Infective Endocarditis with Gerbode Defect and DRESS Syndrome: A Rare Case Report
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Patient Information
2.1.1. Demographics
2.1.2. Presenting Concerns
2.2. Medical History
2.3. Clinical Case
| Day | Stage | Clinical Manifestations | Diagnostic Workup | Therapeutic Interventions |
|---|---|---|---|---|
| 2 weeks before | Initial Presentation | Paranoia, anxiety, cognitive decline, major depressive episode | Risperidone 2 mg od, Escitalopram 10 mg od | |
| Days 1–2 | Infection Onset | Hypotension, fever, leukocytosis | Blood cultures—Staphylococcus aureus TTE—suspicion of endocarditis due to mobile hyperechoic masses identified CT scans of the chest, abdomen, and pelvis showing post-tuberculosis lung scarring | |
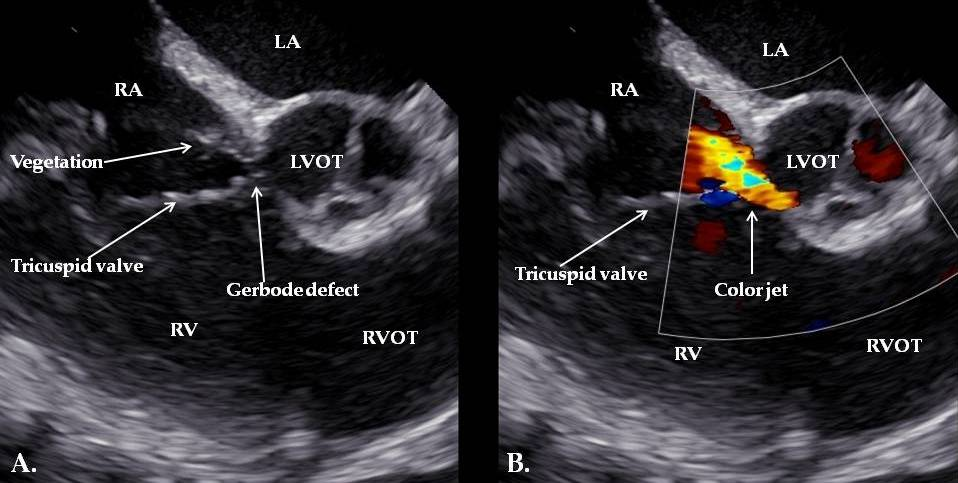
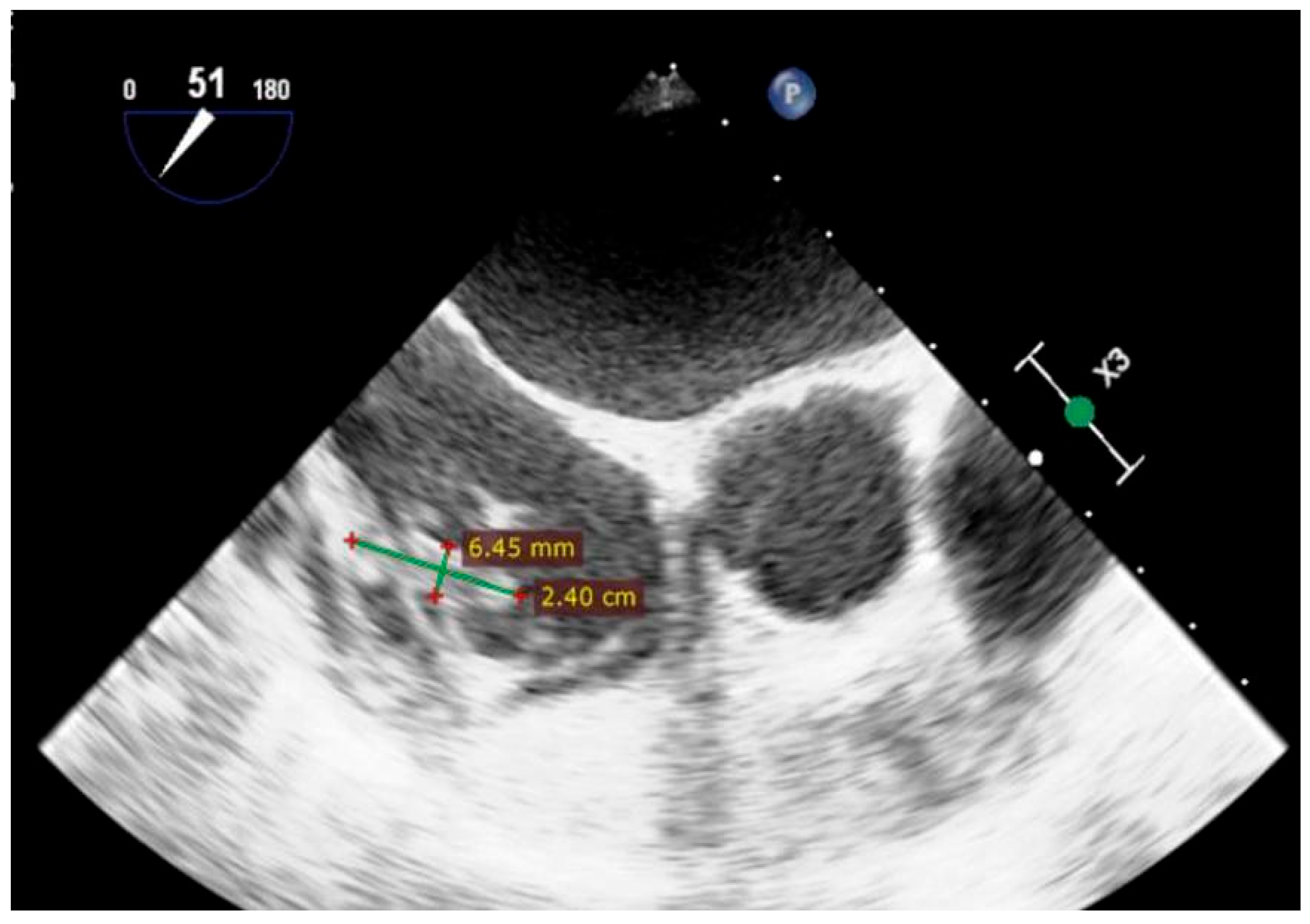
| Day 3 | Diagnosis of IE | Persistent fever, systemic inflammation | TEE: Gerbode defect with a vegetation attached near the tricuspid valve and others involving the tricuspid valve and the pacemaker lead | |
| Days 4–27 | Antibiotic Therapy | Gentamicin 3 mg/kg/day + Oxacillin 12 g/day (dual therapy for 2 weeks), then Oxacillin monotherapy for 2 more weeks); | ||
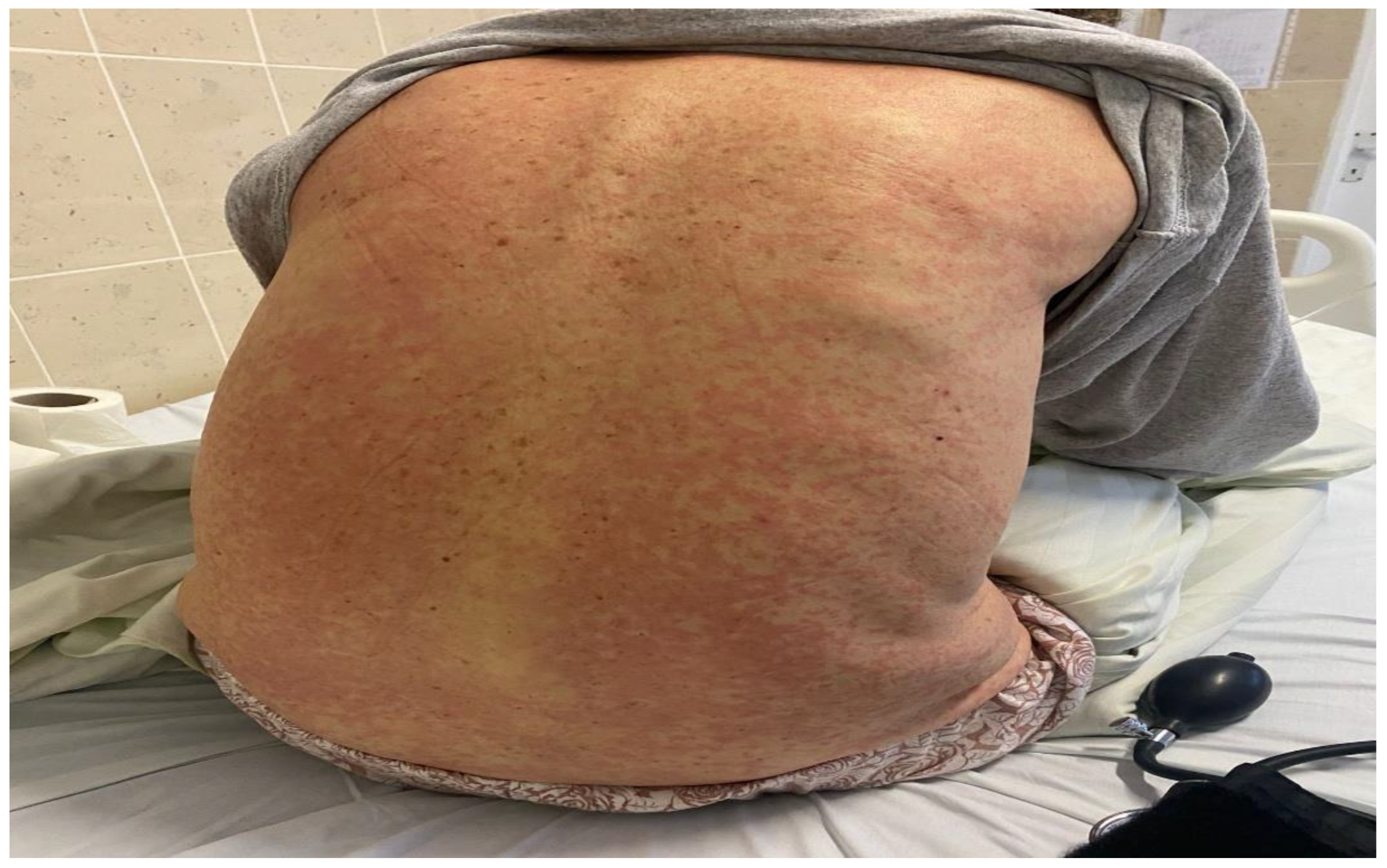
| Day 27 | Development of DRESS Syndrome | Rash, fever, leukopenia, eosinophilia, organ involvement | RegiSCAR score assessment; procalcitonin level | discontinuation of Risperidone; antibiotic switch to Linezolid + Ceftazidime |
| Day 28 | Further Investigations | Persistent symptoms, eosinophilia | Exclusion of fungal superinfection, hematologic disease, serum sickness, repeat blood cultures | |
