Comprehensive Fertility Management After Pituitary Adenoma Surgery: Lessons from a Rural Japanese Case and Practical Review
Abstract
1. Introduction and Clinical Significance
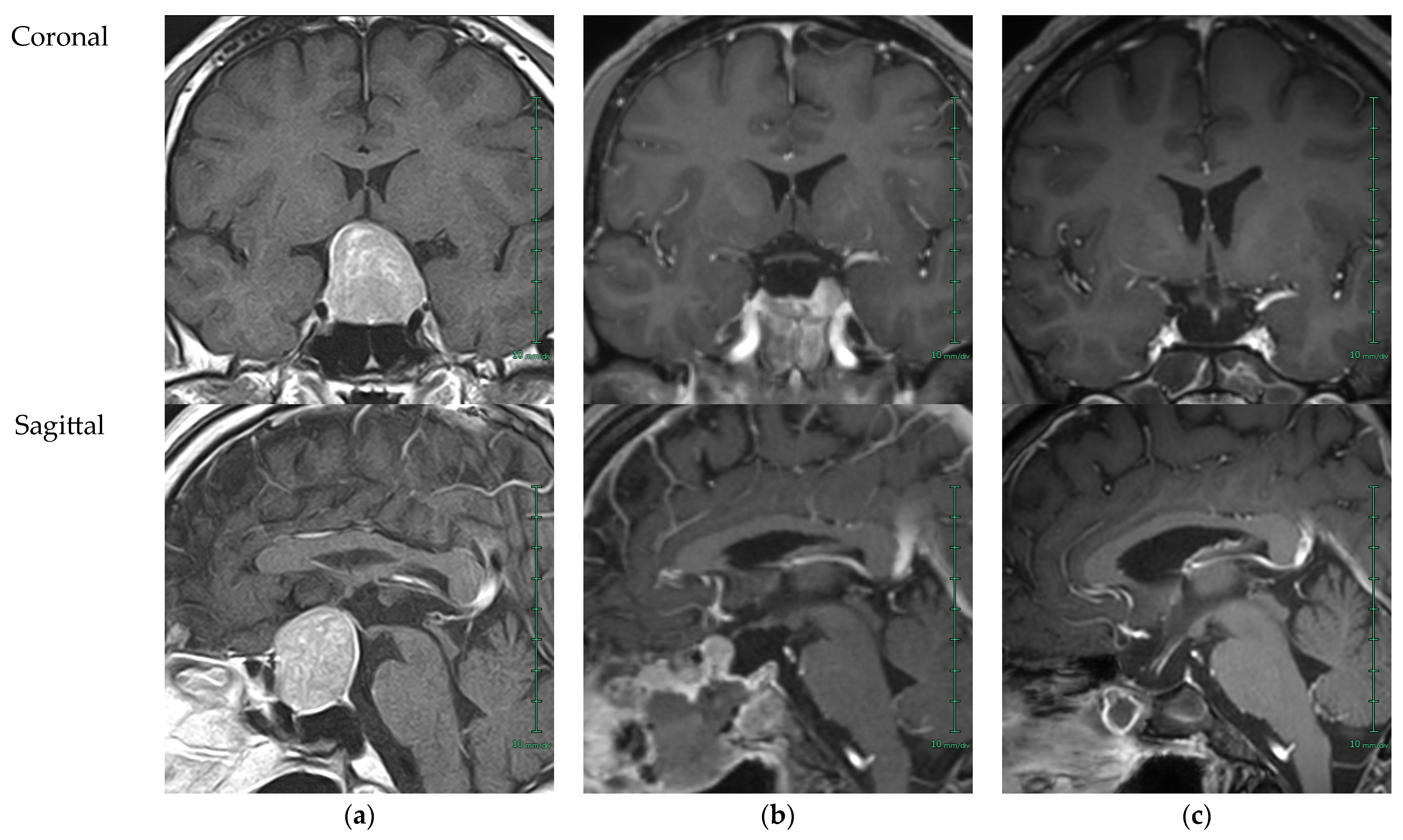
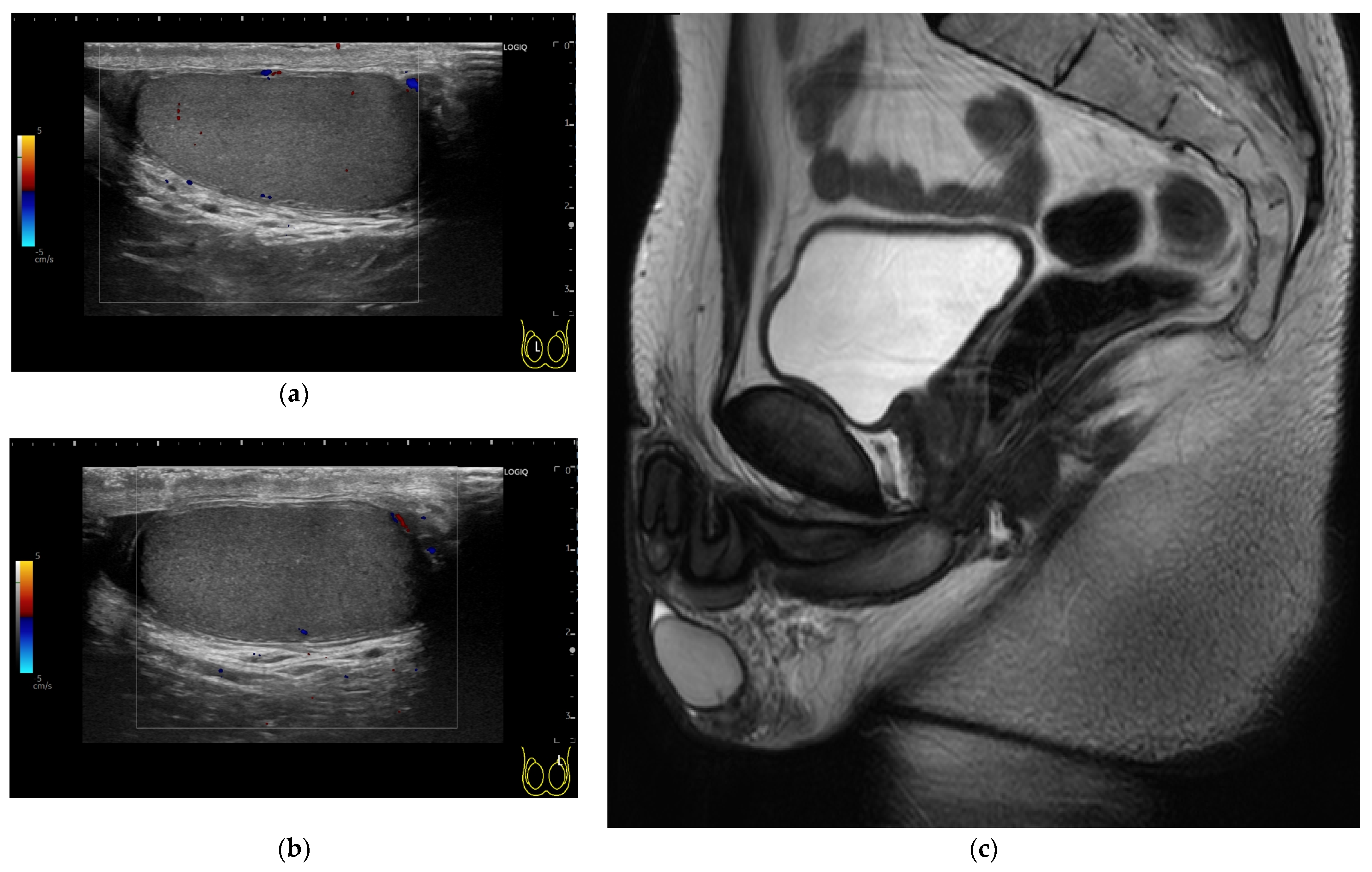
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AMH | Anti-Müllerian hormone |
| AMS | Aging Male’s Symptoms |
| ASCO | American Society of Clinical Oncology |
| CNS | Central nervous system |
| CRH | Corticotropin-releasing hormone |
| CT | Computed tomography |
| E2 | Estoradiol |
| EHS | Erection Hardness Score |
| FSH | Follicle stimulating hormone |
| FT | Free testosterone |
| FT3 | Free triiodothyronine |
| FT4 | Free thyroxine |
| GH | Growth hormone |
| GHRP-2 | Growth hormone-releasing peptide 2 |
| GnRH | Gonadotropin-releasing hormone |
| hCG | Human chorionic gonadotropin |
| HH | Hypogonadotropic hypogonadism |
| HRT | Hormone replacement therapy |
| IIEF-5 | The International Index of Erectile Function-5 |
| IGF-1 | Insulin-like growth factor 1 |
| JES | Japan Endocrine Society |
| JSCO | Japan Society of Clinical Oncology |
| JSGI | Japanese Society of Gender Incongruence |
| JSHP | Japanese Society for Hypothalamic and Pituitary Tumors |
| JSMH | Japanese Society of Men’s Health |
| JSPE | Japanese Society for Pediatric Endocrinology |
| JSPU | Japanese Society of Pediatric Urology |
| JSRE | Japan Society of Reproductive Endocrinology |
| JSRM | Japan Society for Reproductive Medicine |
| JUA | Japanese Urological Association |
| LH | luteinizing hormone |
| LHRH | Luteinizing hormone-releasing hormone |
| LLR | Lower limit of reference intervals |
| MSHQ-EjD-SF | Male Sexual Health Questionnaire-Ejaculatory Dysfunction-Short Form |
| MRI | Magnetic resonance imaging |
| NA | Not available |
| PIF | Prolactin inhibiting factor |
| PitNET | Pituitary neuroendocrine tumor |
| PRL | Prolactin |
| rFSH | Recombinant human follicle-stimulating hormone |
| ST1 | Steroidogenic factor 1 |
| T/E ratio | Ratio of total testosterone to estradiol |
| TESE | Testicular sperm extraction |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| TT | Total testosterone |
| ULR | Upper limit of reference intervals |
| WHO | World Health Organization |
References
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Astaf’eva, L.I.; Zhukov, O.B.; Kadashev, B.A.; Klochkova, I.S.; Kobyakov, G.L.; Poddubskiy, A.A.; Kalinin, P.L. Narusheniya reproduktivnoy funktsii i vozmozhnosti sokhraneniya fertil’nosti muzhchin s dobrokachestvennymi i zlokachestvennymi opukholyami golovnogo mozga [Reproductive disorders and preservation of fertility in males with benign and malignant brain tumors]. Zhurnal Voprosy Neirokhirurgii Imeni N. N. Burdenko 2019, 83, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Astafyeva, L.; Sidneva, Y. Preservation of fertility in men with benign and malignant brain tumors. Endocr. Abstr. 2020, 70, EP408. [Google Scholar] [CrossRef]
- Quinn, G.P.; Vadaparampil, S.T.; Gwede, C.K.; Miree, C.; King, L.M.; Clayton, H.B.; Wilson, C.; Munster, P. Discussion of fertility preservation with newly diagnosed patients: Oncologists’ views. J. Cancer Surviv. 2007, 1, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Furui, T.; Takae, S.; Sugishita, Y.; Kawahara, T.; Shigematsu, K.; Kimura, F.; Horie, A.; Hara, T.; Kato, M.; et al. Gan-Seishoku Iryō Renkei Miseibi Chiiki 24-kasho no Genjō to Kadai — Chiiki Kakusa o Kaishō Suru Tame no Shisaku [Promotion of Equal Access to Medical Services for Children, Adolescent and Young Adult (CAYA) Cancer Patients with Reproductive Problems-A Nationwide Expansion of the Regional Oncofertility Network in Japan]. Gan Kagaku Ryoho 2020, 47, 1691–1696. [Google Scholar] [PubMed]
