Case Report: Spontaneous Pneumomediastinum and Pneumothorax Complicating Severe Ketoacidosis—An Unexpected Presentation
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
3. Discussion
3.1. Inaugural DKA in Adult Patients
3.2. DKA-Associated Spontaneous Pneumomediastinum
3.3. Acute Pancreatitis and DKA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycemic Crises in Adults With Diabetes: A Consensus Report. Diabetes Care 2024, 47, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Singh, S.; Syed, M.H.; Dave, H.; Hasnain, M.; Zahid, D.; Haider, M.; Jilani, S.M.A.; Mirza, M.A.; Kiran, N.; et al. Temporal Trends in the Prevalence of Diabetes Decompensation (Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State) Among Adult Patients Hospitalized with Diabetes Mellitus: A Nationwide Analysis Stratified by Age, Gender, and Race. Cureus 2019, 11, e4353. [Google Scholar] [CrossRef]
- Elendu, C.; David, J.A.; Udoyen, A.-O.; Egbunu, E.O.; Ogbuiyi-Chima, I.C.; Unakalamba, L.O.; Temitope, A.I.; Ibhiedu, J.O.; Ibhiedu, A.O.; Nwosu, P.U.; et al. Comprehensive Review of Diabetic Ketoacidosis: An Update. Ann. Med. Surg. 2023, 85, 2802–2807. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, J.; Wang, G.; Zhong, S.; Su, X.; Huang, Z.; Chen, G.; Zhang, J.; Hou, X.; Yu, X.; et al. Clinical Profile of Diabetic Ketoacidosis in Tertiary Hospitals in China: A Multicentre, Clinic-based Study. Diabet. Med. 2016, 33, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sall, S.A.B.; Ndiaye, N.; Diack, N.D.; Leye, M.Y.; Leye, P.A.; Diallo, V.M.P.C.; Leye, A. Diagnostic and Evolutionary Aspects of Inaugural Diabetic Ketoacidosis in the Dakar Hospital Setting: A Descriptive and Analytical Cross-Sectional Study in the Endocrinology-Metabolism-Nutrition Department of CHN de Pikine. Open J. Endocr. Metab. Dis. 2025, 15, 8–21. [Google Scholar] [CrossRef]
- Susai, C.J.; Banks, K.C.; Alcasid, N.J.; Velotta, J.B. A Clinical Review of Spontaneous Pneumomediastinum. Mediastinum 2024, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.; Ali, S.Z.; Braud, R.; Weiman, D.; Garrett, H.E. Spontaneous Pneumomediastinum: A Comparative Study and Review of the Literature. Ann. Thorac. Surg. 2008, 86, 962–966. [Google Scholar] [CrossRef]
