Mapping the Incidence of Dengue Fever in the State of Pará, Eastern Amazon: Epidemiology and Relationships with Climate
Abstract
1. Introduction
2. Materials and Methods
- i.
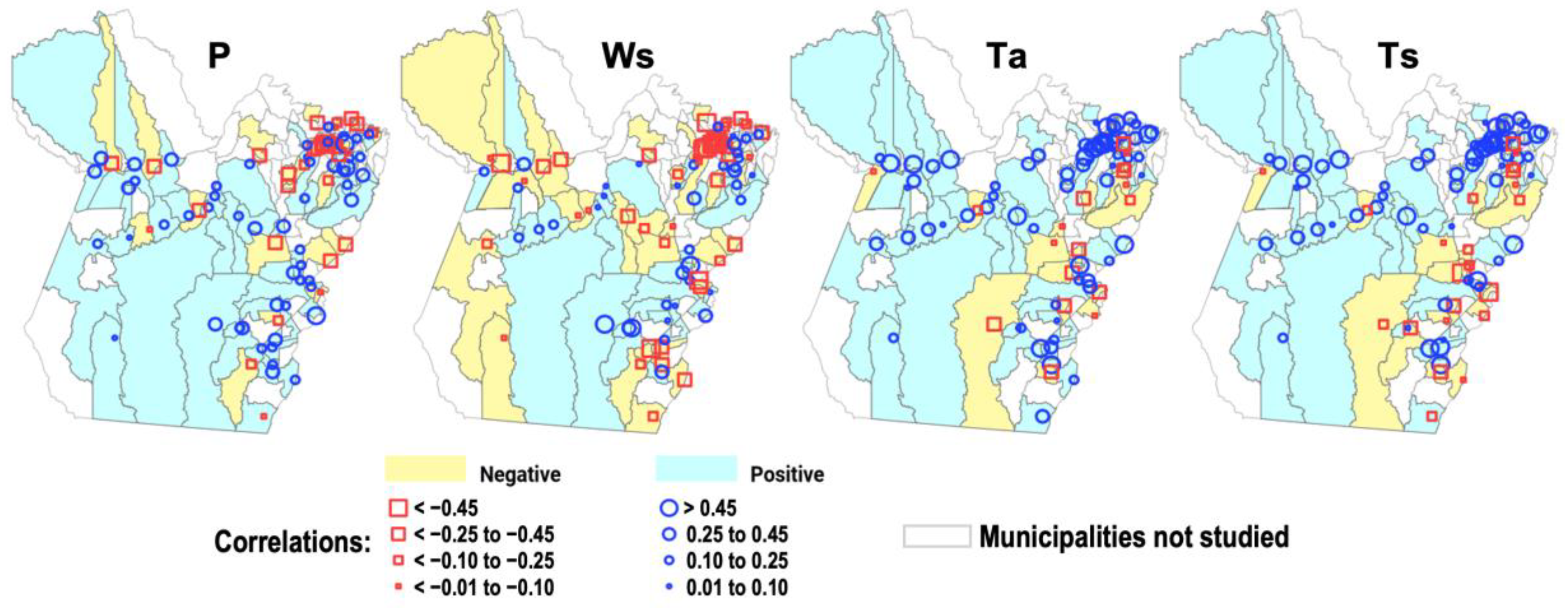
- The group of municipalities with positive values (≥0.1) on the correlation maps must be significantly higher than the group with negative values (≤−0.1);
- ii.
- To evaluate the representativeness of each group, the percentage proportions of the two groups (relative to the entire sample of municipalities = 86) are compared using the chi-square test; i.e., the difference in the proportion of the group with positive correlations must exhibit a statistically significant test value at the 5% level (p-value < 0.05).