- Stone, J.B.; Kelvin, J.F.; DeAngelis, L.M. Fertility preservation in primary brain tumor patients. Neurooncol. Pract. 2017, 4, 40–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casanueva, F.F.; Barkan, A.L.; Buchfelder, M.; Klibanski, A.; Laws, E.R.; Loeffler, J.S.; Melmed, S.; Mortini, P.; Wass, J.; Giustina, A.; et al. Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): A Pituitary Society Statement. Pituitary 2017, 20, 489–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marques, P.; Sagarribay, A.; Tortosa, F.; Neto, L.; Tavares Ferreira, J.; Subtil, J.; Palha, A.; Dias, D.; Sapinho, I. Multidisciplinary Team Care in Pituitary Tumours. Cancers 2024, 16, 950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonneville, J.-F. Nonfunctioning Pituitary Macroadenoma: General Points. In MRI of the Pituitary Gland; Springer: Cham, Switzerland, 2016; pp. 25–33. ISBN 978-3-319-29041-6/978-3-319-29043-0. [Google Scholar] [CrossRef]
- Yuan, L.; Li, P.; Li, J.; Peng, J.; Zhouwen, J.; Ma, S.; Jia, G.; Jia, W.; Kang, P. Identification and gene expression profiling of human gonadotrophic pituitary adenoma stem cells. Acta Neuropathol. Commun. 2023, 11, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sav, A. Editorial: Diagnosis and treatment of non-functioning pituitary tumors. Front. Endocrinol. 2025, 16, 1558988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albano, L.; Losa, M.; Barzaghi, L.R.; Niranjan, A.; Siddiqui, Z.; Flickinger, J.C.; Lunsford, L.D.; Mortini, P. Gamma Knife Radiosurgery for Pituitary Tumors: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charleux, T.; Vendrely, V.; Huchet, A.; Trouette, R.; Ferriere, A.; Tabarin, A.; Jecko, V.; Loiseau, H.; Dupin, C. Management after initial surgery of nonfunctioning pituitary adenoma: Surveillance, radiotherapy or surgery? Radiat. Oncol. 2022, 17, 165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, H. Kasuitai Shuyō no Hōshasen Chiryō — Saishin no Chiken kara [Radiotherapy for Pituitary Tumors:An Overview of the Current Evidence]. No Shinkei Geka 2023, 51, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- The Japan Neurosurgical Society; The Japanese Society of Pathology. Rinshō, Byōri Nōshuyō Toriatsukai Kiyaku [General Rules for Clinical and Pathological Studies on Brain Tumors], 5th ed.; Kaneharashuppan: Tokyo, Japan, 2023; ISBN 978-4-307-20463-7/978-4-307-80463-9. [Google Scholar] [CrossRef]
- Goyal, A.; Kubihal, S.; Gupta, Y.; Jyotsna, V.P.; Khadgawat, R. Dynamic Testing for Evaluation of Adrenal and Gonadal Function in Pediatric and Adult Endocrinology: An Overview. Indian. J. Endocrinol. Metab. 2019, 23, 593–601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwasaki, Y. Kasuitai Shigeki Shiken no Hitsuyō-sei wa? [Why is a pituitary stimulation test necessary?]. Sogo Shinryo 2017, 27, 1084. [Google Scholar] [CrossRef]
- Arima, H. Shitei Nanbyō to Wareware no Torikumi [Designated intractable disease and our efforts]. In The Hypothalamic-Pituitary Dysfunction Study Group; Ministry of Health, Labour and Welfare Science Research Grants: Policy Research Project for Intractable Diseases (Policy Research Project for Intractable Disease): Tokyo, Japan, 2020. Available online: https://kannoukasuitai.jp/archive/file/20200209_1.pdf (accessed on 19 February 2025).
