Treatment of Leptomeningeal Disease with Tepotinib in a Patient with Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation Presenting with Extensive Metastasis Involving Duodenum
Abstract
1. Introduction and Clinical Significance
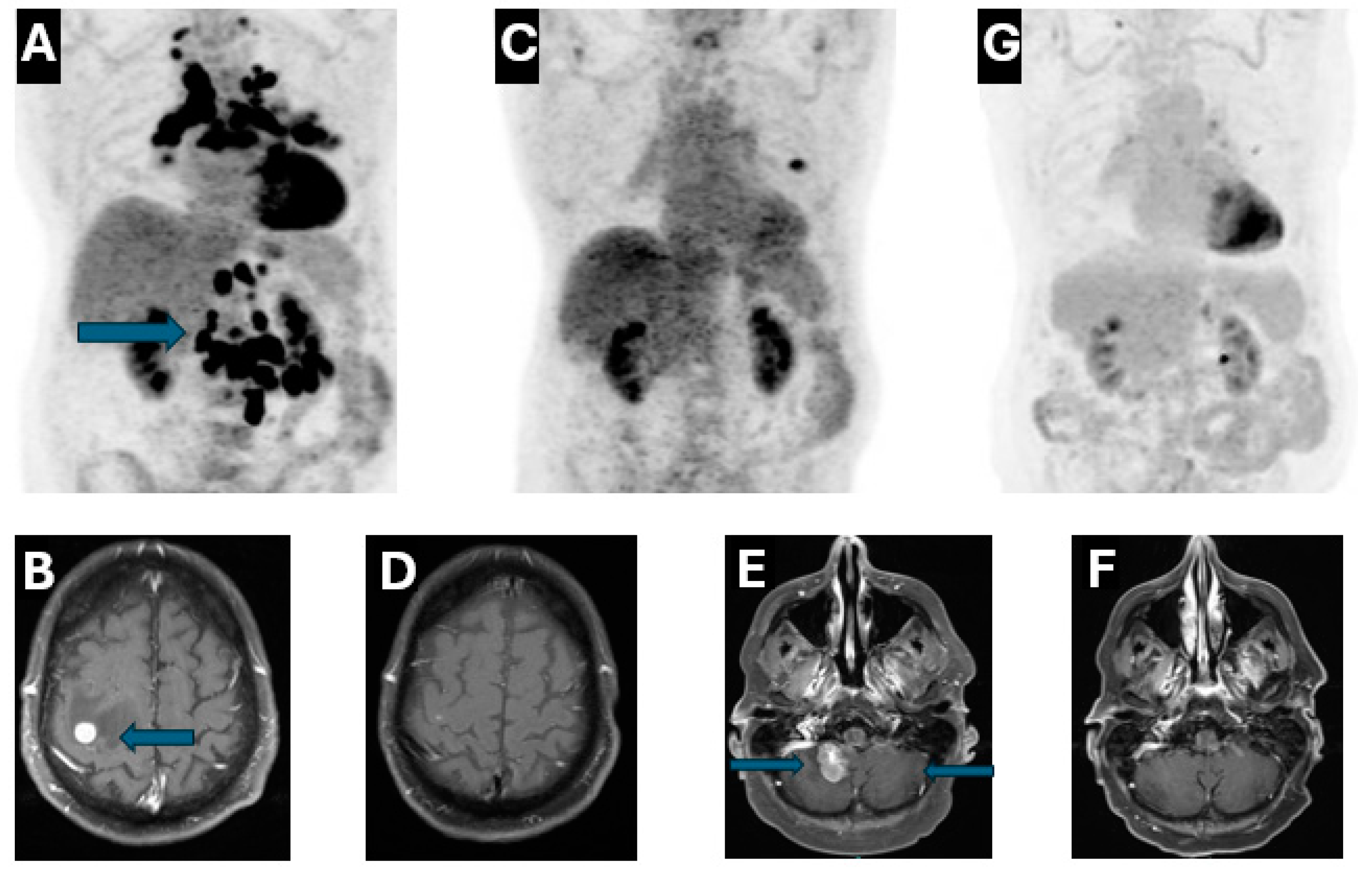
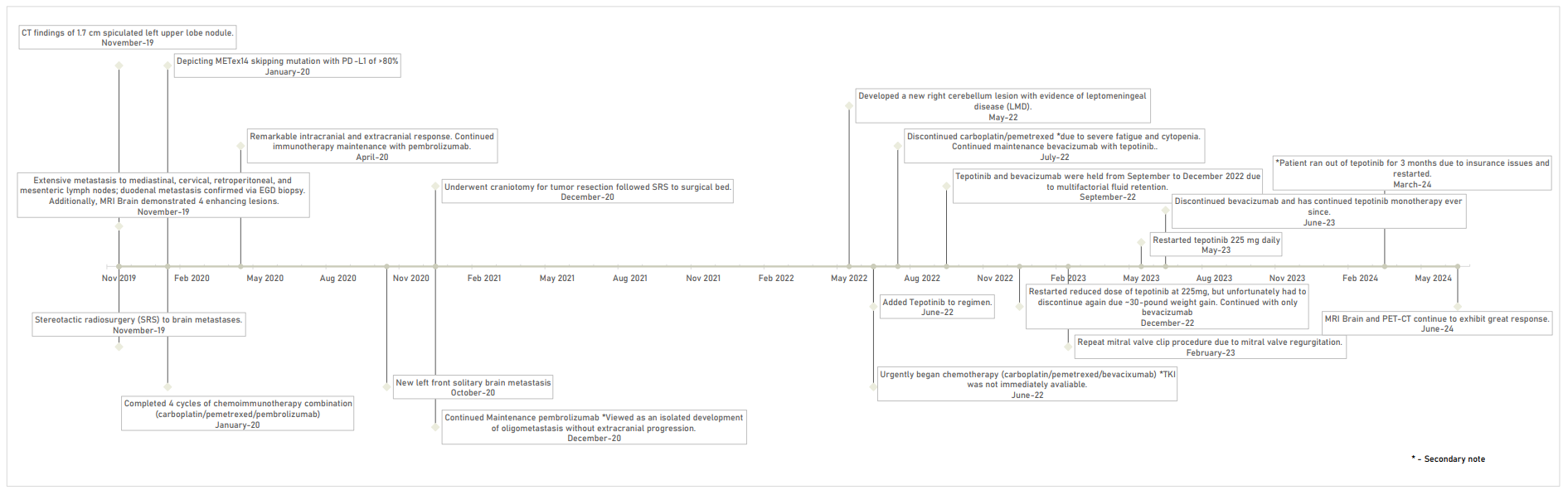
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MET | Mesenchymal–epithelial transition |
| METex14 | Mesenchymal–epithelial transition exon 14 skipping mutation |
| NSCLC | Non-small cell lung cancer |
| TKIs | Tyrosine Kinase Inhibitors |
| LMD | Leptomeningeal Disease |
| ED | Emergency Department |
| CT | Computed Tomography |
| EGD | Esophagogastroduodenoscopy |
| PET | Positron Emission Tomography |
| MRI | Magnetic Resonance Imaging |
| NGS | Next-Generation Sequencing |
| PD-L1 | Programmed Death-Ligand 1 |
| BM | Brain Metastases |
| CNS | Central nervous system |
| EGFR | Epidermal Growth Factor Receptor |
References
- Socinski, M.A.; Pennell, N.A.; Davies, K.D. MET Exon 14 Skipping Mutations in Non–Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021, 5, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients with MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 1260. [Google Scholar] [CrossRef]
- Rossi, G.; Marchioni, A.; Romagnani, E.; Bertolini, F.; Longo, L.; Cavazza, A.; Barbieri, F. Primary Lung Cancer Presenting with Gastrointestinal Tract Involvement: Clinicopathologic and Immunohistochemical Features in a Series of 18 Consecutive Cases. J. Thorac. Oncol. 2007, 2, 115–120. [Google Scholar] [CrossRef]
- Offin, M.; Luo, J.; Guo, R.; Lyo, J.K.; Falcon, C.; Dienstag, J.; Wilkins, O.; Chang, J.; Rudin, C.M.; Riely, G.; et al. CNS Metastases in Patients with MET Exon 14–Altered Lung Cancers and Outcomes with Crizotinib. JCO Precis. Oncol. 2020, 4, 871–876. [Google Scholar] [CrossRef]
- Neves, A.; Mendonça, I.; da Cunha Marques, J.A.; Costa, J.; Almeida, J.S.; Marques, J.A.D.C.; Almeida, J.S., Sr. Gastric Metastasis from Lung Adenocarcinoma: An Uncommon Presentation. Cureus, 2023; 15, Epub ahead of print. [Google Scholar] [CrossRef]
- Khasraw, M.; Yalamanchili, P.; Santhanagopal, A.; Wu, C.; Salas, M.; Meng, J.; Karnoub, M.; Esker, S.; Felip, E. Clinical Management of Patients with Non-Small Cell Lung Cancer, Brain Metastases, and Actionable Genomic Alterations: A Systematic Literature Review. Adv. Ther. 2024, 41, 1815–1842. [Google Scholar] [CrossRef]
