The Long Shadow of Repair: Late-Onset Atrioventricular Block and Atrial Arrhythmias After Scimitar Syndrome and Mitral Annuloplasty
Abstract
1. Introduction and Clinical Significance
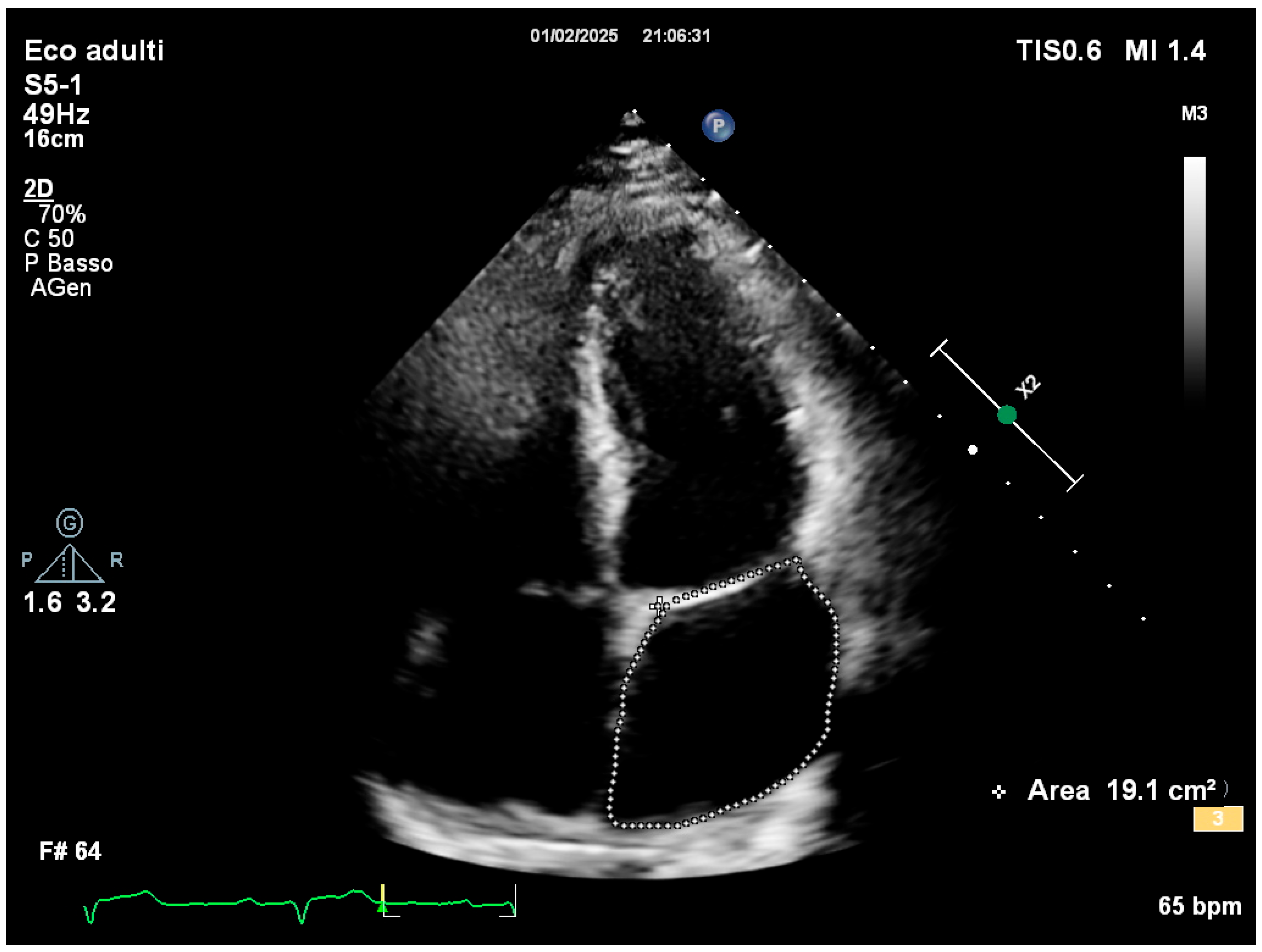
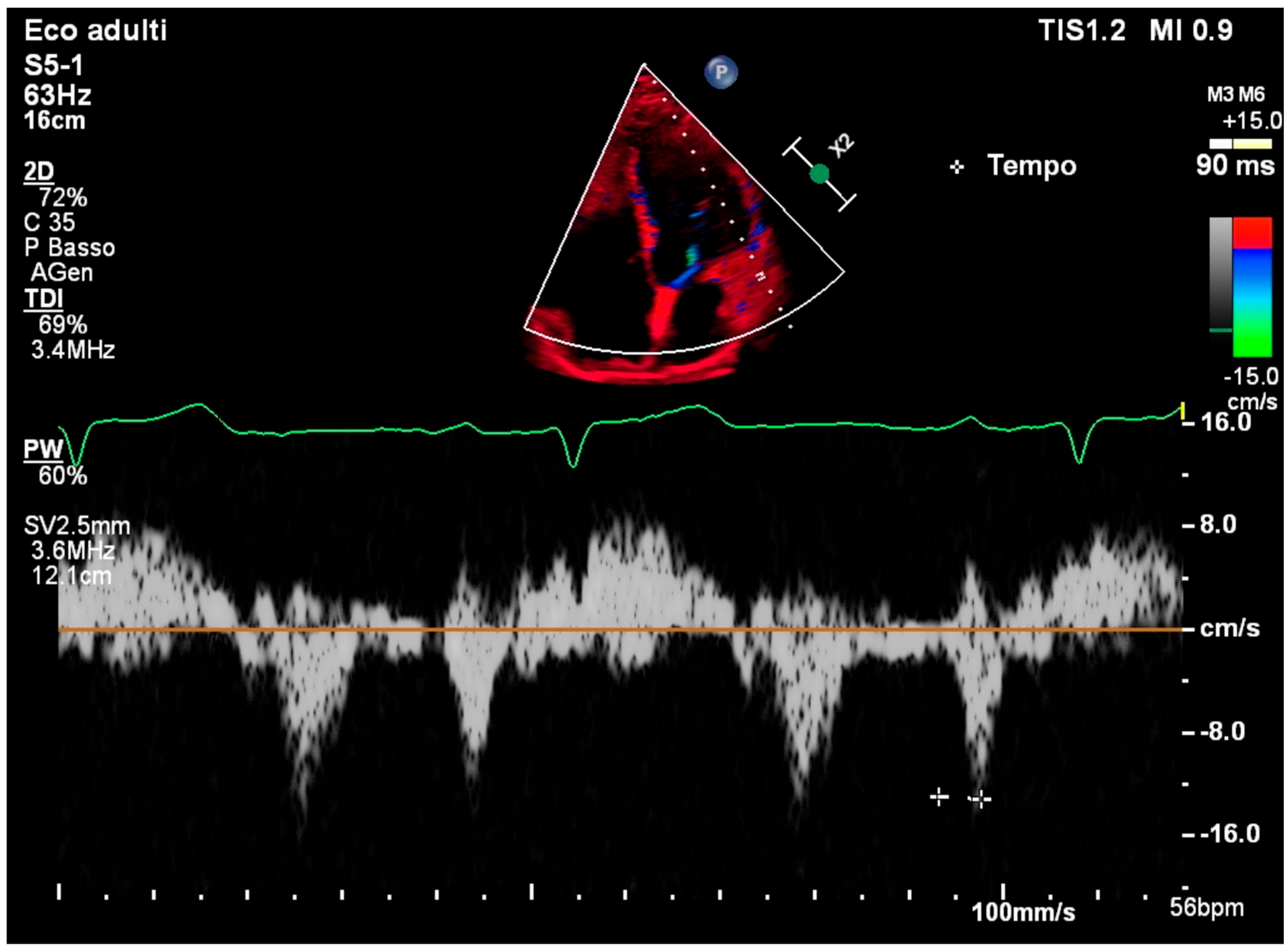
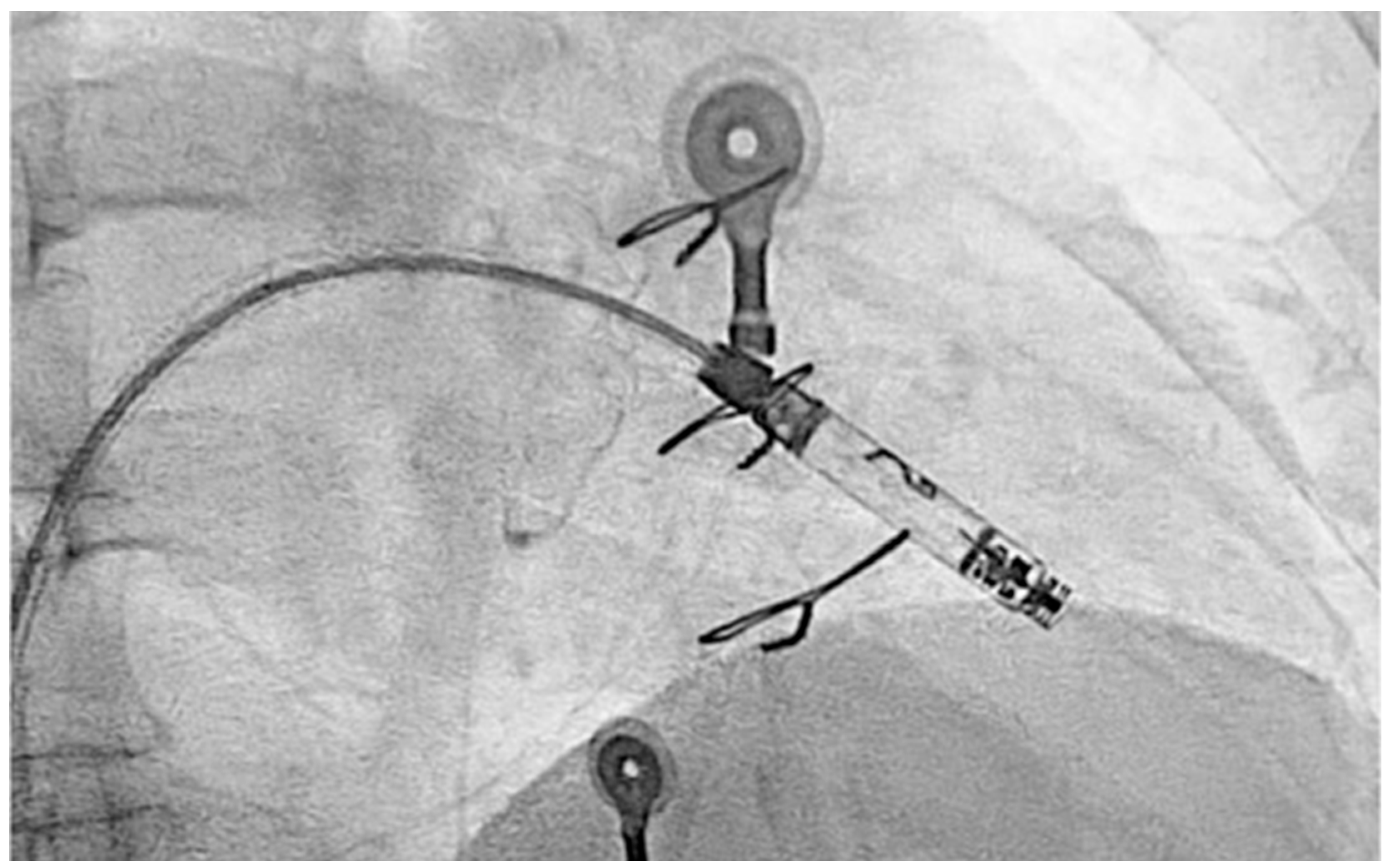
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AV | Atrioventricular |
| AF | Atrial Fibrillation |
| TDI | Tissue Doppler Imaging |
| STE | Speckle-Tracking Echocardiography |
| LA | Left Atrial |
References
- Diaz-Frias, J.; Widrich, J. Scimitar Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gudjonsson, U.; Brown, J.W. Scimitar syndrome. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2006, 9, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Jang, W.S.; Song, K. Surgical correction of total anomalous pulmonary venous return in an adult patient. J. Cardiothorac. Surg. 2022, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Berdajs, D.; Schurr, U.P.; Wagner, A.; Seifert, B.; Turina, M.I.; Genoni, M. Incidence and pathophysiology of atrioventricular block following mitral valve replacement and ring annuloplasty. Eur. J. Cardiothorac. Surg. 2008, 34, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Brodell, G.K.; Cosgrove, D.; Schiavone, W.; Underwood, D.A.; Loop, F.D. Cardiac rhythm and conduction disturbances in patients undergoing mitral valve surgery. Cleve. Clin. J. Med. 1991, 58, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.S.; Murthy, R.; Guleserian, K.J. Scimitar syndrome with atrial fibrillation: Repair in an adult. J. Thorac. Cardiovasc. Surg. 2016, 152, e105–e107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cacciapuoti, F.; Caso, I.; Crispo, S.; Verde, N.; Capone, V.; Gottilla, R.; Materazzi, C.; Volpicelli, M.; Ziviello, F.; Mauro, C.; et al. Linking Epicardial Adipose Tissue to Atrial Remodeling: Clinical Implications of Strain Imaging. Hearts 2025, 6, 3. