A Case of Success: Guidelines-Based Treatment to Control Atrial Fibrillation-Induced Cardiomyopathy—Atrioventricular Node Ablation and Cardiac Resynchronization Therapy to the Rescue
Abstract
1. Introduction and Clinical Significance
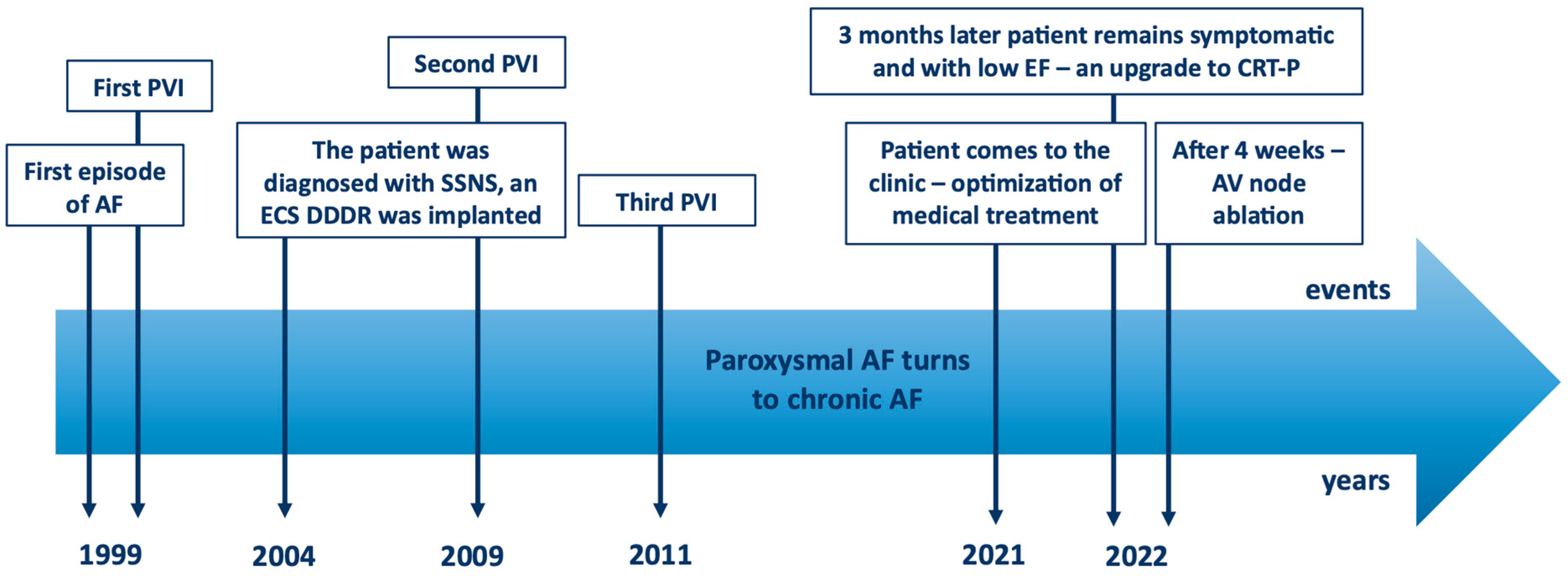
2. Case Presentation
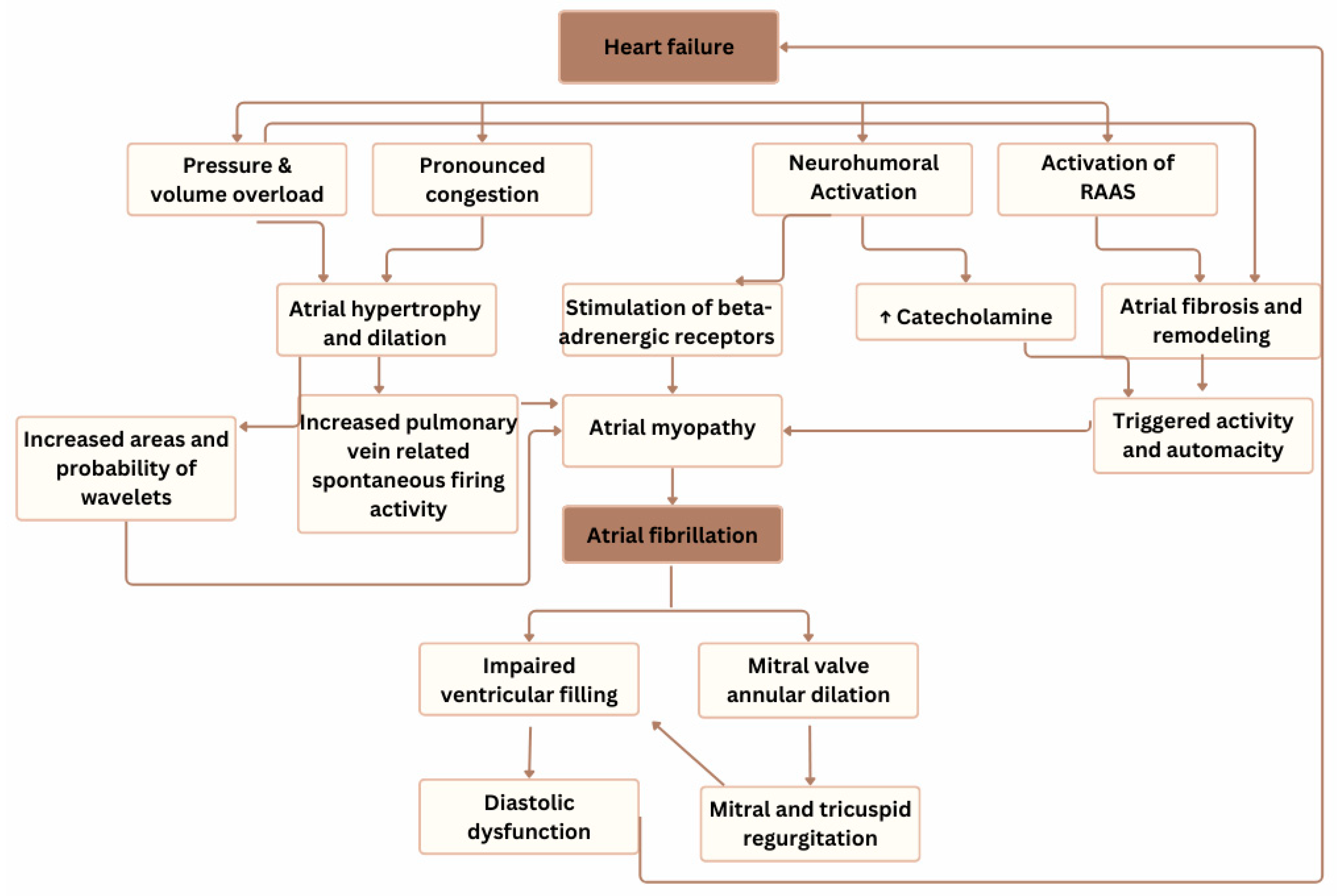
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergau, L.; Bengel, P.; Sciacca, V.; Fink, T.; Sohns, C.; Sommer, P. Atrial fibrillation and heart failure. J. Clin. Med. 2022, 11, 2510. [Google Scholar] [CrossRef]
- Van, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar]
- Mohamed, H.A. Tachycardia-induced cardiomyopathy (tachycardiomyopathy). Libyan J. Med. 2007, 2, 26–29. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chen, Y.C.; Chen, Y.A.; Yeh, Y.-H.; Chen, S.-A.; Chen, Y.-J. B-type natriuretic peptide modulates pulmonary vein arrhythmogenesis: A novel potential contributor to the genesis of atrial tachyarrhythmia in heart failure. J. Cardiovasc. Electrophysiol. 2016, 27, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Kneller, J.; Leon, J.L.; Nattel, S. Substrate size as a determinant of fibrillatory activity maintenance in a mathematical model of canine atrium. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1002–H1012. [Google Scholar] [CrossRef]
- Workman, A.J.; Kane, K.A.; Rankin, A.C. Cellular bases for human atrial fibrillation. Heart Rhythm. 2008, 5 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef]
- Shinagawa, K.; Pang, L.; Leung, T.K.; Cardin, S.; Wang, Z.; Nattel, S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 2001, 104, 2608–2614. [Google Scholar]
- Maggioni, A.P.; Latini, R.; Carson, P.E.; Singh, S.N.; Barlera, S.; Glazer, R.; Masson, S.; Cerè, E.; Tognoni, G.; Cohn, J.N. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: Results from the Valsartan Heart Failure Trial (Val-HeFT). Am. Heart J. 2005, 149, 548–557. [Google Scholar] [CrossRef]
- Del Monte, A.; Sarkozy, A.; Verbrugge, F.H. Atrial fibrillation management with guideline-directed medical therapy and comorbidity treatment in heart failure patients. Card. Electrophysiol. Clin. 2025, 17, 63–73. [Google Scholar] [CrossRef]