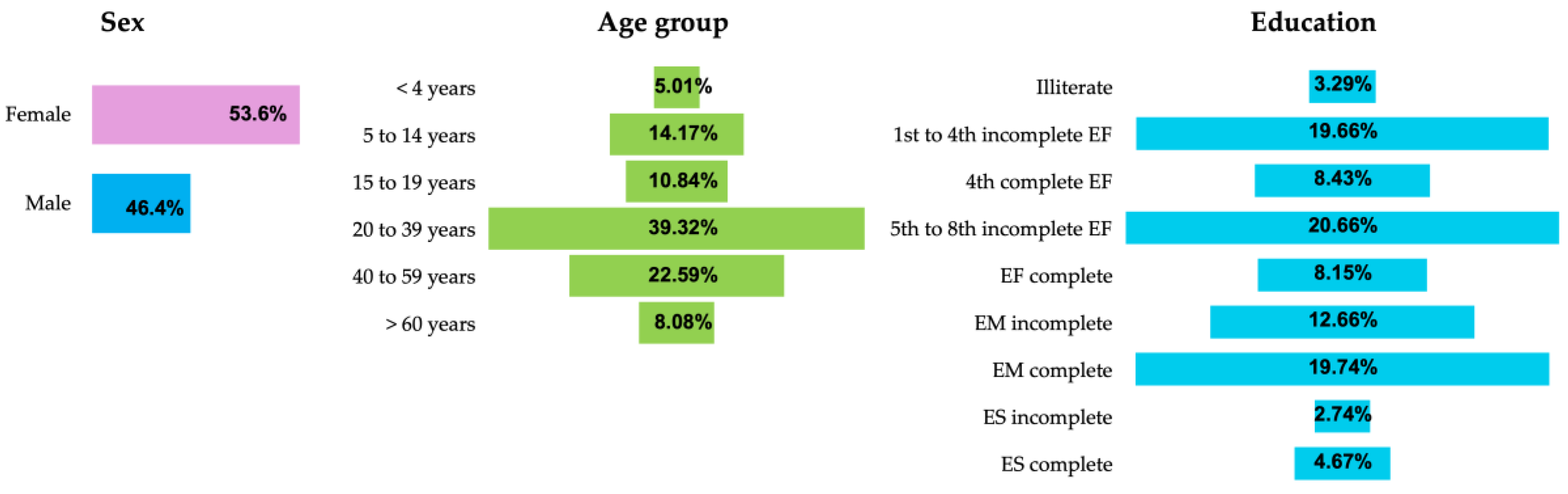
3. Results
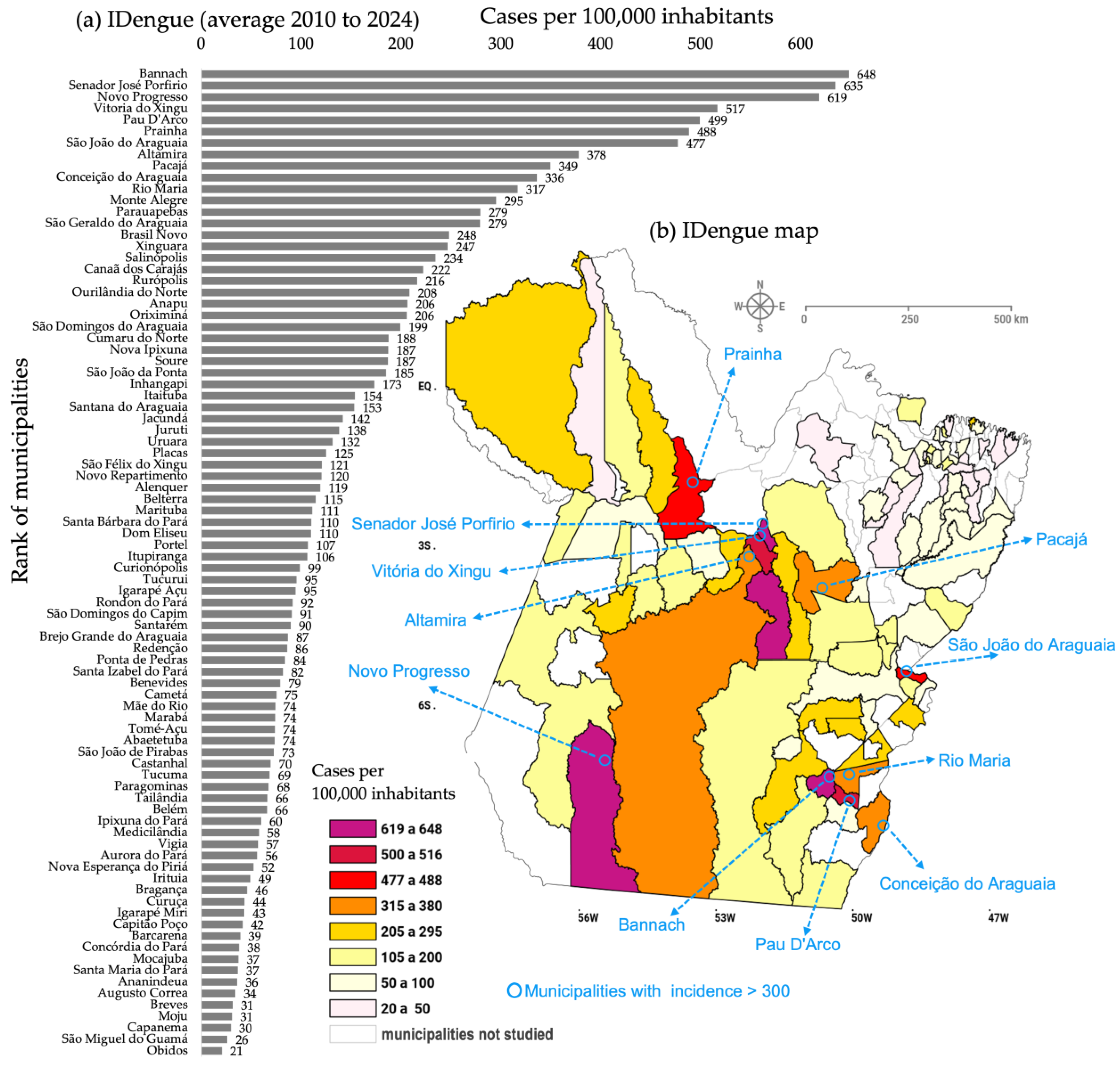
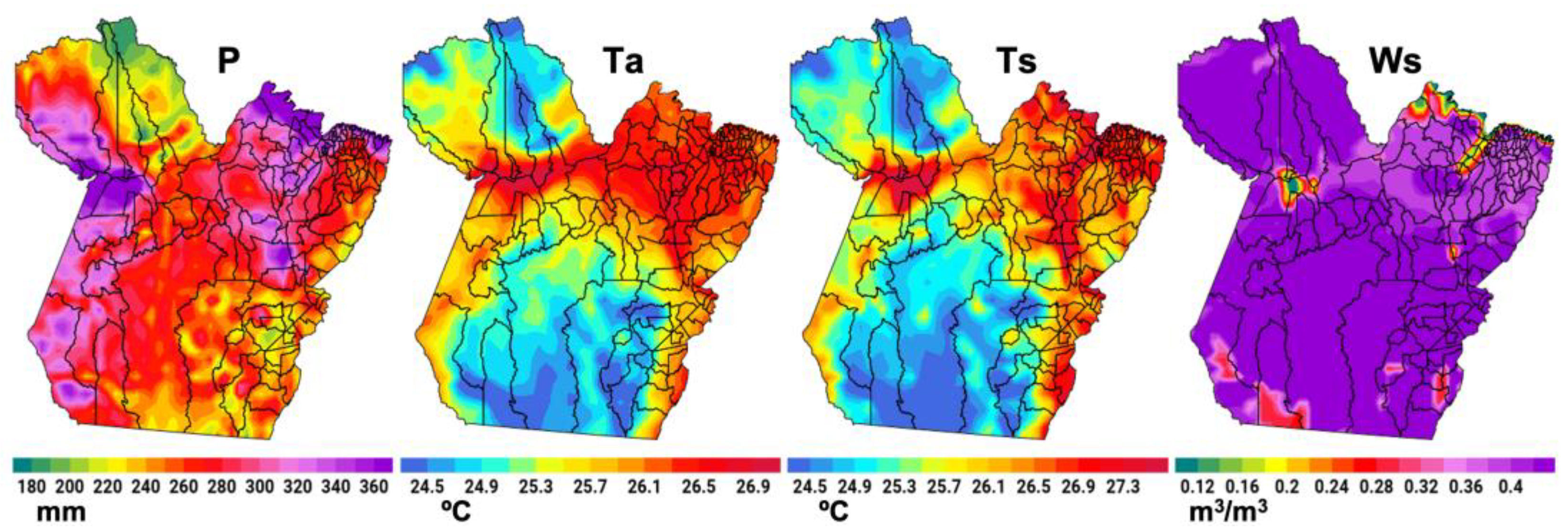
3.1. Mapping Dengue and the Epidemiology of Critical Areas
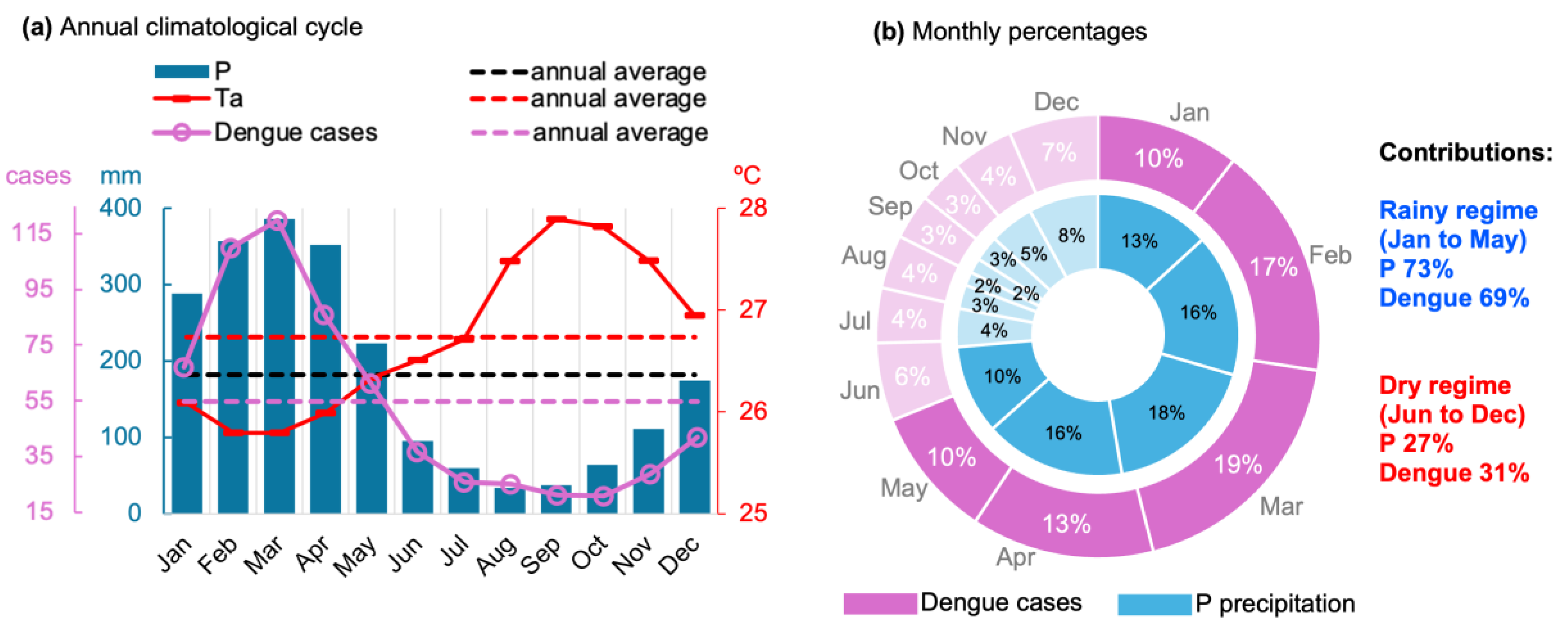
3.2. Relationships Between Climate Variables and Dengue Incidence
3.3. The Effects of the RAINY Regime on Dengue Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Santana, V.S.; Lavezzo, L.C.; Mondini, A.; Terzian, A.C.B.; Bronzoni, R.V.M.; Rossit, A.R.B.; Machado, R.L.D.; Rahal, P.; Nogueira, M.C.L.; Nogueira, M.L. Concurrent dengue and malaria in the Amazon region. Rev. Soc. Bras. Med. Trop. 2010, 43, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, C.; Monteiro, A.M.V.; Corvalán, C.; Gurgel, H.C.; Carvalho, M.S.; Artaxo, P.; Hacon, S.; Ragoni, V. Mudanças climáticas e ambientais e as doenças infecciosas: Cenários e incertezas para o Brasil. Epidemiol. Serviços Saúde 2009, 18, 285–304. [Google Scholar] [CrossRef]
- Penna, G.; Luiz Pinto, L.F.; Soranz, D.; Glatt, R. High incidence of diseases endemic to the Amazon region of Brazil, 2001–2006. Emerg. Infect. Dis. 2009, 15, 626–632. [Google Scholar] [CrossRef]
