Case Report of Successful Extracorporeal CPR (eCPR) in Refractory Cardiac Arrest Caused by Fulminant Pulmonary Embolism with Remarkable Recovery
Abstract
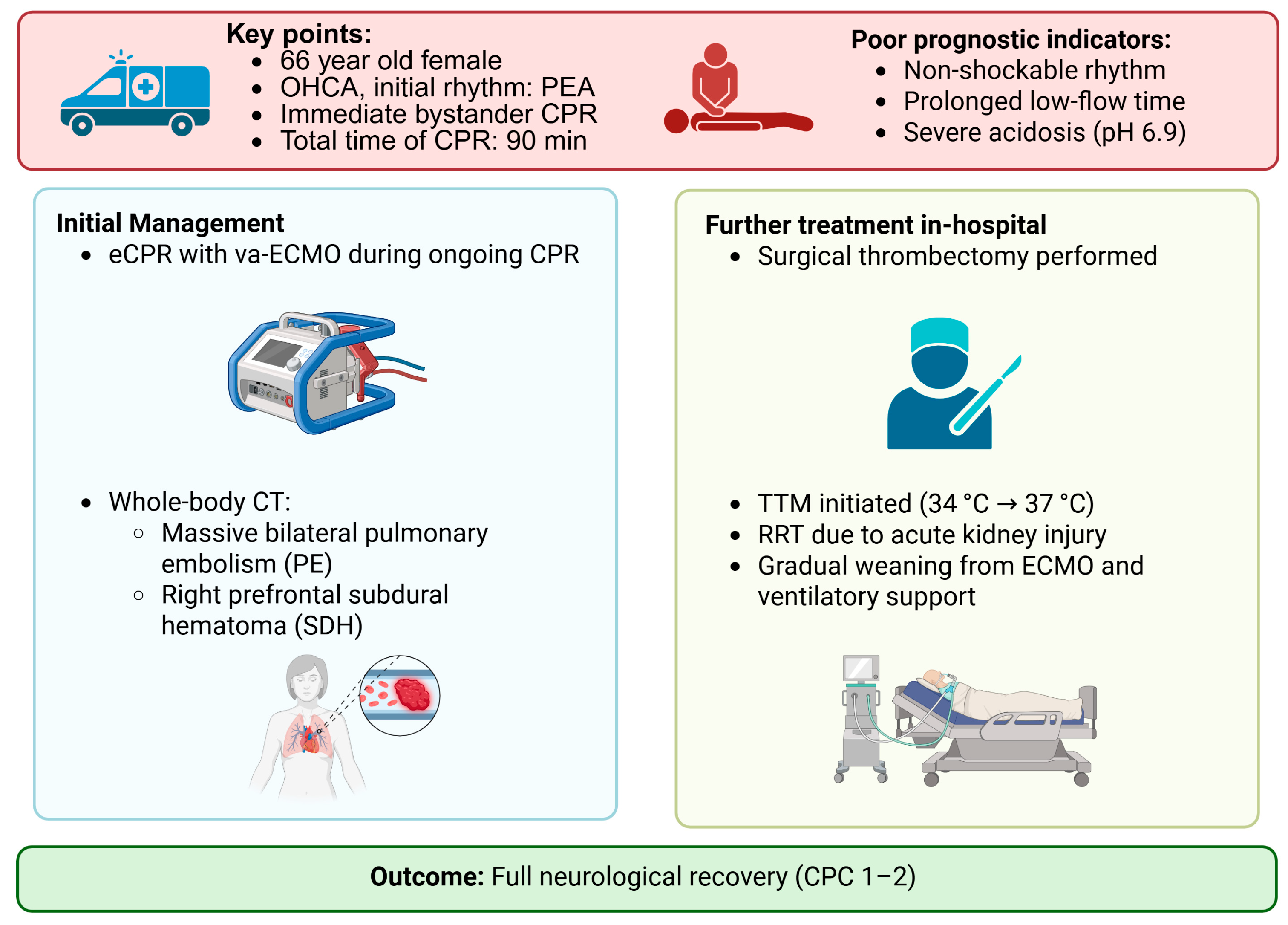
1. Introduction and Clinical Significance
2. Case Presentation
3. Discussion
4. Conclusions
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez Licha, C.R.; McCurdy, C.M.; Maldonado, S.M.; Lee, L.S. Current Management of Acute Pulmonary Embolism. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 65–71. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Luppino Assad, A.P.; Alves-Jr, J.L.; Jardim, C.; de Souza, R. Pulmonary Embolism and Gas Exchange. Respiration 2019, 98, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Zietlow, S.; Wagner, T.O.F.; Laas, J.; Borst, H.G. Fulminant pulmonary embolism: Symptoms, diagnostics, operative technique, and results. Ann. Thorac. Surg. 1991, 52, 1102–1107. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.; Wengenmayer, T.; Hagl, C.; Dohmen, C.; Böttiger, B.W.; Bauersachs, J.; Markewitz, A.; Bauer, A.; Gräsner, J.-T.; Pfister, R.; et al. Recommendations for extracorporeal cardiopulmonary resuscitation (eCPR): Consensus statement of DGIIN, DGK, DGTHG, DGfK, DGNI, DGAI, DIVI and GRC. Clin. Res. Cardiol. 2019, 108, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Debaty, G.; Babaz, V.; Durand, M.; Gaide-Chevronnay, L.; Fournel, E.; Blancher, M.; Bouvaist, H.; Chavanon, O.; Maignan, M.; Bouzat, P.; et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 2017, 112, 1–10. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Zeserson, E.; Goodgame, B.; Hess, J.D.; Schultz, K.; Hoon, C.; Lamb, K.; Maheshwari, V.; Johnson, S.; Papas, M.; Reed, J.; et al. Correlation of Venous Blood Gas and Pulse Oximetry With Arterial Blood Gas in the Undifferentiated Critically Ill Patient. J. Intensive Care Med. 2018, 33, 176–181. [Google Scholar] [CrossRef]
- Cox, L.; Tebbett, A. Videolaryngoscopy versus direct laryngoscopy for endotracheal intubation of cardiac arrest patients in hospital: A systematic literature review. Resusc. Plus 2022, 11, 100297. [Google Scholar] [CrossRef]
- Eberlein, C.M.; Luther, I.S.; Carpenter, T.A.; Ramirez, L.D. First-Pass Success Intubations Using Video Laryngoscopy Versus Direct Laryngoscopy: A Retrospective Prehospital Ambulance Service Study. Air. Med. J. 2019, 38, 356–358. [Google Scholar] [CrossRef]
- Hossfeld, B.; Thierbach, S.; Allgoewer, A.; Gaessler, H.; Helm, M. First pass success of tracheal intubation using the C-MAC PM videolaryngoscope as first-line device in prehospital cardiac arrest compared with other emergencies. Eur. J. Anaesthesiol. 2021, 38, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Kill, C.; Manegold, R.K.; Fistera, D.; Risse, J. Airway management and ventilation techniques in resuscitation during advanced life support: An update. J. Anesth. Analg. Crit. Care 2024, 4, 58. [Google Scholar] [CrossRef]