- Ozcan, G.; Singh, M.; Vredenburgh, J.J. Leptomeningeal Metastasis from Non–Small Cell Lung Cancer and Current Landscape of Treatments. Clin. Cancer Res. 2023, 29, 11–29. [Google Scholar] [CrossRef]
- Pierret, T.; Giaj-Levra, N.; Toffart, A.-C.; Alongi, F.; Moro-Sibilot, D.; Gobbini, E. Immunotherapy in NSCLC Patients with Brain and Leptomeningeal Metastases. Front. Oncol. 2022, 12, 787080. [Google Scholar] [CrossRef]
- Catalano, M.; Marini, A.; Ferrari, K.; Voltolini, L.; Cianchi, F.; Comin, C.E.; Castiglione, M.; Roviello, G.; Mini, E. Gastric and colonic metastasis from NSCLC: A very unusual case report. Medicine 2022, 101, e28249. [Google Scholar] [CrossRef] [PubMed]
- Shih-Chun, C.; Shih-Chiang, H.; Chun-Yi, T.; Shan-Yu, W.; Keng-Hao, L.; Jun-Te, H.; Ta-Sen, Y.; Chun-Nan, Y. Non-small cell lung cancer with gastric metastasis and repeated gastrointestinal bleeding: A rare case report and literature review. Thorac. Cancer 2021, 12, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Matsubara, T.; Fujishita, T.; Ito, K.; Toyozawa, R.; Seto, T.; Yamaguchi, M.; Okamoto, T. Dramatic intracranial response to tepotinib in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation. Thorac. Cancer 2021, 12, 978–980. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Y.; Lin, M.; Chen, H.; Liu, M.; Wang, Y.; Li, S.; Yang, D.; Yang, Z. Clinical and pathological characteristics of 11 NSCLC patients with c-MET exon 14 skipping. Transl. Cancer Res. TCR 2022, 11, 880–887. [Google Scholar] [CrossRef]
- Ninomaru, T.; Okada, H.; Fujishima, M.; Irie, K.; Fukushima, S.; Hata, A. Lazarus Response to Tepotinib for Leptomeningeal Metastases in a Patient with MET Exon 14 Skipping Mutation–Positive Lung Adenocarcinoma: Case Report. JTO Clin. Res. Rep. 2021, 2, 100145. [Google Scholar] [CrossRef]
- Roth, K.G.; Mambetsariev, I.; Salgia, R. Prolonged survival and response to tepotinib in a non-small-cell lung cancer patient with brain metastases harboring MET exon 14 mutation: A research report. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005785. [Google Scholar] [CrossRef]