- Confalonieri, U. Environmental Change and Human Health in the Brazilian Amazon. Glob. Change Hum. Health 2000, 1, 174–183. [Google Scholar] [CrossRef]
- Barreto, M.L.; Teixeira, M.G. Dengue in Brazil: Epidemiological situation and Contribution to a Research agenda. Estud. Avançados 2008, 22, 53–72. [Google Scholar] [CrossRef]
- Moraes, B.C.; Souza, E.B.; Sodré, G.R.C.; Ferreira, D.B.S.; Ribeiro, J.B.M. Sazonalidade nas notificações de dengue das capitais da Amazônia e os impactos do El Niño/La Niña. Cad. Saúde Pública 2019, 35, e00123417. [Google Scholar] [CrossRef]
- Viana, D.V.; Ignotti, E. A ocorrência da dengue e variações meteorológicas no Brasil: Revisão sistemática. Rev. Bras. Epidemiol. 2013, 16, 240–256. [Google Scholar] [CrossRef]
- Stolerman, L.M.; Maia, P.D.; Kutz, J.N. Forecasting dengue fever in Brazil: An assessment of climate conditions. PLoS ONE 2019, 14, e0220106. [Google Scholar] [CrossRef]
- Morin, C.W.; Comrie, A.C.; Ernst, K.C. Climate and dengue transmission: Evidence and implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Cohen, J.M.; Evans, M.V.; Gudapati, P.; Johnson, L.R.; Lippi, C.A.; Murdock, C.C.; Rohr, J.R.; Ryan, S.J.; Savage, V.; et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl. Trop. Dis. 2017, 11, e0005568. [Google Scholar] [CrossRef]
- Duarte, J.L.; Diaz-Quijano, F.A.; Batista, A.C.; Giatti, L.L. Climatic variables associated with dengue incidence in a city of the Western Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180429. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.A.J.; Costa, A.C.L.; Pereira, I.C.N. Associação entre a precipitação pluviométrica e a incidência de dengue em sete municípios do Estado do Pará. Rev. Bras. Geogr. Física 2016, 9, 2264–2276. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Churakov, M.; Villabona-Arenas, C.J.; Kraemer, M.U.G.; Salje, H.; Cauchemez, S. Spatio-temporal dynamics of dengue in Brazil: Seasonal travelling waves and determinants of regional synchrony. PLoS. Negl. Trop. Dis. 2019, 13, e0007012. [Google Scholar] [CrossRef]