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Jiang, C.; Du, F.; Zhang, J.; Chang, P. Usefulness of two-dimensional speckle tracking echocardiography in assessment of left atrial fibrosis degree and its application in atrial fibrillation. Int. J. Cardiovasc. Imaging 2025, 41, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Granchietti, A.G.; Ciardetti, N.; Mazzoni, C.; Garofalo, M.; Mazzotta, R.; Micheli, S.; Chiostri, M.; Orlandi, M.; Biagiotti, L.; Del Pace, S.; et al. Left atrial strain and risk of atrial fibrillation after coronary artery bypass-grafting. Int. J. Cardiol. 2025, 422, 132981. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Hu, H.; Zhu, R.; Hu, W.; Li, X.; Shen, D.; Zhang, A.; Zhou, C. Ultrasound assessment of the association between left atrial remodeling and fibrosis in patients with valvular atrial fibrillation: A clinical investigation. BMC Cardiovasc. Disord. 2025, 25, 149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjamin, M.M.; Rabbat, M.G. Left Atrial Markers in Diagnosing and Prognosticating Non-Ischemic Cardiomyopathies: Ready for Prime Time? Echocardiography 2025, 42, e70088. [Google Scholar] [CrossRef] [PubMed]
- Kruse, K.; Matsubara, M.; Schaeffer, T.; Palm, J.; Klawonn, F.; Osawa, T.; Niedermaier, C.; Heinisch, P.P.; Piber, N.; Balling, G.; et al. Postoperative atrioventricular block after surgery for congenital heart disease: Incidence, recovery and risks. Eur. J. Cardiothorac. Surg. 2025, 67, ezaf059. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Kawamura, M.; Yoshioka, D.; Saito, S.; Kawamura, T.; Kawamura, A.; Misumi, Y.; Taira, M.; Shimamura, K.; Komukai, S.; et al. Impact of the Aortomitral Positional Anatomy on Atrioventricular Conduction Disorder Following Mitral Valve Surgery. J. Am. Heart. Assoc. 2024, 13, e035826. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Kawamura, M.; Yoshioka, D.; Saito, S.; Kawamura, T.; Kawamura, A.; Misumi, Y.; Komukai, S.; Kitamura, T.; Miyagawa, S. Impact of aortic root rotation angle on new-onset first-degree atrioventricular block following mitral valve surgery. Interdiscip. Cardiovasc. Thorac. Surg. 2025, 40, ivaf046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, C.C.; Mori, S.; Gerstenfeld, E.P. Iatrogenic Atrioventricular Block. Cardiol. Clin. 2023, 41, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Liu, Y.B.; Lin, L.Y.; Huang, H.C.; Ho, L.T.; Huang, K.C.; Lai, L.P.; Chen, W.J.; Ho, Y.L.; Lin, L.C.; et al. Left atrial reservoir strain as a surrogate marker for atrial fibrillation burden in patients with non-valvular atrial fibrillation. J. Formos. Med. Assoc. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, H.; Wang, Y.; Yu, T.; Li, J.; Wu, Y.; Yu, Z.; Liang, C.; Yu, D.; Xue, L. Left atrial strain predicts paroxysmal atrial fibrillation recurrence after catheter ablation: A 1-year study using three-dimensional speckle-tracking echocardiography. BMC Cardiovasc. Disord. 2025, 25, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, L.; Ling, L.; Song, Y.; Jiang, T. Efficacy and safety of leadless ventricular pacemaker: A single-center retrospective observational study. Cardiovasc. Diagn. Ther. 2024, 14, 