- The Japanese Society for Hypothalamic and Pituitary tumors. Gonadotoropin Bunpitsu Teika-shō no Shindan to Chiryō no Tebiki (Heisei 22-nendo Kaitei) [Guidelines for the Diagnosis and Treatment of Gonadotropin Deviciency (2010)]. In Guidelines for Hypothalamic Pituitary Dysfunction. 2010. Available online: https://square.umin.ac.jp/kasuitai/guidance/gonadotropin.pdf (accessed on 19 May 2025).
- The Japan Endocrine Society. Kannō Kasuitai Kinō Shōgai no Shindan to Chiryō no Tebiki (Heisei 30-nendo Kaitei) [Diagnosis and treatment of hypothalamic pituitary dysfunction]. Folia Endocrinol. Jpn. 2019, 95, 1–60. [Google Scholar] [CrossRef]
- The Japan Endocrine Society. Kannō Kasuitai Kinō Shōgai to Sentensei Jinsei Nyōhōshō oyobi Kanren Shikkan no Shinryō Gaidorain 2023-nenban [Guideline 2023 for hypothalamic pituitary dysfunction, congenital nephrogenic diabetes insipidus and related diseases]. Folia Endocrinol. Jpn. 2023, 99, 1–171. [Google Scholar] [CrossRef]
- The Japanese Urological Association; The Japanese Society of Men’s Health. Karei Dansei Seisen Kinō Teika-shōkōgun (LOH-shōkōgun) Shinryō no Tebiki: Dansei Horumon Teika ni yoru Dansei Kōnenki Shōgai, ED, Shinshinshō nado no Shinryō Manyuaru [Guidelines for Late-Onset Hypogonadism]; Jiho: Tokyo, Japan, 2007; ISBN 9784840736640. [Google Scholar]
- Namiki, M.; Akaza, H.; Shimazui, T.; Ito, N.; Iwamoto, T.; Baba, K.; Kumano, H.; Koh, E.; Tsujimura, A.; Matsumiya, K.; et al. Clinical practice manual for late-onset hypogonadism syndrome. Int. J. Urol. 2008, 15, 377–388. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Urological Association; The Japanese Society of Men’s Health. LOH-shōkōgun (Karē Dansei Seisen Kinō Teika-shō) Shinryō no Tebiki [Guidelines for Late-Onset Hypogonadism]; Igaku tosho shuppan: Tokyo, Japan, 2022; ISBN 9784865174946. [Google Scholar]
- Ide, H.; Akehi, Y.; Fukuhara, S.; Ohira, S.; Ogawa, S.; Kataoka, T.; Kumagai, H.; Kobayashi, K.; Komiya, A.; Shigehara, K.; et al. Summary of the clinical practice manual for late-onset hypogonadism. Int. J. Urol. 2023, 30, 422–430. [Google Scholar] [CrossRef] [PubMed]
- The Japan Endocrine Society; The japanese Society of Men’s Health. Dansei no Seisen Kinō Teika-shō Gaidorain 2022 [Guideline for male hypogonadism 2022]. Folia Endocrinol. Jpn. 2022, 98, 1–142. [Google Scholar] [CrossRef]
- The Japanese Urological Association; The Japanese Society for Reproductive Medicine. Dansei Funin-shō Shinryō Gaidorain 2024-nenban [Clinical Practice Guideline for Male Infertility]; Medical View Co., Ltd.: Tokyo, Japan, 2024; ISBN 9784779227899. [Google Scholar]
- Tsujimura, A.; Iijima, M.; Umemoto, Y.; Kobayashi, H.; Komiya, A.; Shiraishi, K.; Chiba, K.; Hirota, Y.; Fukuhara, S.; Yumura, Y. Summary of the Clinical Practice Guidelines for Male Infertility by the Japanese Urological Association with the Support of the Japan Society for Reproductive Medicine. Int. J. Urol. 2025, 30, 422–430. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society for Pediatric Endocrinology; The Japan Endocrine Society; The Japanese Society of Pediatric Urology; The Japan Society of Reproductive Endocrinology; Japanese Society of Gender Incongruence. Seibunka Shikkan (DSD) no Shinryō Gaidorain (2025-nenban) [Clinical Practice Guidelines for Differences of Sex Development (2025)]. 2025. Available online: https://jspe.umin.jp/medical/files/guide20250609.pdf (accessed on 7 August 2025).