- Magnet, I.; Poppe, M. Extrakorporale Reanimation—Kriterien, Bedingungen, Outcome. Med. Klin. Intensiv. Notfmed. 2022, 117, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, N.; Abe, D.; Iwama, T.; Suzuki, K.; Sugiyama, K.; Akashi, A.; Hamabe, Y.; Aonuma, K.; Sato, A. Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation 2017, 114, 1–6. [Google Scholar] [CrossRef]
- Ghanem, A.; Andrassy, M.; Dürschmied, D.; Fürnau, G.; Geisler, T.; Hennersdorf, M.; Knorr, M.; Lange, T.; Masri-Zada, A.; Michels, G.; et al. Interventional treatment and multidisciplinary management strategies for acute pulmonary embolism. Kardiologie 2023, 17, 141–159. [Google Scholar] [CrossRef]
- Rivers, J.; Pilcher, D.; Kim, J.; Bartos, J.A.; Burrell, A. Extracorporeal membrane oxygenation for the treatment of massive pulmonary embolism. An analysis of the ELSO database. Resuscitation 2023, 191, 109940. [Google Scholar] [CrossRef] [PubMed]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Verbelen, T.; Binzenhöfer, L.; Goslar, T.; Supady, A.; Spieth, P.M.; Noc, M.; Verstraete, A.; Hoffmann, S.; Schomaker, M.; et al. Management of high-risk acute pulmonary embolism: An emulated target trial analysis. Intensive Care Med. 2025, 51, 490–505. [Google Scholar] [CrossRef]
- Hobohm, L.; Sagoschen, I.; Habertheuer, A.; Barco, S.; Valerio, L.; Wild, J.; Schmidt, F.P.; Gori, T.; Münzel, T.; Konstantinides, S.; et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation 2022, 170, 285–292. [Google Scholar] [CrossRef]
- Inoue, A.; Hifumi, T.; Sakamoto, T.; Kuroda, Y. Extracorporeal Cardiopulmonary Resuscitation for Out-of-Hospital Cardiac Arrest in Adult Patients. J. Am. Heart Assoc. 2020, 9, e015291. [Google Scholar] [CrossRef]