| Day 30 | Corticosteroid Therapy | Skin rash and eosinophilia | Dexamethasone 8 mg/day for 4 days | |
| Day 31+ | Pacemaker-related complications | Vegetation on pacemaker lead | TEE: Persistent vegetations confirmed; lead extraction via transvenous approach | Temporary external pacemaker; continued antibiotic therapy |
| Week 6 | Recovery and Stability | Overall clinical and laboratory improvement | Echocardiography: ejection fraction; coronary angiography | Evaluation for potential cardiac resynchronization therapy |
| Day 38 | Blood Disorders | Anemia and thrombocytopenia | Upper and lower GI endoscopy; platelet function tests; vitamin B12/folate levels | - |
| Week 9 | Spontaneous Remission | Resolution of hematologic abnormalities, | - | |
| 6-month Follow-up | Long-term Outcome | asymptomatic, no signs of recurrence or disease progression | The cardiology follow-up visit; clinical and biochemical stability | Routine follow-up |
3. Follow-Up and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IE | Infective endocarditis |
| DRESS | Drug Reaction with Eosinophilia and Systemic Symptoms |
| RegiSCAR | The European Registry on severe cutaneous adverse drug reactions |
| TTE | Transthoracic echocardiography |
References
- Hammond-Haley, M.; Hartley, A.; Al-Khayatt, B.M.; Delago, A.J.; Ghajar, A.; Ojha, U.; Marshall, D.C.; Salciccioli, J.D.; Prendergast, B.D.; Shalhoub, J. Trends in the incidence and mortality of infective endocarditis in high-income countries between 1990 and 2019. Int. J. Cardiol. 2023, 371, 441–451. [Google Scholar] [CrossRef]