878–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doshi, R.N.; Ip, J.E.; Defaye, P.; Reddy, V.Y.; Exner, D.V.; Canby, R.; Shoda, M.; Bongiorni, M.G.; Hindricks, G.; Neuzil, P.; et al. Dual-chamber leadless pacemaker implant procedure outcomes: Insights from the AVEIR DR i2i study. Heart Rhythm 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Pozios, I.; Vouliotis, A.I.; Dilaveris, P.; Tsioufis, C. Electro-Mechanical Alterations in Atrial Fibrillation: Structural, Electrical, and Functional Correlates. J. Cardiovasc. Dev. Dis. 2023, 10, 149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciapuoti, F.; Mauro, C.; Crispo, S.; Carpinella, G.; Volpicelli, M. The Long Shadow of Repair: Late-Onset Atrioventricular Block and Atrial Arrhythmias After Scimitar Syndrome and Mitral Annuloplasty. Reports 2025, 8, 72. https://doi.org/10.3390/reports8020072
Cacciapuoti F, Mauro C, Crispo S, Carpinella G, Volpicelli M. The Long Shadow of Repair: Late-Onset Atrioventricular Block and Atrial Arrhythmias After Scimitar Syndrome and Mitral Annuloplasty. Reports. 2025; 8(2):72. https://doi.org/10.3390/reports8020072
Chicago/Turabian StyleCacciapuoti, Fulvio, Ciro Mauro, Salvatore Crispo, Gerardo Carpinella, and Mario Volpicelli. 2025. "The Long Shadow of Repair: Late-Onset Atrioventricular Block and Atrial Arrhythmias After Scimitar Syndrome and Mitral Annuloplasty" Reports 8, no. 2: 72. https://doi.org/10.3390/reports8020072
APA StyleCacciapuoti, F., Mauro, C., Crispo, S., Carpinella, G., & Volpicelli, M. (2025). The Long Shadow of Repair: Late-Onset Atrioventricular Block and Atrial Arrhythmias After Scimitar Syndrome and Mitral Annuloplasty. Reports, 8(2), 72. https://doi.org/10.3390/reports8020072