- Yang, J.C.; Kim, S.-W.; Kim, D.-W.; Lee, J.-S.; Cho, B.C.; Ahn, J.-S.; Lee, D.H.; Kim, T.M.; Goldman, J.W.; Natale, R.B.; et al. Osimertinib in Patients with Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J. Clin. Oncol. 2020, 38, 538–547. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Friese-Hamim, M.; Clark, A.; Perrin, D.; Crowley, L.; Reusch, C.; Bogatyrova, O.; Zhang, H.; Crandall, T.; Lin, J.; Ma, J.; et al. Brain penetration and efficacy of tepotinib in orthotopic patient-derived xenograft models of MET-driven non-small cell lung cancer brain metastases. Lung Cancer 2022, 163, 77–86. [Google Scholar] [CrossRef]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.-H.; Melosky, B.; Shih, J.-Y.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; Felip, E.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR -Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Kato, T.; Toyozawa, R.; Hida, T. Management of Peripheral Edema in Patients with MET Exon 14-Mutated Non-small Cell Lung Cancer Treated with Small Molecule MET Inhibitors. Targ. Oncol. 2022, 17, 597–604. [Google Scholar] [CrossRef]
- Chen, M.F.; Harada, G.; Liu, D.; DeMatteo, R.; Falcon, C.; Wilhelm, C.; Kris, M.G.; Drilon, A.; Gutgarts, V. Brief Report: Tyrosine Kinase Inhibitors for Lung Cancers That Inhibit MATE-1 Can Lead to “False” Decreases in Renal Function. J. Thorac. Oncol. 2024, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shugarts, J.; Amado, A.; Praska, T.; Camou, M.; Niu, J. Treatment of Leptomeningeal Disease with Tepotinib in a Patient with Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation Presenting with Extensive Metastasis Involving Duodenum. Reports 2025, 8, 96. https://doi.org/10.3390/reports8020096
Shugarts J, Amado A, Praska T, Camou M, Niu J. Treatment of Leptomeningeal Disease with Tepotinib in a Patient with Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation Presenting with Extensive Metastasis Involving Duodenum. Reports. 2025; 8(2):96. https://doi.org/10.3390/reports8020096
Chicago/Turabian StyleShugarts, Jacquelyn, Aida Amado, Taylor Praska, Monica Camou, and Jiaxin Niu. 2025. "Treatment of Leptomeningeal Disease with Tepotinib in a Patient with Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation Presenting with Extensive Metastasis Involving Duodenum" Reports 8, no. 2: 96. https://doi.org/10.3390/reports8020096
APA StyleShugarts, J., Amado, A., Praska, T., Camou, M., & Niu, J. (2025). Treatment of Leptomeningeal Disease with Tepotinib in a Patient with Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation Presenting with Extensive Metastasis Involving Duodenum. Reports, 8(2), 96. https://doi.org/10.3390/reports8020096