- Delgado, V.; Marsan, N.A.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2023, 44, 3948–4042. Available online: https://academic.oup.com/eurheartj/article/44/39/3948/7243107?login=false (accessed on 12 January 2025). [CrossRef]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management Considerations in Infective Endocarditis: A Review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.; Mourad, B.M.; Hardwick, C.J.; Reilly, T.; Dweck, E.; Bondarsky, E. Oxacillin-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Am. J. Case Rep. 2019, 20, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Abu, H.O.; Ali, S.M.J.; Phuyal, A.; Sherif, A.; Williams, G.T.; Chastain, I. DRESS syndrome in the setting of oxacillin therapy-a call for better patient preparedness: A case report. J. Med. Case Rep. 2021, 15, 613. [Google Scholar] [CrossRef]
- Nguyen, K.; Ahmed, M.S. Drug Rash with Eosinophilia and Systemic Symptoms Syndrome Presenting After the Initiation of Staphylococcus hominis Infectious Endocarditis Treatment: A Case Report and Updated Review of Management Considerations. Cureus 2018, 10, e3679. [Google Scholar] [CrossRef]
- Kroshinsky, D.; Cardones, A.R.G.; Blumenthal, K.G. Drug Reaction with Eosinophilia and Systemic Symptoms. N. Engl. J. Med. 2024, 391, 2242–2254. [Google Scholar] [CrossRef]
- Bocquet, H.; Bagot, M.; Roujeau, J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin. Cutan. Med. Surg. 1996, 15, 250–257. [Google Scholar] [CrossRef]
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS syndrome: A literature review. Am. J. Med. 2011, 124, 588–597. [Google Scholar] [CrossRef]
- Choudhary, S.; McLeod, M.; Torchia, D.; Romanelli, P. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J. Clin. Aesthet. Dermatol. 2013, 6, 31–37. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3718748/ (accessed on 16 January 2025).