- Hage, R.S.; Bohm, B.C.; Casagrande, C.P.; Silva, S.C.M.; Soares, A.T.; Lima, J.V.; Bruhn, N.C.P.; Bruhn, F.R.P. Spatiotemporal expansion of dengue in Brazilian Amazon between 2001 and 2021. Sci. Rep. 2025, 15, 1032. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. DATASUS. Tabnet. Brasília, DF: Ministério da Saúde. 2022. Available online: https://datasus.saude.gov.br/informacoes-de-saude-tabnet (accessed on 3 January 2025).
- IBGE—Instituto Brasileiro de Geografia e Estatistica. Estimativa da População. Rio de Janeiro, RJ. 2022. Available online: https://www.ibge.gov.br/estatisticas/sociais/populacao/9103-estimativas-de-populacao.html (accessed on 5 January 2025).
- IBGE—Instituto Brasileiro de Geografia e Estatistica. Monitoramento da Cobertura e Uso da Terra do Brasil: 2018/2020; IBGE: Rio de Janeiro, RJ, Brazil, 2020; 39p, ISBN 978-85-240-4544-8.
- World Health Organization—New Edition. DENGUE Guidelines for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009; 160p, Available online: https://www.who.int/publications/i/item/9789241547871 (accessed on 29 January 2025).