- Scott, J.H.; Gordon, M.; Vender, R.; Pettigrew, S.; Desai, P.; Marchetti, N.; Mamary, A.J.; Panaro, J.; Cohen, G.; Bashir, R.; et al. Venoarterial Extracorporeal Membrane Oxygenation in Massive Pulmonary Embolism-Related Cardiac Arrest: A Systematic Review. Crit. Care Med. 2021, 49, 760–769. [Google Scholar] [CrossRef] [PubMed]
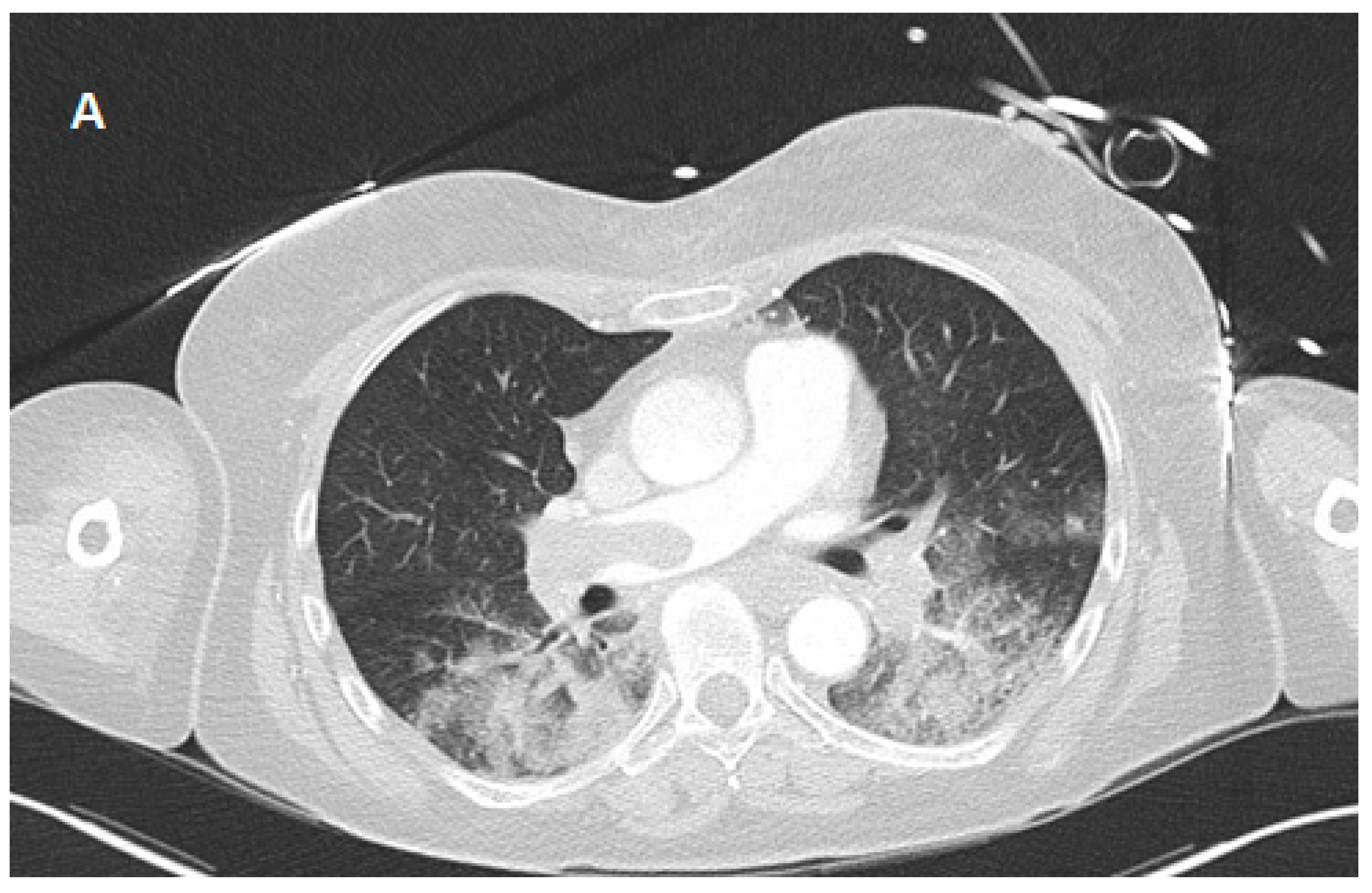
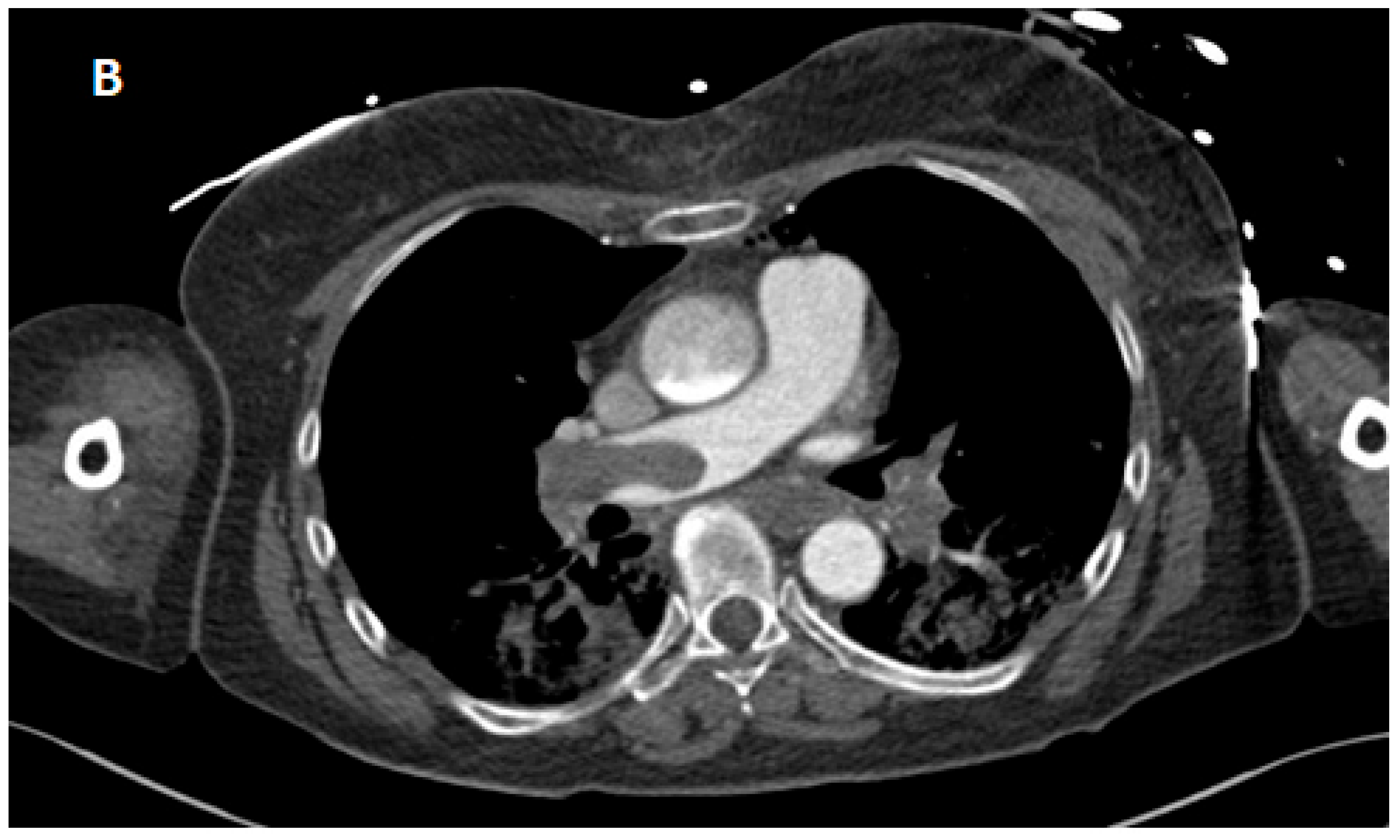
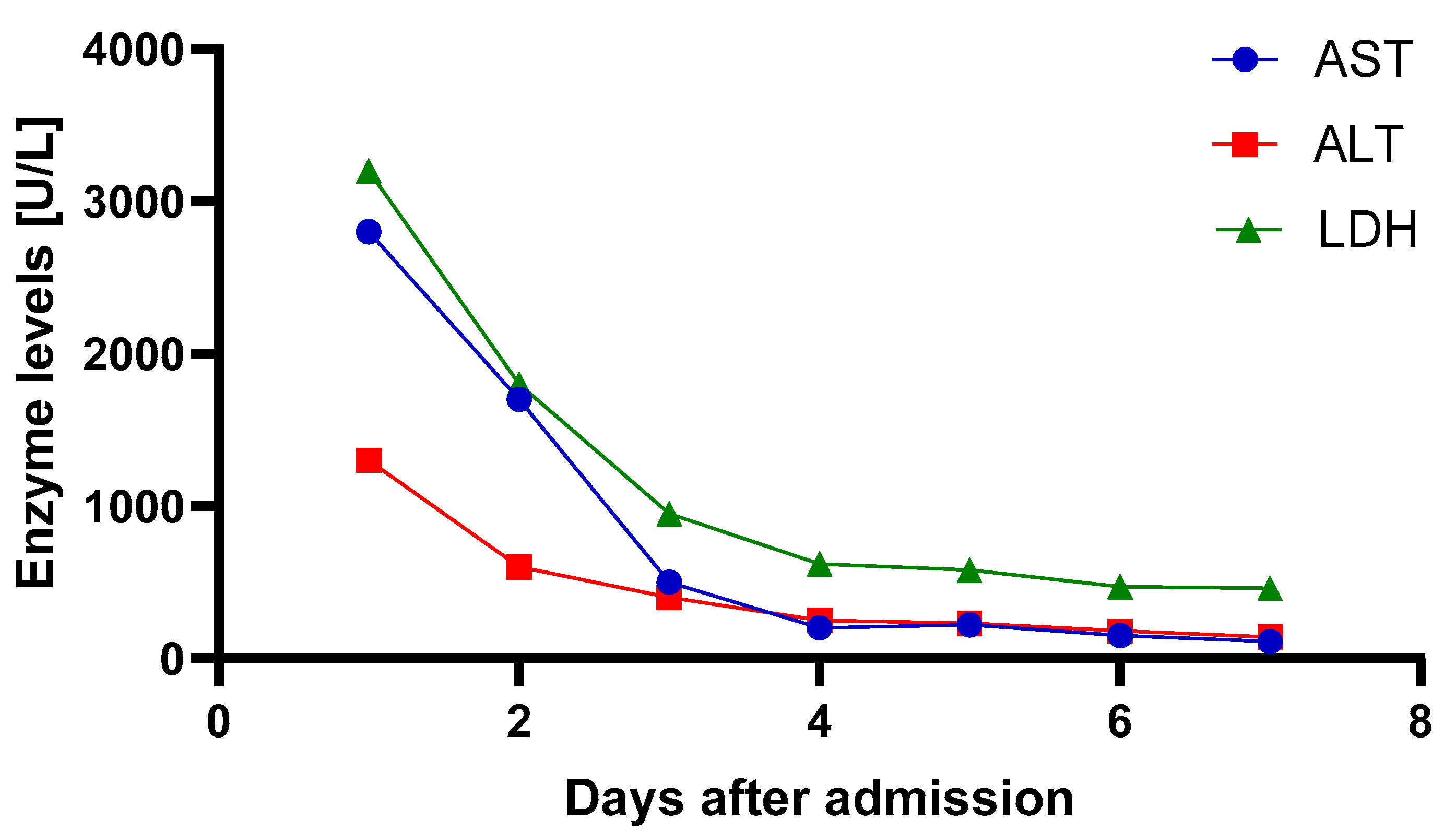
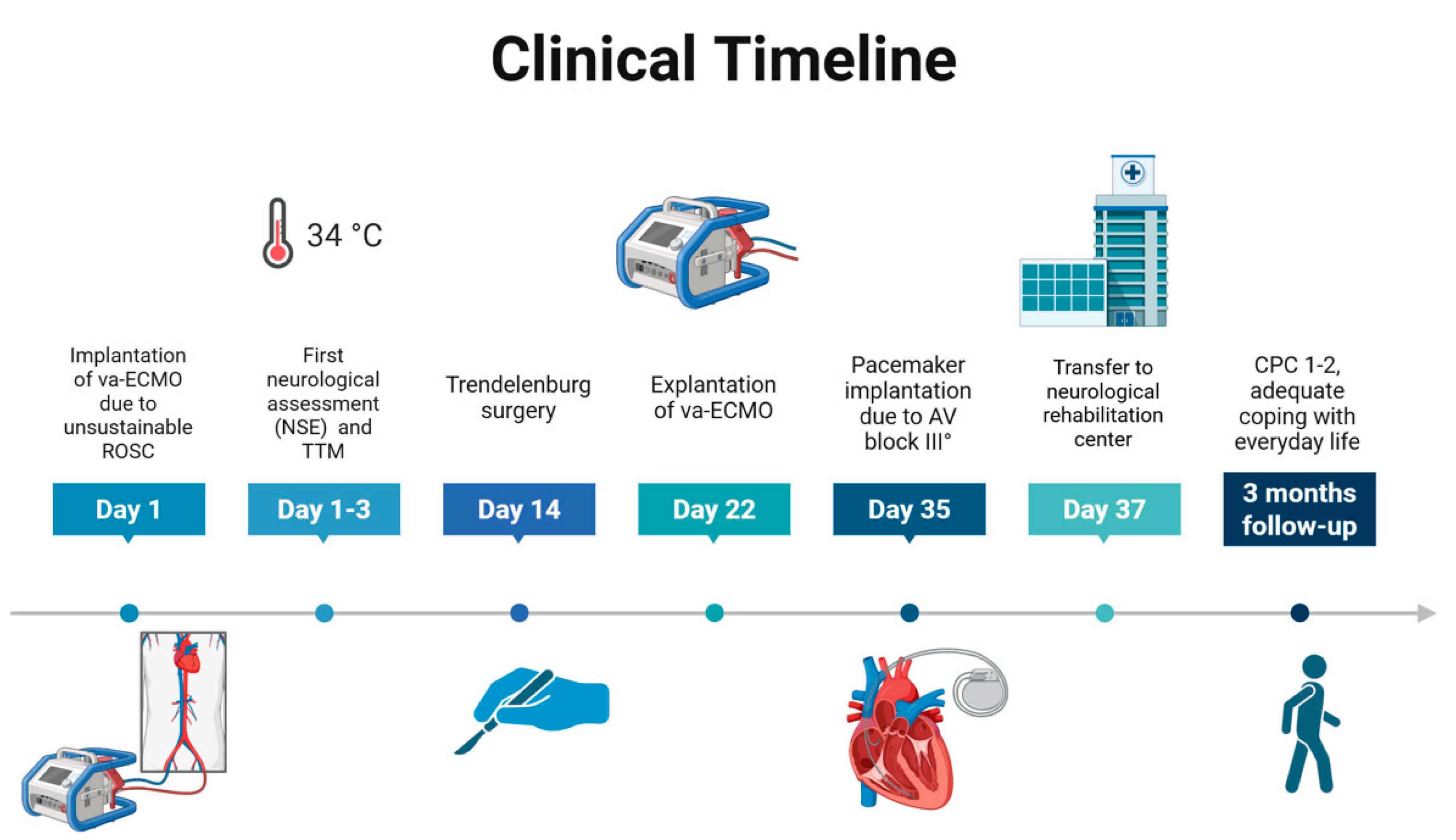
| ABG 1 (Immediately After Va-ECMO Implantation) | ABG 2 (3 h After Va-ECMO Implantation) | ABG 3 (24 h After Va-ECMO Implantation) | |
|---|---|---|---|
| pH | 6.90 | 7.30 | 7.52 |
| pCO2 (mmHg) | 34.5 | 17.9 | 29.0 |
| pO2 (mmHg) | 423 | 312 | 121 |
| Lactate (mmol/L) | >30.0 | 22.0 | 2.6 |
| BE | −19.0 | −16.4 | +1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harbaum, L.; Mihali, K.; Ausbüttel, F.; Schieffer, B.; Kreutz, J. Case Report of Successful Extracorporeal CPR (eCPR) in Refractory Cardiac Arrest Caused by Fulminant Pulmonary Embolism with Remarkable Recovery. Reports 2025, 8, 100. https://doi.org/10.3390/reports8030100
Harbaum L, Mihali K, Ausbüttel F, Schieffer B, Kreutz J. Case Report of Successful Extracorporeal CPR (eCPR) in Refractory Cardiac Arrest Caused by Fulminant Pulmonary Embolism with Remarkable Recovery. Reports. 2025; 8(3):100. https://doi.org/10.3390/reports8030100
Chicago/Turabian StyleHarbaum, Lukas, Klevis Mihali, Felix Ausbüttel, Bernhard Schieffer, and Julian Kreutz. 2025. "Case Report of Successful Extracorporeal CPR (eCPR) in Refractory Cardiac Arrest Caused by Fulminant Pulmonary Embolism with Remarkable Recovery" Reports 8, no. 3: 100. https://doi.org/10.3390/reports8030100
APA StyleHarbaum, L., Mihali, K., Ausbüttel, F., Schieffer, B., & Kreutz, J. (2025). Case Report of Successful Extracorporeal CPR (eCPR) in Refractory Cardiac Arrest Caused by Fulminant Pulmonary Embolism with Remarkable Recovery. Reports, 8(3), 100. https://doi.org/10.3390/reports8030100