- Sharifzadeh, S.; Mohammadpour, A.H.; Tavanaee, A.; Elyasi, S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A literature review. Eur. J. Clin. Pharmacol. 2021, 77, 275–289. [Google Scholar] [CrossRef]
- Lisowska, A.; Kamińska, M.; Knapp, M.; Sobkowicz, B. The Gerbode defect—A left ventricle to right atrium communication. Kardiol. Pol. 2008, 66, 1118–1120. Available online: https://pubmed.ncbi.nlm.nih.gov/19006037/ (accessed on 16 January 2025). [PubMed]
- Houssine, H.B.; Samih, A.; Louizi, W.; Rami, H.; Amri, R.; Zarzur, J.; Cherti, M. Congenital Gerbode defects complicated by infective endocarditis: 2 case reports. RA J. Appl. Res. 2021, 7, 2449–2452. [Google Scholar] [CrossRef]
- Gerbode, F.; Hultgren, H.; Melrose, D.; Osborn, J. Syndrome of left ventricular-right atrial shunt: Successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann. Surg. 1958, 148, 433. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, N.; El-Medany, A.; Temporal, J.; Pannell, L.; Doolub, G.; Nelson, M.; Vohra, H. The Gerbode defect: A case series. Eur. Heart J. Case Rep. 2021, 5, ytaa548. [Google Scholar] [CrossRef]
- Winter, L.; Strizek, B.; Recker, F. Congenital Gerbode Defect: A Left Ventricular to Right Atrial Shunt—State-of-the-Art Review of Its General Data, Diagnostic Modalities, and Treatment Strategies. J. Cardiovasc. Dev. Dis. 2024, 11, 166. [Google Scholar] [CrossRef]
- Wasserman, S.M.; Fann, J.I.; Atwood, J.E.; Burdon, T.A.; Fadel, B.M. Acquired left ventricular-right atrial communication: Gerbode-type defect. Echocardiography 2002, 19, 67–72. [Google Scholar] [CrossRef]
- Fleming, P.; Marik, P.E. The DRESS syndrome: The great clinical mimicker. Pharmacotherapy 2011, 31, 332. [Google Scholar] [CrossRef]
- Bayer, A.S.; Theofilopoulos, A.N.; Eisenberg, R.; Dixon, F.J.; Guze, L.B. Circulating immune complexes in infective endocarditis. N. Engl. J. Med. 1976, 295, 1500–1505. [Google Scholar] [CrossRef]
- Kretzer, A.; Amhaz, H.; Nicoara, A.; Kendall, M.; Glower, D.; Jones, M.-M. A Case of Gerbode Ventricular Septal Defect Endocarditis. CASE Cardiovasc. Imaging Case Rep. 2018, 2, 207. [Google Scholar] [CrossRef]
- Colomba, D.; Cardillo, M.; Raffa, A.; Argano, C.; Licata, G. A hidden echocardiographic pitfall: The Gerbode defect. Intern. Emerg. Med. 2014, 9, 237–238. [Google Scholar] [CrossRef]
- Wu, M.-H.; Wang, J.-K.; Lin, M.-T.; Wu, E.-T.; Lu, F.L.; Chiu, S.-N.; Lue, H.-C. Ventricular Septal Defect with Secondary Left Ventricular–to–Right Atrial Shunt Is Associated with a Higher Risk for Infective Endocarditis and a Lower Late Chance of Closure. Pediatrics 2006, 117, e262–e267. [Google Scholar] [CrossRef]