- World Health Organization. Disease Outbreak News; Dengue—Global Situation. 21 December 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (accessed on 14 April 2025).
- Stević, I.; Milenković, D.; Petrović, A.; Pejčić, A.; Dodevska, M.; Ilić, M.P.; Ivanović, N. The role of nutrition education in the pharmacy curriculum using the example of knowledge about the health benefits of nuts. Front. Public Health 2024, 12, 1481265. [Google Scholar] [CrossRef]
- Rosa-Freitas, M.G.; Schreiber, K.V.; Tsouris, P.; Weimann, E.T.; Luitgards-Moura, J.F. Associations between dengue and combinations of weather factors in a city in the Brazilian Amazon. Pan Am. J. Public Health 2006, 20, 256–267. [Google Scholar] [CrossRef]
- Miyazaki, R.D.; Ribeiro, A.L.M.; Pignatti, M.G.; Campelo Júnior, J.H.; Pignati, M. Monitoramento do mosquito Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae), por meio de ovitrampas no Campus da Universidade Federal de Mato Grosso, Cuiabá, Estado de Mato Grosso. Rev. Soc. Bras. Med. Trop. 2009, 42, 392–397. [Google Scholar] [CrossRef]
- de Azevedo, T.S.; Lorenz, C.; Chiaravalloti-Neto, F. Spatiotemporal evolution of dengue outbreaks in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 593–602. [Google Scholar] [CrossRef]
- Mathulamuthu, S.S.; Asirvadam, V.S.; Dass, S.C.; Gill, B.S.; Loshini, T. Predicting dengue incidences using cluster based re-gression on climate data. In Proceedings of the 2016 6th IEEE International Conference on Control System, Computing and Engineering (ICCSCE), Penang, Malaysia, 25–27 November 2016; pp. 245–250. [Google Scholar] [CrossRef]
- de Souza, E.B.; Ferreira, D.B.S.; Guimarães, J.T.F.; Franco, V.S.; Azevedo, F.T.M.; Souza, P.J.O.P. Padrões climatológicos e tendências da precipitação nos regimes chuvoso e seco da Amazônia oriental. Rev. Bras. Climatol. 2017, 21, 81–93. [Google Scholar] [CrossRef]