- Talwar, A.; Rajeev, A.; Rachapudi, S.; Khan, S.; Singh, V.; Talwar, A. Spontaneous Pneumomediastinum: A Comprehensive Review of Diagnosis and Management. Intractable Rare Dis. Res. 2024, 13, 138–147. [Google Scholar] [CrossRef]
- Marza, A.M.; Cindrea, A.C.; Petrica, A.; Stanciugelu, A.V.; Barsac, C.; Mocanu, A.; Critu, R.; Botea, M.O.; Trebuian, C.I.; Lungeanu, D. Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves. J. Pers. Med. 2023, 13, 1497. [Google Scholar] [CrossRef]
- Al-Mufarrej, F.; Badar, J.; Gharagozloo, F.; Tempesta, B.; Strother, E.; Margolis, M. Spontaneous Pneumomediastinum: Diagnostic and Therapeutic Interventions. J. Cardiothorac. Surg. 2008, 3, 59. [Google Scholar] [CrossRef]
- Dirol, H.; Keskin, H. Risk Factors for Mediastinitis and Mortality in Pneumomediastinum. J. Cardiovasc. Thorac. Res. 2022, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Yonezawa, N.; Fukasawa, M.; Fujisawa, M. Extensive Epidural Pneumatosis and Pneumomediastinum Combined with Diabetic Ketoacidosis. Clin. Case Rep. 2023, 11, e7356. [Google Scholar] [CrossRef] [PubMed]
- Alkhuja, S.; Gazizov, N.; Charles, G. Pneumomediastinum Complicating Diabetic Ketoacidosis and Boerhaave’s Syndrome. Case Rep. Med. 2013, 2013, 598720. [Google Scholar] [CrossRef] [PubMed]
- Murayama, S. Spontaneous Pneumomediastinum and Macklin Effect: Overview and Appearance on Computed Tomography. World J. Radiol. 2014, 6, 850. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Wu, X.; Chen, L.; Wei, J.; Xue, M.; Liang, Q. Analysing the Clinical Features of Pneumomediastinum Associated with Diabetic Ketoacidosis in 79 Cases. Diabetes Metab. Syndr. Obes. 2020, 13, 405–412. [Google Scholar] [CrossRef]
- Usman, A.; Angelopoulos, J.; Kumar, R. Spontaneous Pneumomediastinum and Subcutaneous Emphysema Associated With Diabetic Ketoacidosis: A Case Report. Cureus 2024, 16, e61001. [Google Scholar] [CrossRef]
- Xu, W.L.; Sun, L.C.; Zang, X.X.; Wang, H.; Li, W. Spontaneous Pneumomediastinum in Diabetic Ketoacidosis: A Case Series of 10 Patients. World J. Emerg. Med. 2022, 13, 141–143. [Google Scholar] [CrossRef]
