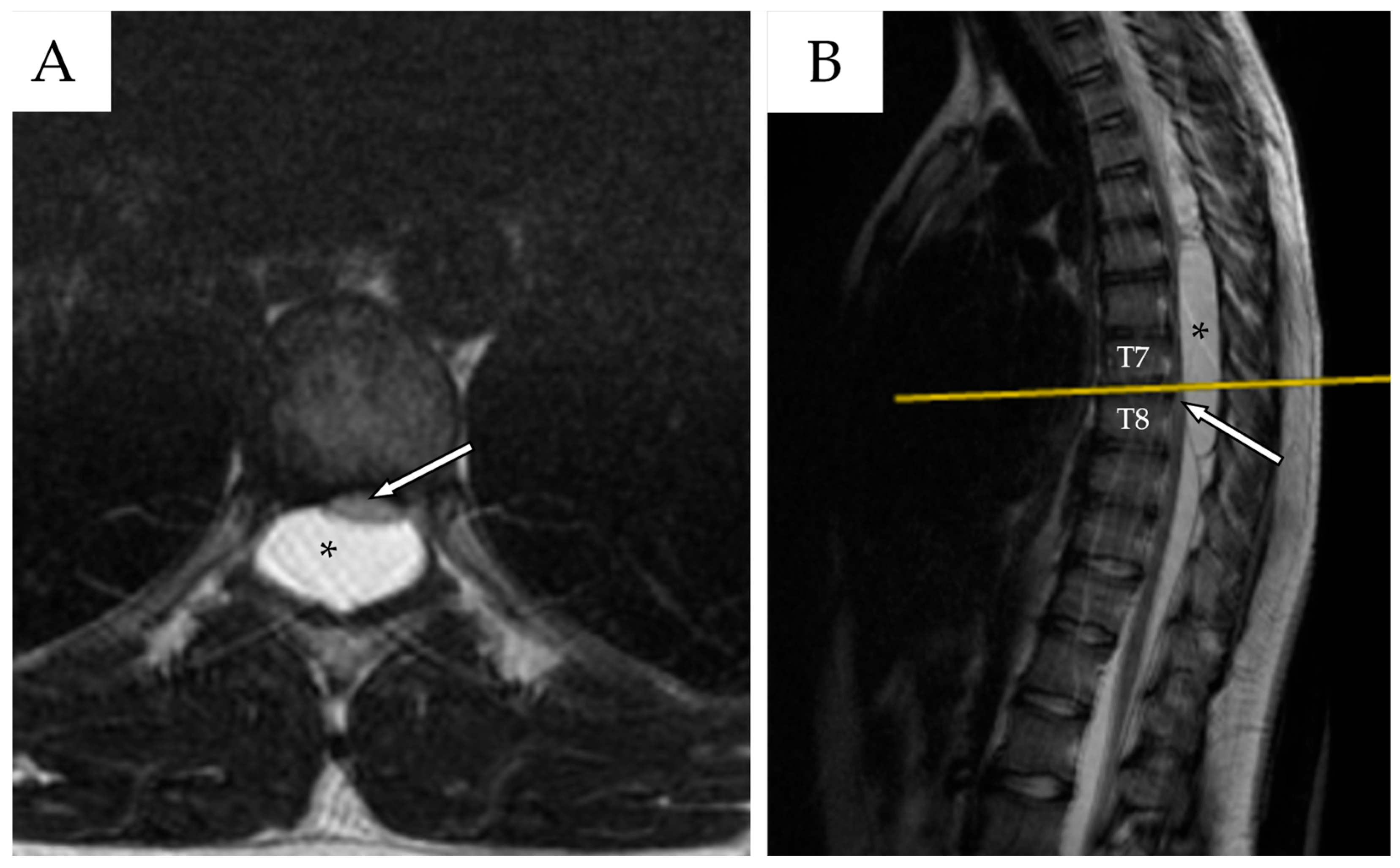
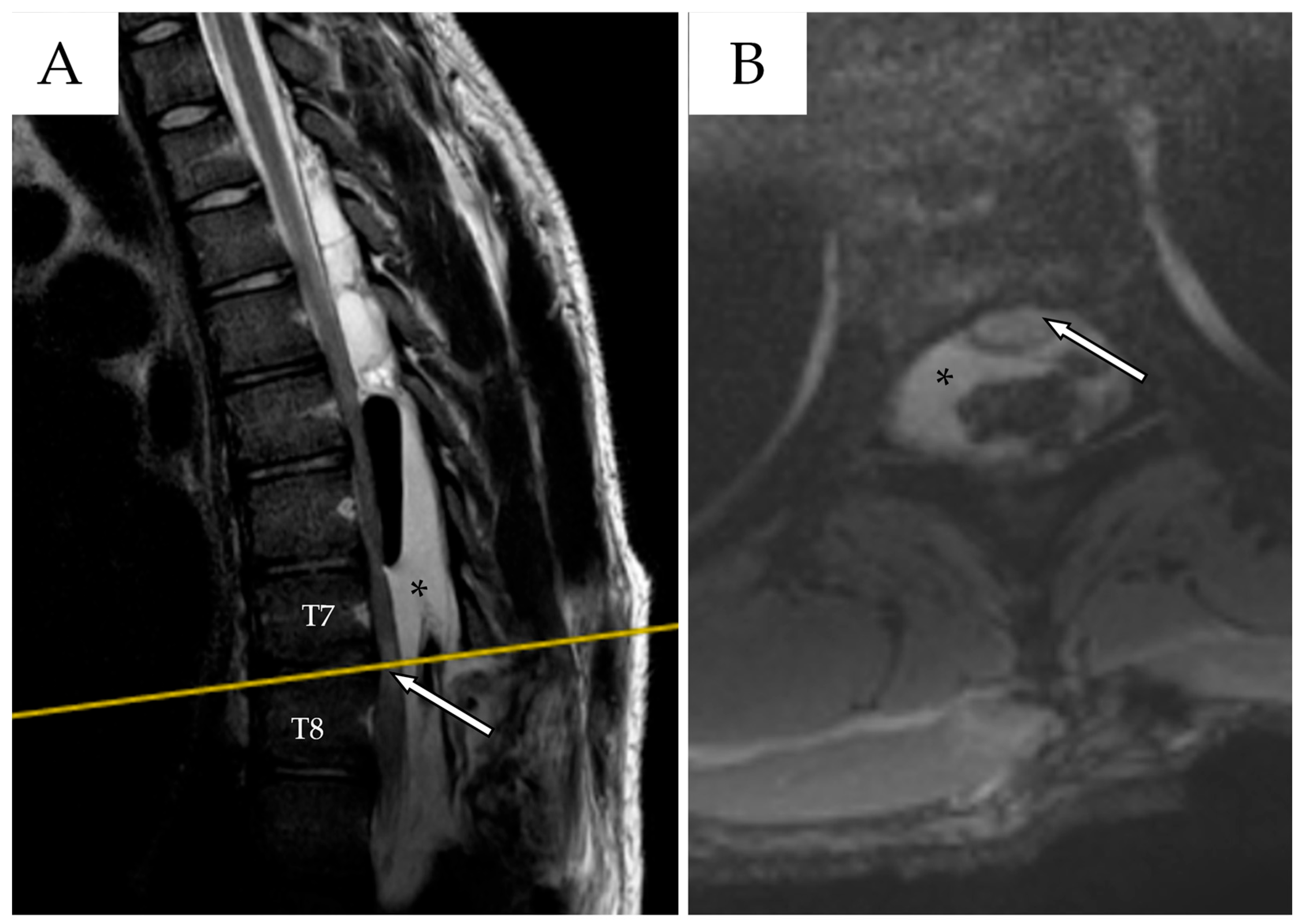
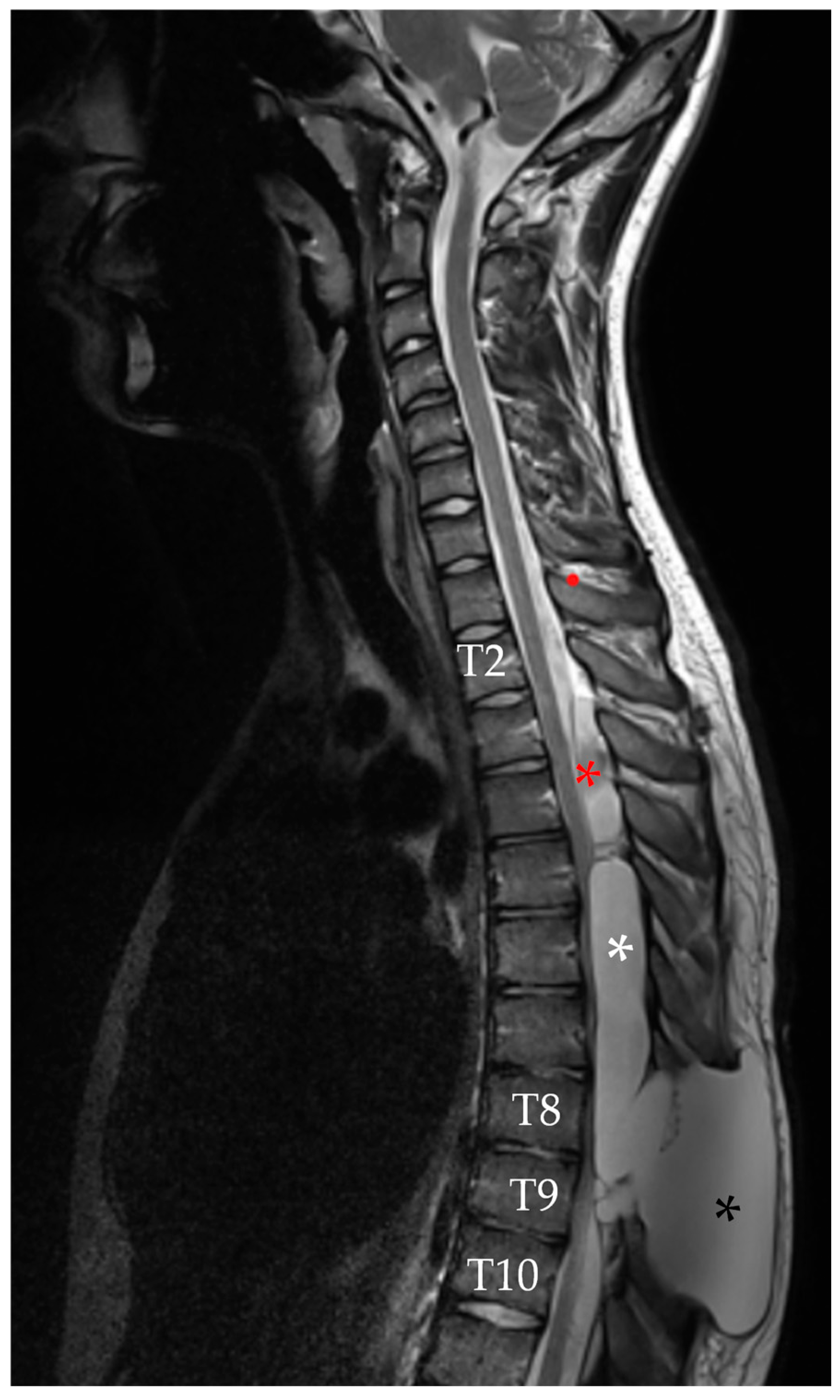
| Author | Country/Year | N° | Age * | Gender | Risk Factors | Location of the Cyst | Type of Cyst ** | Symptoms | Management |
|---|---|---|---|---|---|---|---|---|---|
| Ichinose T et al. [24] | Japan, 2020 | 1 | 4 | M | No | Cervical | Type III | Tetraparesis, bladder/bowel dysfunction | Fenestration |
| Novak L et al. [25] | Hungary, 2005 | 1 | 15 | F | Spine trauma | Thoracic | Type I | Spastic paraparesis inferior limbs, sensory loss | Excision |
| Lee HG et al. [26] | Korea, 2019 | 1 | 50 | M | Spine surgery | Lumbar | Type III | LBP, right S1 radiculopathy | Fenestration, excision |
| Krstačić A et al. [27] | Croatia, 2016 | 1 | 50 | F | No | Lumbar | Type III | LBP, right S1 radiculopathy, right leg hyposthenia | NA |
| Lee & Cho [28] | China, 2001 | 3 | 8, 9, 9 | M, M, F | No, No, No | Thoracic–lumbar, cervical–thoracic, thoracic | Type III | 1: Cauda equina 1: Spastic gait 1: Quadriplegia | Fenestration, excision |
| Bond AE et al. [29] | USA, 2012 | 31 | 6, 9 (median) | 17 F, 14 M | 16 CNS anomalies | 11 thoracic, 2 lumbar, 3 sacral, 4 cervical–thoracic, 4 thoracic–lumbar, 4 lumbar–sacral, 2 cervical | 19: Type III 11: Type I, 1: Type III/I | 21: Radiculopathy/myelopathy 3: Asymptomatic | 29: Excision 2: Fenestration, excision |
| Liu JK et al. [30] | USA, 2005 | 1 | 11 | F | No | Thoracic | Type I | Bladder dysfunction, gait ataxia, lower limbs hyposthenia | Excision |
| El-Hajj VG et al. [31] | Sweden, 2023 | 1 | 35 | F | Surgery for cranial arachnoid cyst | Cervical–thoracic | Type III | Neck and back pain, upper limbs weakness | Excision |