- Nakagawa, N. Infective Endocarditis in Congenital Heart Disease. Endocarditis—Diagnosis and Treatment; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Mert, G.; Dural, M.; Gorenek, B.; Mert, K. Gerbode defect: A rare complication of infective endocarditis. J. Postgrad. Med. 2019, 65, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Orion, E.; Marcos, B.; Matz, H. Life-threatening acute adverse cutaneous drug reactions. Clin. Dermatol. 2005, 23, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chiu, H.-C.; Chu, C.-Y. Drug Reaction with Eosinophilia and Systemic Symptoms: A Retrospective Study of 60 Cases. Arch. Dermatol. 2010, 146, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, R.; Ramírez, E.; Sendagorta, E.; Alamar, R.; Barranco, R.; Blanca-López, N.; Doña, I.; Fernández, J.; Garcia-Nunez, I.; García-Samaniego, J.; et al. Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome. J. Investig. Allergol. Clin. Immunol. 2020, 30, 229–253. [Google Scholar] [CrossRef]
- Yacoub, M.-R.; Berti, A.; Campochiaro, C.; Tombetti, E.; Ramirez, G.A.; Nico, A.; Di Leo, E.; Fantini, P.; Sabbadini, M.G.; Nettis, E.; et al. Drug induced exfoliative dermatitis: State of the art. Clin. Mol. Allergy 2016, 14, 9. [Google Scholar] [CrossRef]
- Della-Torre, E.; Yacoub, M.-R.; Pignatti, P.; Della-Torre, F.; Sabbadini, M.-G.; Colombo, G.; Tresoldi, M. Optimal management of DRESS syndrome in course of infectious endocarditis. Ann. Allergy Asthma Immunol. 2013, 110, 303–305. [Google Scholar] [CrossRef]
- Calle, A.M.; Aguirre, N.; Ardila, J.C.; Villa, R.C. DRESS syndrome: A literature review and treatment algorithm. World Allergy Organ. J. 2023, 16, 100673. [Google Scholar] [CrossRef]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome: Part II. Management and therapeutics. J. Am. Acad. Dermatol. 2013, 68, 709.e1–709.e8, quiz 718–720. [Google Scholar] [CrossRef]
- Shiohara, T.; Kano, Y.; Takahashi, R.; Ishida, T.; Mizukawa, Y. Drug-induced hypersensitivity syndrome: Recent advances in the diagnosis, pathogenesis and management. Chem. Immunol. Allergy 2012, 97, 122–138. [Google Scholar] [CrossRef]
- Yildirim Arslan, S.; Bal, Z.S.; Ozenen, G.G.; Bilen, N.M.; Avcu, G.; Erci, E.; Kurugol, Z.; Gunay, H.; Tamsel, I.; Ozkinay, F. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome secondary to antimicrobial therapy in pediatric bone and joint infections. World Allergy Organ. J. 2024, 17, 100850. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureche, C.; Moldovan, D.L.; Vița, I.; Guila, V.; Nicola-Varo, T. Infective Endocarditis with Gerbode Defect and DRESS Syndrome: A Rare Case Report. Reports 2025, 8, 127. https://doi.org/10.3390/reports8030127
Ureche C, Moldovan DL, Vița I, Guila V, Nicola-Varo T. Infective Endocarditis with Gerbode Defect and DRESS Syndrome: A Rare Case Report. Reports. 2025; 8(3):127. https://doi.org/10.3390/reports8030127
Chicago/Turabian StyleUreche, Corina, Diana Lavinia Moldovan, Ionel Vița, Valeria Guila, and Teodora Nicola-Varo. 2025. "Infective Endocarditis with Gerbode Defect and DRESS Syndrome: A Rare Case Report" Reports 8, no. 3: 127. https://doi.org/10.3390/reports8030127
APA StyleUreche, C., Moldovan, D. L., Vița, I., Guila, V., & Nicola-Varo, T. (2025). Infective Endocarditis with Gerbode Defect and DRESS Syndrome: A Rare Case Report. Reports, 8(3), 127. https://doi.org/10.3390/reports8030127