- Pain, A.R.; Pomroy, J.; Benjamin, A. Hamman’s Syndrome in Diabetic Ketoacidosis. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 17-0135. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chang, C.-T.; Chen, C.-C.; Wang, T.-Y. Diabetic Ketoacidosis Complicated by Pneumomediastinum and Mallory-Weiss Tear in a Young Boy with New Onset Type 2 Diabetes. South Med. J. 2008, 101, 769–770. [Google Scholar] [CrossRef]
- Hu, J.-X.; Zhao, C.-F.; Wang, S.-L.; Tu, X.-Y.; Huang, W.-B.; Chen, J.-N.; Xie, Y.; Chen, C.-R. Acute Pancreatitis: A Review of Diagnosis, Severity Prediction and Prognosis Assessment from Imaging Technology, Scoring System and Artificial Intelligence. World J. Gastroenterol. 2023, 29, 5268–5291. [Google Scholar] [CrossRef]
- Zerem, E.; Kurtcehajic, A.; Kunosić, S.; Zerem Malkočević, D.; Zerem, O. Current Trends in Acute Pancreatitis: Diagnostic and Therapeutic Challenges. World J. Gastroenterol. 2023, 29, 2747–2763. [Google Scholar] [CrossRef]
- Wen, Y.; Luo, Y.; Huang, Y.; Zhang, Z.; Xiong, L.; Wang, Y. Global, Regional, and National Pancreatitis Burden and Health Inequality of Pancreatitis from 1990 to 2019 with a Prediction from 2020 to 2034. BMC Public Health 2024, 24, 3329. [Google Scholar] [CrossRef]
- Carroll, J.K.; Herrick, B.; Gipson, T.; Lee, S.P. Acute Pancreatitis: Diagnosis, Prognosis, and Treatment. Am. Fam. Physician 2007, 75, 1513–1520. [Google Scholar] [PubMed]
- Khan, A.A.; Ata, F.; Yousaf, Z.; Aljafar, M.S.; Seijari, M.N.; Matarneh, A.; Dakkak, B.; Halabiya, M.; Muthanna, B.; Maliyakkal, A.M.; et al. A Retrospective Study on Comparison of Clinical Characteristics and Outcomes of Diabetic Ketoacidosis Patients with and without Acute Pancreatitis. Sci. Rep. 2023, 13, 4347. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Murphy, M.B.; Kitabchi, A.E. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome. Diabetes Spectr. 2002, 15, 28–36. [Google Scholar] [CrossRef]
- Shahid, W.; Khan, F.; Makda, A.; Kumar, V.; Memon, S.; Rizwan, A. Diabetic Ketoacidosis: Clinical Characteristics and Precipitating Factors. Cureus 2020, 12, e10792. [Google Scholar] [CrossRef]