| Özdemir M et al. [32] | Turkey, 2019 | 1 | 22 | M | No | Cervical–thoracic | Type I | Neck pain, numbness and weakness of the left upper arm | Excision |
| Ebot J et al. [33] | USA, 2020 | 1 | 80 | F | No | Thoracic | Type III | Lower extremity weakness, decreased sensation and hyperreflexia | Fenestration |
| Alugolu R et al. [34] | India, 2016 | 1 | 54 | F | No | Thoracic | Type III | Spastic weakness of lower limbs, decreased sensations below T12 | Excision |
| Nayak R et al. [35] | India, 2015 | 1 | 7 | F | No | Thoracic | Type I | Numbness and weakness of lower limbs | Excision |
| Sharif S et al. [36] | Pakistan, 2017 | 1 | 25 | M | No | Lumbar | Type III | LBP, numbness of lower limbs, cauda equina | Excision |
| Lin & Jason [37] | Malaysia, 2018 | 1 | 35 | M | No | Thoracic | Type I | Numbness and weakness of lower limbs | Excision |
| Shaaban A et al. [38] | Quatar, 2019 | 1 | 31 | M | No | Thoracic | Type III | T6 sensory level, LBP, bladder/bowel disfunction | Excision |
| Raes & Oostra [39] | Belgium, 2021 | 2 | 38, 45 | F, M | Spine trauma with T12 and L1 fracture | Thoracic | Type III | Loss of strength of lower limbs, sensory disturbances in the right leg, paraplegia and T11 neurological level | Fenestration |
| Taccone MS et al. [40] | Canada, 2018 | 1 | 72 | M | TLIF L4-L5 | Lumbar | Type III | Lower limbs weakness in first day after surgery | Fenestration |
| Turel MK et al. [41] | USA, 2017 | 1 | 36 | F | Epidural steroid injections | Lumbar | Type III | Asymptomatic | Conservative managed |