- Ministry of Justice (Japan). Act on Holidays of Administrative Organs (Act No. 91 of 1988). In Japanese Law Translation Database System. Available online: https://www.japaneselawtranslation.go.jp/ja/laws/view/1170 (accessed on 19 February 2025).
- Arab News Japan. New Year is a family holiday in Japan. Arab News Japan. 2024. Available online: https://www.arabnews.jp/en/arts-culture/article_138118/ (accessed on 2024 December 31).
- Shimada-Sammori, K.; Shimada, T.; Miura, R.E.; Kawaguchi, R.; Yamao, Y.; Oshima, T.; Oami, T.; Tomita, K.; Shinozaki, K.; Nakada, T.A. Machine learning algorithms for predicting days of high incidence for out-of-hospital cardiac arrest. Sci. Rep. 2023, 13, 9950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The Japan News. Stations, Airports Packed with Travelers; Scores Head Home for Holiday Season. The Japan News. 2024. Available online: https://japannews.yomiuri.co.jp/society/general-news/20241228-230307/ (accessed on 2025 February 19).
- Kyodo News. Japan transport hubs overflow as 9-day New Year break begins. Kyodo News. 2024. Available online: https://english.kyodonews.net/articles/-/51949 (accessed on 2025 February 19).
- Markar, S.R.; Wahlin, K.; Mattsson, F.; Lagergren, P.; Lagergren, J. Surgery during holiday periods and prognosis in oesophageal cancer: A population-based nationwide Swedish cohort study. BMJ Open 2016, 6, e013069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasinzai, A.Q.K.; Crispen, P.; Das, D.G.; Staras, S.A.S.; O’Malley, P.; Nassour, I.; Hitchcock, K.; Salloum, R.; Guo, Y.; George, T.J. Impact of year-end holidays on delayed cancer diagnoses and survival implications: A population-based study. J. Clin. Oncol. 2025, 43, e13793. [Google Scholar] [CrossRef]
- Takahashi, K.; Shimadzu, H. The daily incidence of out-of-hospital cardiac arrest unexpectedly increases around New Year’s Day in Japan. Resuscitation 2015, 96, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Fu, Z.; Luo, X.; Wang, J.; Ren, Z.; Wang, Y.; Mei, G.; Xiao, S. Evidence-based management strategies for endocrine complications after pituitary adenoma surgery. Ibrain 2025, 11, 245–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarzour, F.; Hage, M.; Sanson, M.R.; Baussart, B.; Chakhtoura, M. A suggested protocol for the endocrine postoperative management of patients undergoing pituitary surgery. Ann. Endocrinol. 2023, 84, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Sugihara, H. Kasuitai Zen’yō Kinō Kensa (GH Bunpitsu Shigeki Shiken, Sansha Fuka Shiken) [Anterior pituitary function test (GH secretion stimulation test, triple loading test)]. Rinsho Zasshi Naika 2020, 125, 982–983. [Google Scholar] [CrossRef]
- Hizuka, N. Naibunpitsu Rinshō Kensa Manyuaru [Manual of Clinical Laboratory Tests for Endocrine Diseases]; Nihon’ijishinposha: Tokyo, Japan, 2017; ISBN 9784784955459. [Google Scholar]
- Hashimoto, K.; Makino, S.; Hirasawa, R.; Takao, T.; Kageyama, J.; Ogasa, T.; Ota, Z. Combined anterior pituitary function test using CRH, GRH, LH-RH, TRH and vasopressin in patients with non-functioning pituitary tumors. Acta Med. Okayama 1990, 44, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Biller, B.M.; Samuels, M.H.; Zagar, A.; Cook, D.M.; Arafah, B.M.; Bonert, V.; Stavrou, S.; Kleinberg, D.L.; Chipman, J.J.; Hartman, M.L. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J. Clin. Endocrinol. Metab. 2002, 87, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Dzialach, L.; Sobolewska, J.; Zak, Z.; Respondek, W.; Witek, P. Prolactin-secreting pituitary adenomas: Male-specific differences in pathogenesis, clinical presentation and treatment. Front. Endocrinol. 2024, 15, 1338345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyu, L.; Yin, S.; Hu, Y.; Chen, C.; Jiang, Y.; Yu, Y.; Ma, W.; Wang, Z.; Jiang, S.; Zhou, P. Hyperprolactinemia in clinical non-functional pituitary macroadenomas: A STROBE-compliant study. Medicine 2020, 99, e22673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, S.; Suzuki, S.; Kami, S.; Naka, M.; Okano, K.; Harama, T.; Sugo, M.; Igarashi, K.; Ruike, Y.; Ishiwata, K.; et al. GHRP2 Fuka Shiken no PRL Bunpitsu Shōgai Shindan ni okeru Yūyōsei [Usefulness of the GHRP-2 Stimulation Test for the Diagnosis of Prolactin Secretion Disorders]. Folia Endocrinol. Jpn. 2025, 101, 9–11. [Google Scholar] [CrossRef]
- Atmaca, H.; Tanriverdi, F.; Gokce, C.; Unluhizarci, K.; Kelestimur, F. Do we still need the TRH stimulation test? Thyroid 2007, 17, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Sawin, C.T.; Hershman, J.M. The TSH response to thyrotropin-releasing hormone (TRH) in young adult men: Intra-individual variation and relation to basal serum TSH and thyroid hormones. J. Clin. Endocrinol. Metab. 1976, 42, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.H.; Ellis, R.D.; Beck, P.R. Exaggerated and prolonged thyrotrophin releasing hormone (TRH) test responses in tertiary hypothyroidism. J. Clin. Pathol. 