- Ahmad, R.; Narwaria, M.; Singh, A.; Kumar, S.; Haque, M. Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools. Diagnostics 2023, 13, 2441. [Google Scholar] [CrossRef]
- El-Remessy, A. Diabetic Ketoacidosis Management: Updates and Challenges for Specific Patient Population. Endocrines 2022, 3, 801–812. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic Ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 40. [Google Scholar] [CrossRef]
- Kikani, N.; Balasubramanyam, A. Remission in Ketosis-Prone Diabetes. Endocrinol. Metab. Clin. N. Am. 2023, 52, 165–174. [Google Scholar] [CrossRef]
- Makahleh, L.; Othman, A.; Vedantam, V.; Vedantam, N. Ketosis-Prone Type 2 Diabetes Mellitus: An Unusual Presentation. Cureus 2022, 14, e30031. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, Å. Ketosis-Prone Type 2 Diabetes: A Case Series. Front. Endocrinol. 2019, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Bunduc, S.; Veres, D.S.; Palinkas, D.; Gagyi, E.B.; Hegyi, P.J.; Eross, B.; Mihaly, E.; Hegyi, P.; Hosszufalusi, N. One Third of Cases of New-onset Diabetic Ketosis in Adults Are Associated with Ketosis-prone Type 2 Diabetes—A Systematic Review and Meta-analysis. Diabetes Metab. Res. Rev. 2024, 40, e3743. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E.; Banerji, M.A. Ketosis-Prone Diabetes (Flatbush Diabetes): An Emerging Worldwide Clinically Important Entity. Curr. Diab. Rep. 2018, 18, 120. [Google Scholar] [CrossRef]
- Benoit, S.R.; Hora, I.; Pasquel, F.J.; Gregg, E.W.; Albright, A.L.; Imperatore, G. Trends in Emergency Department Visits and Inpatient Admissions for Hyperglycemic Crises in Adults With Diabetes in the U.S., 2006–2015. Diabetes Care 2020, 43, 1057–1064. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jin, S.W.; Jang, S.H.; Jang, Y.S.; Lee, E.K.; Kim, Y.J.; Lee, M.Y.; Park, J.C.; Rho, T.H.; Kim, J.H.; et al. A Case of Spontaneous Pneumomediastinum and Pneumopericardium in a Young Adult. Korean J. Intern. Med. 2001, 16, 205–209. [Google Scholar] [CrossRef]