| Kong WK et al. [42] | Korea, 2013 | 1 | 65 | M | Spine trauma | Thoracic–lumbar | Type I | Weakness of lower limbs, bladder dysfunction | Fenestration |
| Fischer KM et al. [43] | USA, 2022 | 1 | 7 | F | No | Thoracic | Type III | Urinary and fecal incontinence | Excision |
| Sharma R et al. [44] | India, 2023 | 1 | 16 | M | No | Cervical–thoracic–lumbar | Type I | Weakness of lower limbs, numbness of the right leg | Excision |
| Engelhardt J et al. [45] | France, 2015 | 1 | 18 | M | No | Cervical | Type III | Cervical pain, paresthesia and weakness of both arms | Excision |
| Zekaj E et al. [46] | Italy, 2016 | 1 | 47 | F | No | Thoracic | Type III | Right dorsal radicular pain, weakness of the left lower limb | Fenestration |
| Watanabe A et al. [47] | Japan, 2019 | 1 | 37 | F | No | Thoracic | Type III | Abdominal pain, numbness and weakness of both lower extremities | Excision |
| Cavalcante-Neto JF et al. [48] | Brazil, 2021 | 1 | 38 | F | No | Thoracic–lumbar | Type I | Transient weakness of lower limbs, bilateral patellar hyperreflexia | Excision |
| Marrone S et al. [49] | Italy, 2022 | 1 | 70 | M | No | Thoracic | Type I | Bilateral lower limb weakness and paresthesia | Fenestration, excision |
| Alanazi RF et al. [50] | Saudi Arabia, 2022 | 1 | 47 | F | No | Thoracic–lumbar | Type I | Progressive lower extremity paraparesis | Excision |
| Sawaya J et al. [51] | USA, 2024 | 1 | 15 | F | No | Thoracic–lumbar | Type I | LBP radiating bilateral in posterior thighs and knees | Fenestration, excision |
| Ayantayo TO et al. [52] | Nigeria, 2023 | 1 | 49 | M | No | Thoracic | Type III | Gait instability, urinary retention and paraplegia | Excision |