- Sossalla, S.; Vollmann, D. Arrhythmia-induced cardiomyopathy. Dtsch. Arztebl. Int. 2018, 115, 335–341. [Google Scholar] [CrossRef]
- Ellis, E.R.; Josephson, M.E. What about tachycardia-induced cardiomyopathy? Arrhythm. Electrophysiol. Rev. 2013, 2, 82–90. [Google Scholar] [CrossRef]
- Dandamudi, G.; Rampurwala, A.Y.; Mahenthiran, J.; Miller, J.M.; Das, M.K. Persistent left ventricular dilatation in tachycardia-induced cardiomyopathy patients after appropriate treatment and normalization of ejection fraction. Heart Rhythm 2008, 5, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Vlachakis, P.K.; Theofilis, P.; Apostolos, A.; Karakasis, P.; Ktenopoulos, N.; Boulmpou, A.; Drakopoulou, M.; Leontsinis, I.; Xydis, P.; Kordalis, A.; et al. Beyond pulmonary vein reconnection: Exploring the dynamic pathophysiology of atrial fibrillation recurrence after catheter ablation. J. Clin. Med. 2025, 14, 2919. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- De Vecchis, R.; Paccone, A.; Di Maio, M. Favorable effects of sacubitril/valsartan on peak atrial longitudinal strain in patients with chronic heart failure and a history of atrial fibrillation: A retrospective cohort study. J. Clin. Med. Res. 2020, 12, 100–107. [Google Scholar] [CrossRef]
- Docherty, K.F.; Vaduganathan, M.; Solomon, S.D.; McMurray, J.J.V. Sacubitril/valsartan: Neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020, 8, 800–810. [Google Scholar] [CrossRef]
- Wachter, R.; Senni, M.; Belohlavek, J.; Straburzynska-Migaj, E.; Witte, K.K.; Kobalava, Z. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: Primary results of the randomised TRANSITION study. Eur. J. Heart Fail. 2019, 21, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Dolladille, C.; Douesnel, L.; Font, J.; Dabrowski, R.; Shavit, L.; Legallois, D.; Funck-Brentano, C.; Champ-Rigot, L.; Ollitrault, P.; et al. Effects of mineralocorticoid receptor antagonists on atrial fibrillation occurrence: A systematic review, meta-analysis, and meta-regression to identify modifying factors. J. Am. Heart Assoc. 2019, 8, e013267. [Google Scholar] [CrossRef]