- de Moraes, A.G.; Surani, S. Effects of Diabetic Ketoacidosis in the Respiratory System. World J. Diabetes 2019, 10, 16–22. [Google Scholar] [CrossRef]
- Deshwal, H.; Elkhapery, A.; Ramanathan, R.; Nair, D.; Singh, I.; Sinha, A.; Vashisht, R.; Mukherjee, V. Patient-Self Inflicted Lung Injury (P-SILI): An Insight into the Pathophysiology of Lung Injury and Management. J. Clin. Med. 2025, 14, 1632. [Google Scholar] [CrossRef]
- Carteaux, G.; Parfait, M.; Combet, M.; Haudebourg, A.-F.; Tuffet, S.; Mekontso Dessap, A. Patient-Self Inflicted Lung Injury: A Practical Review. J. Clin. Med. 2021, 10, 2738. [Google Scholar] [CrossRef]
- Marongiu, I.; Slobod, D.; Leali, M.; Spinelli, E.; Mauri, T. Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring. J. Clin. Med. 2024, 13, 4018. [Google Scholar] [CrossRef]
- Sklienka, P.; Frelich, M.; Burša, F. Patient Self-Inflicted Lung Injury—A Narrative Review of Pathophysiology, Early Recognition, and Management Options. J. Pers. Med. 2023, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hongo, T.; Nojima, T.; Yumoto, T.; Nakao, A.; Naito, H. Hamman’s Syndrome Accompanied by Diabetic Ketoacidosis; a Case Report. Arch. Acad. Emerg. Med. 2022, 10, e68. [Google Scholar] [CrossRef] [PubMed]
- Garcipérez de Vargas, F.J.; Gómez Barrado, J.J.; Moyano Calvente, S.L.; Amaya García, M.J.; Marcos, G. Pneumopericardium and Pneumomediastinum in a Diabetic and Cocaine User Patient. Endocrinol. Nutr. (Engl. Ed.) 2013, 60, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Pooyan, P.; Puruckherr, M.; Summers, J.A.; Byrd, R.P.; Roy, T.M. Pneumomediastinum, Pneumopericardium, and Epidural Pneumatosis in DKA. J. Diabetes Complicat. 2004, 18, 242–247. [Google Scholar] [CrossRef]
- Sato, M.; Toyoshima, K.; Tamura, Y.; Araki, A. Spontaneous Pneumoperitoneum and Diabetic Ketoacidosis in Fulminant Type 1 Diabetes: A Case Report. Oxf. Med. Case Rep. 2023, 2023, omad079. [Google Scholar] [CrossRef]
- Marchon, K.A.; Nunn, M.O.; Chakera, A.J. Images of the Month: An Incidental Finding of Spontaneous Pneumomediastinum (Hamman’s Syndrome) Secondary to Diabetic Ketoacidosis during the Coronavirus Pandemic. Clin. Med. 2020, 20, e275–e277. [Google Scholar] [CrossRef]