1991, 44, 522–523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanase, T.; Tajima, T.; Katabami, T.; Iwasaki, Y.; Tanahashi, Y.; Sugawara, A.; Hasegawa, T.; Mune, T.; Oki, Y.; Nakagawa, Y.; et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: A Japan Endocrine Society clinical practice guideline [Opinion]. Endocr. J. 2016, 63, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Schulte, H.M.; Chrousos, G.P.; Avgerinos, P.; Oldfield, E.H.; Gold, P.W.; Cutler, G.B., Jr.; Loriaux, D.L. The corticotropin-releasing hormone stimulation test: A possible aid in the evaluation of patients with adrenal insufficiency. J. Clin. Endocrinol. Metab. 1984, 58, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.H.; Zheng, Y.; Zhang, X.L.; Mu, Y.M. Role of Gonadotropin-releasing Hormone Stimulation Test in Diagnosing Gonadotropin Deficiency in Both Males and Females with Delayed Puberty. Chin. Med. J. 2015, 128, 2439–2443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.J.; Lee, S.Y. Relationship between prolactin level and puberty in girls with early breast development. J. Pediatr. Endocrinol. Metab. 2022, 35, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Chiba, K.; Miyake, H. Tei Gonadotoropin-sei Danshi Seisen Kinō Teikashō (MHH) [Male hypogonadotropic hypogonadism (MHH)]. Jpn. J. Clin. Urol. 2024, 78, 125–127. [Google Scholar] [CrossRef]
- Enatsu, N.; Chiba, K.; Fujisawa, M. Dansei Tei Gonadotoropin-sei Seisen Kinō Teikashō no Kiso to Rinshō [Basic and Clinical Aspects of Male Hypogonadotropic Hypogonadism]. Jpn. J. Reprod. Endocrinol. 2016, 21, 17–22. Available online: https://jsre.umin.jp/16_21kan/9-review4.pdf (accessed on 19 May 2025).
- Iwamoto, T.; Matsuda, T. Dansei Funinshō no Rinshō [Diagnosis and Treatment of Male Infertility]; Medical View Co., Ltd.: Tokyo, Japan, 2007; ISBN 9784758305631. [Google Scholar]
- Kunitake, T.; Kitamura, T. hCG Fuka Shiken (Dansei) [The hCG Stimulation Test in Males]. Medicina 1996, 33, 2364–2365. [Google Scholar] [CrossRef]
- Fukutani, K.; Ishida, H.; Shinohara, M.; Minowada, S.; Niijima, T.; Isurugi, K. Responses of serum testosterone levels to human chorionic gonadotrophin stimulation in patients with Klinefelter’s syndrome after long-term androgen replacement therapy. Int. J. Androl. 1983, 6, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Isurugi, K.; Fukutani, K.; Hosoi, Y.; Takayasu, H. Kakushu danshi seisen kinō fuzenshō, oyobi sei bunka ijō shikkan ni okeru hCG tesuto ni tsuite [Short term hCG stimulation test in patients with various types of testicular insufficiency and abnormal genital development (author’s transl)]. Nihon Hinyokika Gakkai Zasshi 1978, 69, 6–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rey, R.A. Biomarkers of male hypogonadism in childhood and adolescence. Adv. Lab. Med. 2020, 1, 20200024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishii, T.; Matsuo, N.; Sato, S.; Ogata, T.; Tamai, S.; Anzo, M.; Kamimaki, T.; Sasaki, G.; Inokuchi, M.; Hori, N.; et al. Human Chorionic Gonadotropin Stimulation Test in Prepubertal Children with Micropenis Can Accurately Predict Leydig Cell Function in Pubertal or Postpubertal Adolescents. Horm. Res. Paediatr. 2015, 84, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tanda, H.; Kato, S.; Onishi, S.; Nakajima, H.; Nanbu, A.; Nitta, T.; Koroku, M.; Akagashi, K.; Hanzawa, T.; et al. Serum testosterone levels using the radioimmunoassay method in healthy Japanese male volunteers. Reprod. Med. Biol. 2006, 5, 37–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Celik, M.; Ozcelik, S.; Bas, S.; Sariaydin, M.; Ozcelik, M.; Gozu, H. Role of testosterone to estradiol ratio in predicting the efficacy of recombinant human chorionic gonadotropin and testosterone treatment in male hypogonadism. Arch. Endocrinol. Metab. 2021, 65, 617–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Takihara, H.; Sakatoku, J.; Fujii, M.; Nasu, T.; Cosentino, M.J.; Cockett, A.T. Significance of testicular size measurement in andrology. I. A new orchiometer and its clinical application. Fertil. Steril. 1983, 39, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Belay, R.E.; Huang, G.O.; Shen, J.K.; Ko, E.Y. Diagnosis of clinical and subclinical varicocele: How has it evolved? Asian J. Androl. 2016, 18, 182–185. [Google Scholar] [CrossRef]
- Kim, J.; So, B.; Heo, Y.; So, H.; Jo, J.K. Penile Erection Morphometry: The Need for a Novel Approach. World J. Mens. Health 2024, 42, 667–680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otani, T. Clinical review of ejaculatory dysfunction. Reprod. Med. Biol. 2019, 18, 331–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heinemann, L.A.; Saad, F.; Zimmermann, T.; Novak, A.; Myon, E.; Badia, X.; Potthoff, P.; T’Sjoen, G.; Pollanen, P.; Goncharow, N.P.; et al. The Aging Males’ Symptoms (AMS) scale: Update and compilation of international versions. Health Qual. Life Outcomes 2003, 1, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Zhang, X.; Wang, H.; Yang, B.; Li, N.; Ji, Z. A Chinese Cross-Sectional Study on Symptoms in Aging Males: Prevalence and Associated Factors. Am. J. Mens. Health 2019, 13, 1557988319838113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, K.; Hashimoto, K.; Kato, R.; Tanaka, T.; Hirose, T.; Masumori, N.; Itoh, N.; Mori, M.; Tsukamoto, T. The aging males’ symptoms scale for Japanese men: Reliability and applicability of the Japanese version. Int. J. Impot. Res. 2008, 20, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Okawara, M.; Tateishi, S.; Horie, S.; Yasui, T.; Fujino, Y. Association between andropause symptoms and work functioning impairment: A cross-sectional study in two Japanese companies. Ind. Health 2025, 63, 288–297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulhall, J.P.; Goldstein, I.; Bushmakin, A.G.; Cappelleri, J.C.; Hvidsten, K. Validation of the erection hardness score. J. Sex. Med. 2007, 4, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Shimura, S.; Tai, T.; Kobayashi, H.; Baba, S.; Kano, M.; Nagao, K. A web-based survey of erection hardness score and its relationship to aging, sexual behavior, confidence, and risk factors in Japan. Sex. Med. 2013, 1, 76–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosen, R.C.; Cappelleri, J.C.; Smith, M.D.; Lipsky, J.; Pena, B.M. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int. J. Impot. Res. 1999, 11, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Catania, J.A.; Althof, S.E.; Pollack, L.M.; O’Leary, M.; Seftel, A.D.; Coon, D.W. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007, 69, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L.; Endocrine, S. Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef] [PubMed]
- Kobori, Y.; Ota, S.; Okada, H.; Tanaka, T.; Group, M.H.H.S. Investigation of treatment for azoospermia due to male hypogonadotropic hypogonadism in Japan. Int. J. Urol. 2019, 26, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.V.; Garcia, N.Z.; Gutierrez Romero, J.M.; Diaz-Fierros, P.R.; Lozano Arana, M.D.; Perez, T.R.; Alvarez, J.S.; Rodriguez, G.B.; Bernardo, V.C.; Moyano Gallego, M.J. Sperm recovery from urine in men with retrograde ejaculation. Adv. Lab. Med. 2024, 5, 356–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Mbizvo, M.; Festin, M.P.; Bjorndahl, L.; Toskin, I.; Other Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Evolution of the WHO “Semen” processing manual from the first (1980) to the sixth edition (2021). Fertil. Steril. 2022, 117, 237–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valvachev, A.A.; Pranovich, A.A. Clinical rationale for the importance of assessing estradiol levels in men with hypogonadism syndrome during therapy to stabilize testosterone level. Exp. Clin. Urol. 2024, 17, 80–85. [Google Scholar] [CrossRef]
- Kacker, R.; Traish, A.M.; Morgentaler, A. Estrogens in men: Clinical implications for sexual function and the treatment of testosterone deficiency. J. Sex. Med. 2012, 9, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Lee, K.; Prince, M.; Jetty, V.; Shah, P.; Wang, P. Four Thrombotic Events Over 5 Years, Two Pulmonary Emboli and Two Deep Venous Thrombosis, When Testosterone-HCG Therapy Was Continued Despite Concurrent Anticoagulation in a 55-Year-Old Man With Lupus Anticoagulant. J. Investig. Med. High. Impact Case Rep. 2016, 4, 2324709616661833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figueira, M.I.; Carvalho, T.M.A.; Macario-Monteiro, J.; Cardoso, H.J.; Correia, S.; Vaz, C.V.; Duarte, A.P.; Socorro, S. The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens. Biomedicines 2024, 12, 1636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orlova, I.A.; Sorokin, E.D.; Pavlova, Z.S.; Plisyuk, A.G.; Kamalov, A.A. Estradiol Level as a Risk Factor for Cardiovascular Endpoints in Men: A Systematic Review. Ann. Russ. Acad. Med. Sci. 2024, 79, 205–215. [Google Scholar] [CrossRef]
- Basheer, B.; Ila, V.; Barros, R.; Mesquita, F.; Lopes, L.S.; Lima, V.F.N.; Favorito, L.A.; Ramasamy, R. Management of Adverse Effects in Testosterone Replacement Therapy. Int. Braz. J. Urol. 2025, 51, e20259904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zucker, I.; Rainer, Q.; Pai, R.K.; Ramasamy, R.; Masterson, T.A. Efficacy and Safety of Human Chorionic Gonadotropin Monotherapy for Men With Hypogonadal Symptoms and Normal Testosterone. Cureus 2022, 14, e25543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Kimura, F.; Takai, Y.; Nakajima, T.; Ushijima, K.; Kobayashi, H.; Satoh, T.; Tozawa, A.; Sugimoto, K.; Saji, S.; et al. Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for fertility preservation in childhood, adolescent, and young adult cancer patients: Part 1. Int J Clin Oncol 2022, 27, 265–280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shnorhavorian, M.; Harlan, L.C.; Smith, A.W.; Keegan, T.H.; Lynch, C.F.; Prasad, P.K.; Cress, R.D.; Wu, X.C.; Hamilton, A.S.; Parsons, H.M.; et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: A population-based study. Cancer 2015, 121, 3499–3506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruda, R.; Trevisan, E.; Soffietti, R. Epilepsy and brain tumors. Curr. Opin. Oncol. 2010, 22, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Osborne-Grinter, M.; Sanghera, J.K.; Bianca, O.C.; Kaliaperumal, C. Fertility preserving techniques in neuro-oncology patients: A systematic review. Neurooncol. Adv. 2024, 6, vdae124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeshima, T.; Ueno, H.; Yamamoto, M.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Kawahara, T.; Miyoshi, Y.; et al. Sperm cryopreservation for fertility preservation in male patients with cancer at a single-center in Japan. Glob. Reprod. Health 2019, 4, e34. [Google Scholar] [CrossRef]