- Elayi, C.S.; Shohoudi, A.; Moodie, E.; Etaee, F.; Guglin, M.; Roy, D.; Khairy, P.; AF-CHF Investigators. Digoxin, mortality, and cardiac hospitalizations in patients with heart failure with reduced ejection fraction and atrial fibrillation: An AF-CHF analysis. Int. J. Cardiol. 2020, 313, 48–54. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Bristow, M.R.; Freemantle, N.; Olshansky, B.; Gras, D.; Saxon, L.; Tavazzi, L.; Boehmer, J.; Ghio, S.; Feldman, A.M.; et al. The effect of cardiac resynchronization without a defibrillator on morbidity and mortality: An individual patient data meta-analysis of COMPANION and CARE-HF. Eur. J. Heart Fail. 2022, 24, 1080–1090. [Google Scholar] [CrossRef]
- Brignole, M.; Pentimalli, F.; Palmisano, P.; Landolina, M.; Quartieri, F.; Occhetta, E.; Calò, L.; Mascia, G.; Mont, L.; Vernooy, K.; et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: The APAF-CRT mortality trial. Eur. Heart J. 2021, 42, 4731–4739, Erratum in Eur. Heart J. 2021, 42, 4768; Erratum in Eur. Heart J. 2022, 43, 386. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonaitienė, N.; Ramantauskaitė, G.; Laukaitienė, J. A Case of Success: Guidelines-Based Treatment to Control Atrial Fibrillation-Induced Cardiomyopathy—Atrioventricular Node Ablation and Cardiac Resynchronization Therapy to the Rescue. Reports 2025, 8, 150. https://doi.org/10.3390/reports8030150
Jonaitienė N, Ramantauskaitė G, Laukaitienė J. A Case of Success: Guidelines-Based Treatment to Control Atrial Fibrillation-Induced Cardiomyopathy—Atrioventricular Node Ablation and Cardiac Resynchronization Therapy to the Rescue. Reports. 2025; 8(3):150. https://doi.org/10.3390/reports8030150
Chicago/Turabian StyleJonaitienė, Neda, Grytė Ramantauskaitė, and Jolanta Laukaitienė. 2025. "A Case of Success: Guidelines-Based Treatment to Control Atrial Fibrillation-Induced Cardiomyopathy—Atrioventricular Node Ablation and Cardiac Resynchronization Therapy to the Rescue" Reports 8, no. 3: 150. https://doi.org/10.3390/reports8030150
APA StyleJonaitienė, N., Ramantauskaitė, G., & Laukaitienė, J. (2025). A Case of Success: Guidelines-Based Treatment to Control Atrial Fibrillation-Induced Cardiomyopathy—Atrioventricular Node Ablation and Cardiac Resynchronization Therapy to the Rescue. Reports, 8(3), 150. https://doi.org/10.3390/reports8030150