- Pauw, R.G.; van der Werf, T.S.; van Dullemen, H.M.; Dullaart, R.P.F. Mediastinal Emphysema Complicating Diabetic Ketoacidosis: Plea for Conservative Diagnostic Approach. Neth. J. Med 2007, 65, 368–371. [Google Scholar]
- Gaglia, J.L.; Wyckoff, J.; Abrahamson, M.J. Acute Hyperglycemic Crisis in the Elderly. Med. Clin. N. Am. 2004, 88, 1063–1084. [Google Scholar] [CrossRef]
- Ma, L.P.; Liu, X.; Cui, B.C.; Liu, Y.; Wang, C.; Zhao, B. Diabetic Ketoacidosis With Acute Pancreatitis in Patients With Type 2 Diabetes in the Emergency Department: A Retrospective Study. Front. Med. 2022, 9, 813083. [Google Scholar] [CrossRef]
- Imburgio, S.; Vedire, A.; Sanekommu, H.; Johal, A.; Taj, S.; Lesniak, C.; Mushtaq, A. Acute Pancreatitis as an Unusual Culprit of Diabetic Ketoacidosis in a Nondiabetic: A Case-Based Review. Case Rep. Endocrinol. 2023, 2023, 9122669. [Google Scholar] [CrossRef]
- de Pretis, N.; Amodio, A.; Frulloni, L. Hypertriglyceridemic Pancreatitis: Epidemiology, Pathophysiology and Clinical Management. United Eur. Gastroenterol. J. 2018, 6, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tollard, C.; Champenois, V.; Delemer, B.; Carsin-Vu, A.; Barraud, S. An Inaugural Diabetic Ketoacidosis with Acute Pancreatitis during COVID-19. Acta Diabetol. 2021, 58, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Benjaminov, F.; Yassin, S.; Stein, A.; Naftali, T.; Konikoff, F.M. Presence of Small and Multiple Gallstones Increases the Risk of Biliary Complications. Int. J. Gastrointest. Interv. 2024, 13, 37–40. [Google Scholar] [CrossRef]
- Diehl, A.K. Gallstone Size and Risk of Pancreatitis. Arch. Intern. Med. 1997, 157, 1674. [Google Scholar] [CrossRef]
- Rana, S.S.; Sharma, R.K.; Gupta, P.; Gupta, R. Natural Course of Asymptomatic Walled off Pancreatic Necrosis. Dig. Liver Dis. 2019, 51, 730–734. [Google Scholar] [CrossRef]
- Boškoski, I.; Costamagna, G. Walled-off Pancreatic Necrosis: Where Are We? Ann. Gastroenterol. 2014, 27, 93–94. [Google Scholar]
- Stamatakos, M. Walled-off Pancreatic Necrosis. World J. Gastroenterol. 2010, 16, 1707. [Google Scholar] [CrossRef]