| Werner C et al. [53] | USA, 2020 | 1 | 58 | M | Spinal schwannoma Post-surgical meningitis | Thoracic | Type III | Paraplegia and loss of sensation below T10, urinary retention | Fenestration |
| Pillai MK [54] | Oman, 2016 | 1 | 29 | F | No | Cervical–thoracic | Type III | Posterior column dysfunction | Fenestration |
| French H et al. [55] | Australia, 2017 | 10 | 60 (mean) | F:M 2:1 | NA | Thoracic | Type III | Thoracic myelopathy, gait ataxia 3: thoracic radicular pain 1: sphincter dysfunction | 6: Fenestration 4: Excision |
| Shi L et al. [56] | China, 2021 | 41 | 41 (mean) | 24F, 17M | NA | Thoracic–lumbar | Type I | Radicular pain, gait ataxia, myelopathy 1: cauda equina syndrome | Excision |
| Yılmaz E et al. [57] | Turkey, 2023 | 8 | 8 (mean) | 4F, 4M | NA | Thoracic–lumbar | Type III | Motor weakness, urinary incontinence, sensory disturbance | 7: Excision 1: Shunt placement |
| Tokmak M et al. [58] | Turkey, 2024 | 10 | 43 (mean) | 6F, 4M | NA | Thoracic–lumbar | Type I | Numbness and progressive weakness of the lower extremities, back pain | 9: Excision 1: Conservative managed |
| Menezes AH et al. [59] | USA, 2017 | 2 | 14, 34 | M, F | Back trauma | Thoracic–lumbar, lumbar | Type I | Gait instability, urinary retention, pain in the left leg | Excision |
| Kalsi P et al. [60] | Canada, 2022 | 11 | 47 (mean) | 3F, 8M | 3 presented with associated syrinx | 10 cervical–thoracic, 1 cervical | Type III | Neuropathic pain, back pain, weakness, gait and balance problems, sensory issues, sphincter disturbance, radicular pain, long tract signs, gait ataxia | 10: Fenestration 1: Excision 1: Shunt placement |