- Ascoli, P.; Cavagnini, F. Hypopituitarism. Pituitary 2006, 9, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Mavromati, M.; Mavrakanas, T.; Jornayvaz, F.R.; Schaller, K.; Fitsiori, A.; Vargas, M.I.; Lobrinus, J.A.; Merkler, D.; Egervari, K.; Philippe, J.; et al. The impact of transsphenoidal surgery on pituitary function in patients with non-functioning macroadenomas. Endocrine 2023, 81, 340–348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreassen, M.; Juul, A.; Feldt-Rasmussen, U.; Jorgensen, N. Semen quality in patients with pituitary disease and adult-onset hypogonadotropic hypogonadism. Endocr. Connect. 2018, 7, 523–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meistrich, M.L. Male gonadal toxicity. Pediatr. Blood Cancer 2009, 53, 261–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esteves, S.C.; Viana, M.C.; Achermann, A.P.P.; Santi, D. Human chorionic gonadotropin-based clinical treatments for infertile men with non-obstructive azoospermia. Andrology 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.W.; Brannigan, R.E. Oncofertility and the male cancer patient. Curr. Treat. Options Oncol. 2012, 13, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Behre, H.M. Clinical Use of FSH in Male Infertility. Front. Endocrinol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kordzadeh, A.; Liu, M.O.; Jayanthi, N.V. Male infertility following inguinal hernia repair: A systematic review and pooled analysis. Hernia 2017, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.N.; Pallotti, F.; Faja, F.; Buonacquisto, A.; Cicolani, G.; Conflitti, A.C.; Di Chiano, S.; Lenzi, A.; Lombardo, F.; Paoli, D. Bladder Neck Obstruction: Experience and Management in a Sperm Bank. Life 2023, 13, 842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okamoto, K.; Kojo, K.; Kurobe, M.; Nakazato, Y.; Inai, H.; Uchida, K.; Miyazaki, J.; Takayama, T. Zokuhatsusei Funinshō no Seisa o Kiki ni Ryūkisei Byōhen toshite Hakken sareta Zōshokusei Bōkōen no Ichi-rei [A Case of Proliferative Cystitis Discovered as Protruding Lesion during Inspection of Secondary Infertility]. Hinyokika Kiyo 2024, 70, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Kaluzna, M.; Kompf, P.; Rabijewski, M.; Moczko, J.; Kaluzny, J.; Ziemnicka, K.; Ruchala, M. Reduced Quality of Life and Sexual Satisfaction in Isolated Hypogonadotropic Hypogonadism. J. Clin. Med. 2021, 10, 2622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borgert, B.J.; Bacchus, M.W.; Hernandez, A.D.; Potts, S.N.; Campbell, K.J. The availability of gonadotropin therapy from FDA-approved pharmacies for men with hypogonadism and infertility. Sex. Med. 2023, 11, qfad004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanatani, Y.; Tomita, N.; Sato, Y.; Eto, A.; Omoe, H.; Mizushima, H. National Registry of Designated Intractable Diseases in Japan: Present Status and Future Prospects. Neurol. Med. Chir. 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsujimura, A.; Nonomura, N. Recent topics related to testosterone deficiency syndrome in Japan. Asian J. Androl. 2011, 13, 558–562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lunenfeld, B. Gonadotropin stimulation: Past, present and future. Reprod. Med. Biol. 2012, 11, 11–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mamiya, H.; Izutsu, K.I.; Mitani, D. Draft Guideline for Industry to Manage Drug Shortages in Japan. Ther. Innov. Regul. Sci. 2024, 58, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K. The Oncofertility Consortium--addressing fertility in young people with cancer. Nat. Rev. Clin. Oncol. 2010, 7, 466–475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furui, T.; Takenaka, M. Gan-Seishoku Iryō Nettowāku no ishi kettei shien kinō no shitsuteki kintenka to Sustainability no kōjō o mezashite―Oncofertility Consortium Japan no torikumi [Qualitative Equalization and Sustainability of Decision Support Functions for AYA Patients in Oncofertility Networks- Initiatives of the Oncofertility Consortium Japan]. Gan Kagaku Ryoho 2023, 50, 1253–1259. [Google Scholar] [PubMed]
- Soni, A.; De Silva, S.R.; Allen, K.; Byrne, J.V.; Cudlip, S.; Wass, J.A. A case of macroprolactinoma encasing an internal carotid artery aneurysm, presenting as pituitary apoplexy. Pituitary 2008, 11, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Sadovsky, R. The role of the primary care clinician in the management of erectile dysfunction. Rev. Urol. 2002, 4 (Suppl. S3), S54–S63. [Google Scholar] [PubMed] [PubMed Central]
- Korse, N.S.; Nicolai, M.P.; Both, S.; Vleggeert-Lankamp, C.L.; Elzevier, H.W. Discussing reproductive health in spinal care, part II: Fertility issues. Eur. Spine J. 2016, 25, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Owada, Y.; Kojo, K.; Sanuki, M.; Takiguchi, Y.; Shirabe, K.; Sekine, I.; Nishiyama, H.; Abe, H.; Kato, K.; et al. Kantō Gan Senmon Iryōjin Yōsei Kyoten―Gan Puro Zenkoku E-Learning kuraudo no jukusei to hatten [Maturation and Development of the”All Japan E-Learning Cloud of the Training Program for Oncology Professionals]. Gan Kagaku Ryoho 2022, 49, 520–524. [Google Scholar] [PubMed]