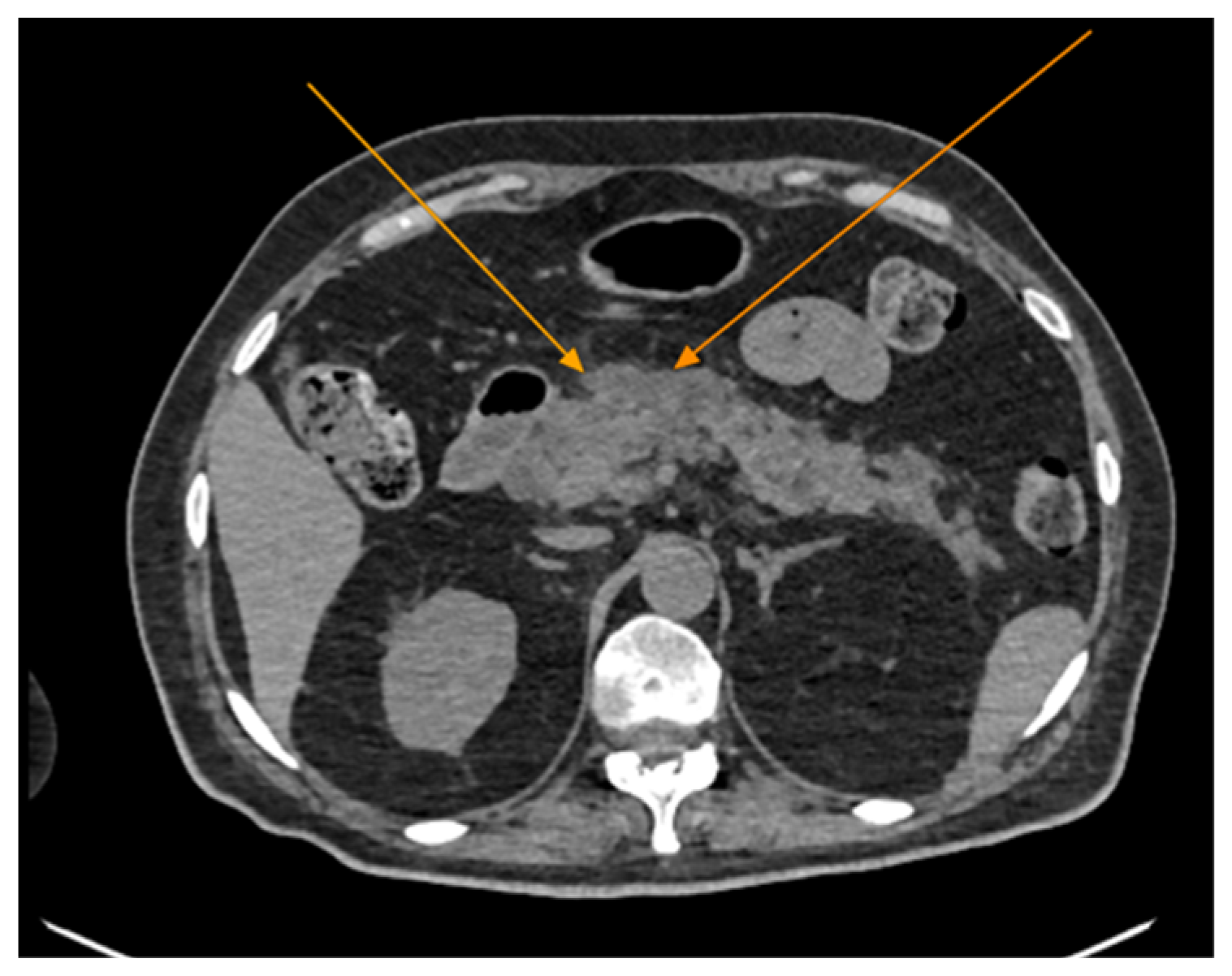
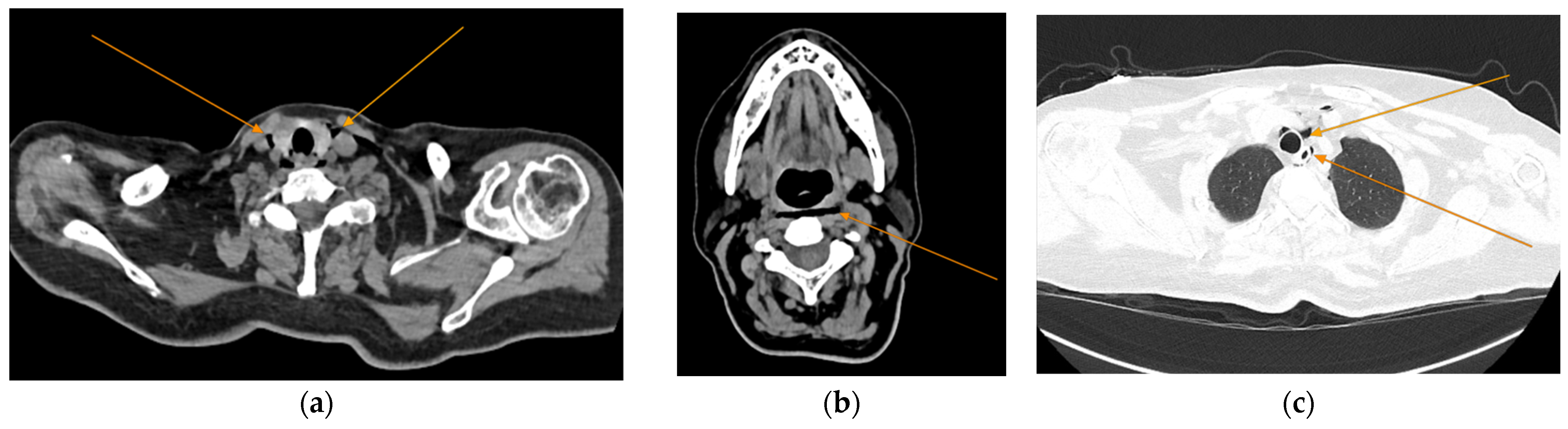
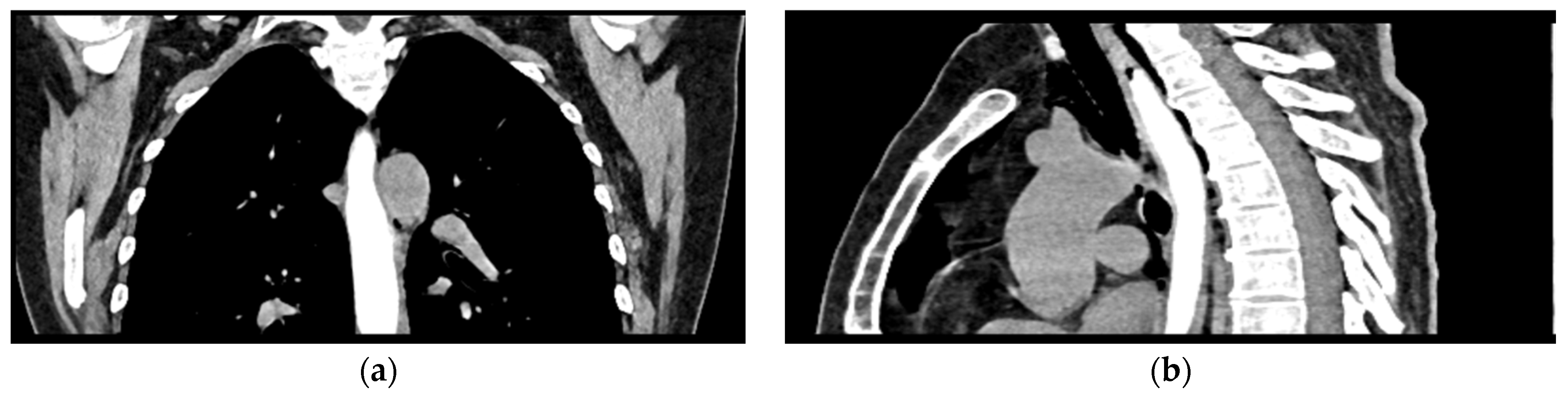

| Bloodwork | Reference Range and Measure Unit | Initial | 7 days | Discharge |
|---|---|---|---|---|
| White blood cells | 4–10 × 103/µL | 29.55 | 23.35 | 7.26 |
| Neutrophiles | 2–7 × 103/µL | 24.19 | 19.34 | 4.74 |
| Hemoglobin | 12–15 g/dL | 16.09 | 14.25 | 12.32 |
| Platelets | 150–410 × 103/µL | 473.2 | 230.5 | 454 |
| Potassium | 3.5–5.1 mmol/L | 3.5 | 2.3 | 4.3 |
| Sodium | 136–145 mmol/L | 137 | 133 | 135 |
| Creatinine | 0.55–1.02 mg/dL | 1.58 | 0.6 | 0.6 |
| Urea | 15–39 mg/dL | 90 | 27 | 6 |
| Glycemia | 74–106 mg/dL | 569 | 390 | 110 |
| Lipase | 16–77 U/L | 687 | 421 | 213 |
| ALT | 14–59 U/L | 47 | 25 | 20 |
| AST | 15–37 U/L | 15 | 18 | 18 |
| Total Bilirubin | 0.2–1.00 mg/dL | 0.66 | 0.9 | 0.5 |
| C reactive protein | 0–5 mg/L | 34.4 | 73 | 34.3 |
| Procalcitonin | 0.1–0.25 ng/mL | 0.14 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cindrea, A.C.; Marza, A.M.; Borita, A.M.; Armega-Anghelescu, A.; Mederle, O.A. Case Report: Spontaneous Pneumomediastinum and Pneumothorax Complicating Severe Ketoacidosis—An Unexpected Presentation. Reports 2025, 8, 95. https://doi.org/10.3390/reports8020095
Cindrea AC, Marza AM, Borita AM, Armega-Anghelescu A, Mederle OA. Case Report: Spontaneous Pneumomediastinum and Pneumothorax Complicating Severe Ketoacidosis—An Unexpected Presentation. Reports. 2025; 8(2):95. https://doi.org/10.3390/reports8020095
Chicago/Turabian StyleCindrea, Alexandru Cristian, Adina Maria Marza, Alexandra Maria Borita, Antonia Armega-Anghelescu, and Ovidiu Alexandru Mederle. 2025. "Case Report: Spontaneous Pneumomediastinum and Pneumothorax Complicating Severe Ketoacidosis—An Unexpected Presentation" Reports 8, no. 2: 95. https://doi.org/10.3390/reports8020095
APA StyleCindrea, A. C., Marza, A. M., Borita, A. M., Armega-Anghelescu, A., & Mederle, O. A. (2025). Case Report: Spontaneous Pneumomediastinum and Pneumothorax Complicating Severe Ketoacidosis—An Unexpected Presentation. Reports, 8(2), 95. https://doi.org/10.3390/reports8020095