| Improvement | Relapse and Complications |
|---|---|
| No | No |
| Yes | No |
| Yes | NA |
| NA | No, no, no |
| 2: Yes 1: Partial | 1: Fistula 1: Pseudomeningocele 1: Relapse and shunt |
| 27: Yes 4: No | No |
| Yes | Yes |
| Yes | No |
| Yes | No |
| Yes | Pseudomeningocele |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | Yes, yes |
| Yes, yes | No |
| Yes | NA |
| NA | No |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | Yes |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | No |
| Yes | Yes |
| Yes | No |
| Yes | 1: Pseudomeningocele 1: Syrinx |
| Yes | 2: Yes |
| Yes | No |
| Yes | 1: Fistula |
| Yes | No |
| Yes | No |
| 4: No 7: Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellino, F.; Bradaschia, L.; Ajello, M.; Garbossa, D. Twice the Leak: Managing CSF Fistulas in a Recurrent Thoracic Arachnoid Cyst—A Case Report. Reports 2025, 8, 152. https://doi.org/10.3390/reports8030152
Bellino F, Bradaschia L, Ajello M, Garbossa D. Twice the Leak: Managing CSF Fistulas in a Recurrent Thoracic Arachnoid Cyst—A Case Report. Reports. 2025; 8(3):152. https://doi.org/10.3390/reports8030152
Chicago/Turabian StyleBellino, Federica, Leonardo Bradaschia, Marco Ajello, and Diego Garbossa. 2025. "Twice the Leak: Managing CSF Fistulas in a Recurrent Thoracic Arachnoid Cyst—A Case Report" Reports 8, no. 3: 152. https://doi.org/10.3390/reports8030152
APA StyleBellino, F., Bradaschia, L., Ajello, M., & Garbossa, D. (2025). Twice the Leak: Managing CSF Fistulas in a Recurrent Thoracic Arachnoid Cyst—A Case Report. Reports, 8(3), 152. https://doi.org/10.3390/reports8030152