- Ozimek, N.; Salama, M.; Woodruff, T.K. National oncofertility registries around the globe: A pilot survey. Front. Endocrinol. 2023, 14, 1148314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Pre-op | Post-1st op | Post-2nd op (Days from 1st op in Parentheses) | LLR | ULR | |||
|---|---|---|---|---|---|---|---|
| POD 6 | POD 19 (33) | POD 47 (61) | POD 89 (103) | ||||
| GH | 0.47 | 0.90 | 0.26 | 0.19 | 0.16 | 0.03 | 2.47 |
| IGF-1 | 113 | 138 | 64 | 47 | 60 | 111 | 309 |
| PRL | 38.1 | 19.2 | 19.2 | 17.8 | 19.5 | 4.29 | 13.69 |
| TSH | 0.57 | 0.01 | 0.06 | 0.07 | 0.03 | 0.50 | 5.00 |
| FT3 | 3.28 | 2.55 | 2.54 | 3.20 | 4.17 | 2.30 | 4.00 |
| FT4 | 1.39 | 1.11 | 0.69 | 0.74 | 0.91 | 0.90 | 1.70 |
| ACTH | 26.2 | 4.6 | 1.6 | 4.7 | 1.9 | 7.2 | 63.3 |
| Cortisol | 8.3 | 0.5 | 0.1 | 0.2 | 0.2 | 7.07 | 19.6 |
| LH | 1.58 | 0.12 | <0.10 | 0.13 | 0.12 | 0.79 | 5.72 |
| FSH | 21.40 | 1.99 | 0.34 | 0.37 | 0.46 | 2.00 | 8.30 |
| TT | NA | NA | NA | NA | <0.03 | 1.31 | 8.71 |
| Title | Main Publisher | Year | References |
|---|---|---|---|
| [Guidelines for the Diagnosis and Treatment of Gonadotropin Deficiency] † | JES, JHPT | 2010 | [20] |
| 2019 | [21] | ||
| 2023 | [22] | ||
| [Clinical Practice Manual for Late-Onset Hypogonadism] | JUA, JSMH | 2007 | [23,24] |
| 2022 | [25,26] | ||
| [Guidelines for Male Hypogonadism] | JES, JSMH | 2022 | [27] |
| [Clinical Practice Guidelines for Male Infertility] | JUA, JSRM | 2024 | [28,29] |
| [Clinical Practice Guidelines for Differences of Sex Development] | JSPE, JES, JSPU, JSRE, JSGI | 2025 | [30] |
| Pre | 15 min | 30 min | 45 min | 60 min | 90 min | 120 min | Peak/Pre | |
|---|---|---|---|---|---|---|---|---|
| GH | 0.24 | 0.20 | 0.23 | 0.33 | 0.40 † | 0.35 | 0.25 | 1.67 |
| PRL | 19.7 | 21.5 | 22.7 † | 22.2 | 22.0 | 20.5 | 19.6 | 1.15 |
| TSH | 0.26 | 0.47 | 0.84 | 1.08 | 1.13 † | 1.13 † | 1.01 | 4.35 |
| ACTH | 6.6 | 54.2 | 58.3 † | 55.0 | 37.6 | 25.8 | 22.2 | 8.83 |
| LH | 0.18 | 0.37 | 0.54 | 0.66 | 0.67 † | 0.62 | 0.54 | 3.72 |
| FSH | 0.55 | 0.66 | 0.78 | 0.87 | 1.00 | 1.02 | 1.04 ‡ | 1.89 |
| Day 1 (Pre-hCG) | Day 2 | Day 3 | Day 4 | LLR | ULR | |
|---|---|---|---|---|---|---|
| TT | <0.03 | 0.83 | 2.99 | 3.87 | 1.31 | 8.71 |
| FT | <0.2 | 1.6 | 5.1 | 8.5 | 7.6 | 23.8 |
| E2 | <5.0 | 6.6 | 14.5 | 14.4 | 14.6 | 48.8 |
| T/E ratio | NA | 12.6 | 20.6 | 26.9 | NA | NA |
| Items | No. | Symptoms | Pre | Day 42 | Day 84 |
|---|---|---|---|---|---|
| AMS-som | 1 | Impaired well-being | 1 | NA | 1 |
| AMS-som | 2 | Joint complaints | 1 | NA | 1 |
| AMS-som | 3 | Excessive sweating | 1 | NA | 1 |
| AMS-som | 4 | Frequent sleep disturbance | 1 | NA | 1 |
| AMS-som | 5 | Need for sleep | 1 | NA | 1 |
| AMS-psy | 6 | Irritability | 2 | NA | 1 |
| AMS-psy | 7 | Nervousness | 2 | NA | 1 |
| AMS-psy | 8 | Anxiety | 1 | NA | 1 |
| AMS-som | 9 | Physical exhaustion | 1 | NA | 1 |
| AMS-som | 10 | Muscular weakness | 2 | NA | 1 |
| AMS-psy | 11 | Depressive mood | 1 | NA | 1 |
| AMS-sex | 12 | Having passed the peak | 1 | NA | 1 |
| AMS-psy | 13 | Feeling burnt out | 1 | NA | 1 |
| AMS-sex | 14 | Decreased beard growth | 2 | NA | 1 |
| AMS-sex | 15 | Impaired sexual potency | 2 | NA | 1 |
| AMS-sex | 16 | Less frequent morning erections | 4 | NA | 1 |
| AMS-sex | 17 | Disturbed libido | 4 | NA | 1 |
| AMS | Somatic subscale (7–35) | 8 | NA | 7 | |
| AMS | Psychological subscale (5–25) | 7 | NA | 5 | |
| AMS | Sexual subscale (5–25) | 13 | NA | 5 | |
| AMS | Total (17–85) | 28 | NA | 17 | |
| EHS | 1 | 2 | 4 | ||
| IIEF-5 | 1 | Erection confidence | 1 | NA | NA |
| IIEF-5 | 2 | Erection hardness | 1 | NA | NA |
| IIEF-5 | 3 | Erection maintenance | 1 | NA | NA |
| IIEF-5 | 4 | Maintenance difficulty | 1 | NA | NA |
| IIEF-5 | 5 | Intercourse satisfaction | 1 | NA | NA |
| IIEF-5 | Total (5–25) | 5 | NA | NA | |
| MSHQ-EjD-SF | 1 | Ejaculation frequency | 1 | NA | NA |
| Semen volume [mL] | 0.6 † | NA | 4.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numahata, D.; Kojo, K.; Ishikawa, S.-e.; Kuramae, T.; Nakazono, A.; Yanagida, K.; Nishiyama, H.; Takayama, T. Comprehensive Fertility Management After Pituitary Adenoma Surgery: Lessons from a Rural Japanese Case and Practical Review. Reports 2025, 8, 144. https://doi.org/10.3390/reports8030144
Numahata D, Kojo K, Ishikawa S-e, Kuramae T, Nakazono A, Yanagida K, Nishiyama H, Takayama T. Comprehensive Fertility Management After Pituitary Adenoma Surgery: Lessons from a Rural Japanese Case and Practical Review. Reports. 2025; 8(3):144. https://doi.org/10.3390/reports8030144
Chicago/Turabian StyleNumahata, Daisuke, Kosuke Kojo, San-e Ishikawa, Takumi Kuramae, Ayumi Nakazono, Kaoru Yanagida, Hiroyuki Nishiyama, and Tatsuya Takayama. 2025. "Comprehensive Fertility Management After Pituitary Adenoma Surgery: Lessons from a Rural Japanese Case and Practical Review" Reports 8, no. 3: 144. https://doi.org/10.3390/reports8030144
APA StyleNumahata, D., Kojo, K., Ishikawa, S.-e., Kuramae, T., Nakazono, A., Yanagida, K., Nishiyama, H., & Takayama, T. (2025). Comprehensive Fertility Management After Pituitary Adenoma Surgery: Lessons from a Rural Japanese Case and Practical Review. Reports, 8(3), 144. https://doi.org/10.3390/reports8030144