- Oneda, R.M.; Basso, S.R.; Frasson, L.R.; Mottecy, N.M.; Saraiva, L.; Bassani, C. Epidemiological profile of dengue in Brazil between the years 2014 and 2019. Rev. Assoc. Médica Bras. 2021, 67, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Paixão, F.A.W.; Oliveira, M.A. Dengue cases in the Amazonas in the years 2018 to 2022. Res. Soc. Dev. 2022, 11, e30111932053. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Cabidelle, A.S.A.; Borges, P.C.L.; Lang, C.F.; Calenti, F.G.; Nogueira, L.O.; Falqueto, A.; Cerutti Junior, C. Dengue: Clinical forms and risk groups in a high incidence city in the southeastern region of Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.B.; Vitorino, M.I.; Sousa, A.M.L.; Santos, J.T.; Ferreira, G.R.; Gonçalves, F.L.T.; Santos, M.R.S. Influência da variabilidade climática na densidade de mosquitos vetores no leste da Amazônia. Rev. Bras. Climatol. 2020, 26, 801–816. [Google Scholar] [CrossRef]
- Honório, N.A.; Castro, M.G.; Barros, F.S.M.; Magalhães, M.A.F.M.; Sabroza, P.C. The spatial distribution of Aedes aegypti and Aedes albopictus in a transition zone, Rio de Janeiro, Brazil. Cad. Saúde Pública 2009, 25, 1203–1214. [Google Scholar] [CrossRef]
- Chen, S.C.; Hsieh, M.H. Modeling the transmission dynamics of dengue fever: Implications of temperature effects. Sci. Total Environ. 2012, 431, 385–391. [Google Scholar] [CrossRef]
- Costa, F.S.; Silva, J.J.; Souza, C.M.; Mendes, J. Dinâmica populacional de Aedes aegypti (L) em área urbana de alta incidência de dengue. Rev. Soc. Bras. Med. Trop. 2008, 41, 309–312. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Li, L.; He, J.; Guo, J.; Wang, Z.; Huang, Y.; Xi, Z.; Yuan, F.; Li, Y.; et al. The effect of temperature on dengue virus transmission by Aedes mosquitoes. Front. Cell. Infect. Microbiol. 2023, 13, 1242173. [Google Scholar] [CrossRef]

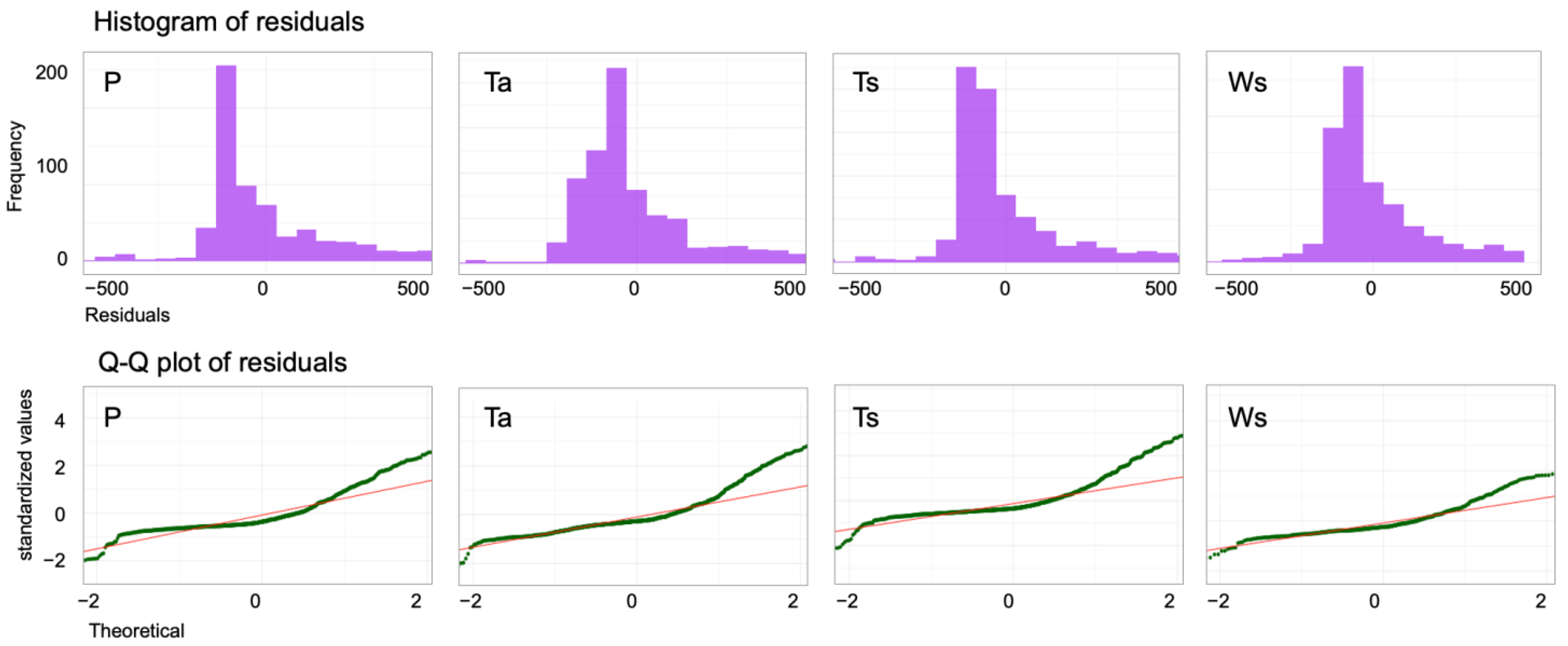
| Groups | Results | P | Ws | Ta | Ts |
|---|---|---|---|---|---|
| Group of positive correlations | count | 51 * | 30 | 59 * | 57 * |
| Group of negative correlations | 27 | 37 | 12 | 16 | |
| Group of positive correlations | proportion | 59.3% | 34.9% | 68.6% * | 63.3% * |
| Group of negative correlations | 31.4% | 43.0% | 14.0% | 18.6% | |
| Group of positive correlations | chi-square | 7.38 * | 0.73 | 31.11 * | 23.03 * |
| Group of negative correlations | p-value | 0.007 | 0.392 | 0.000 | 0.001 |
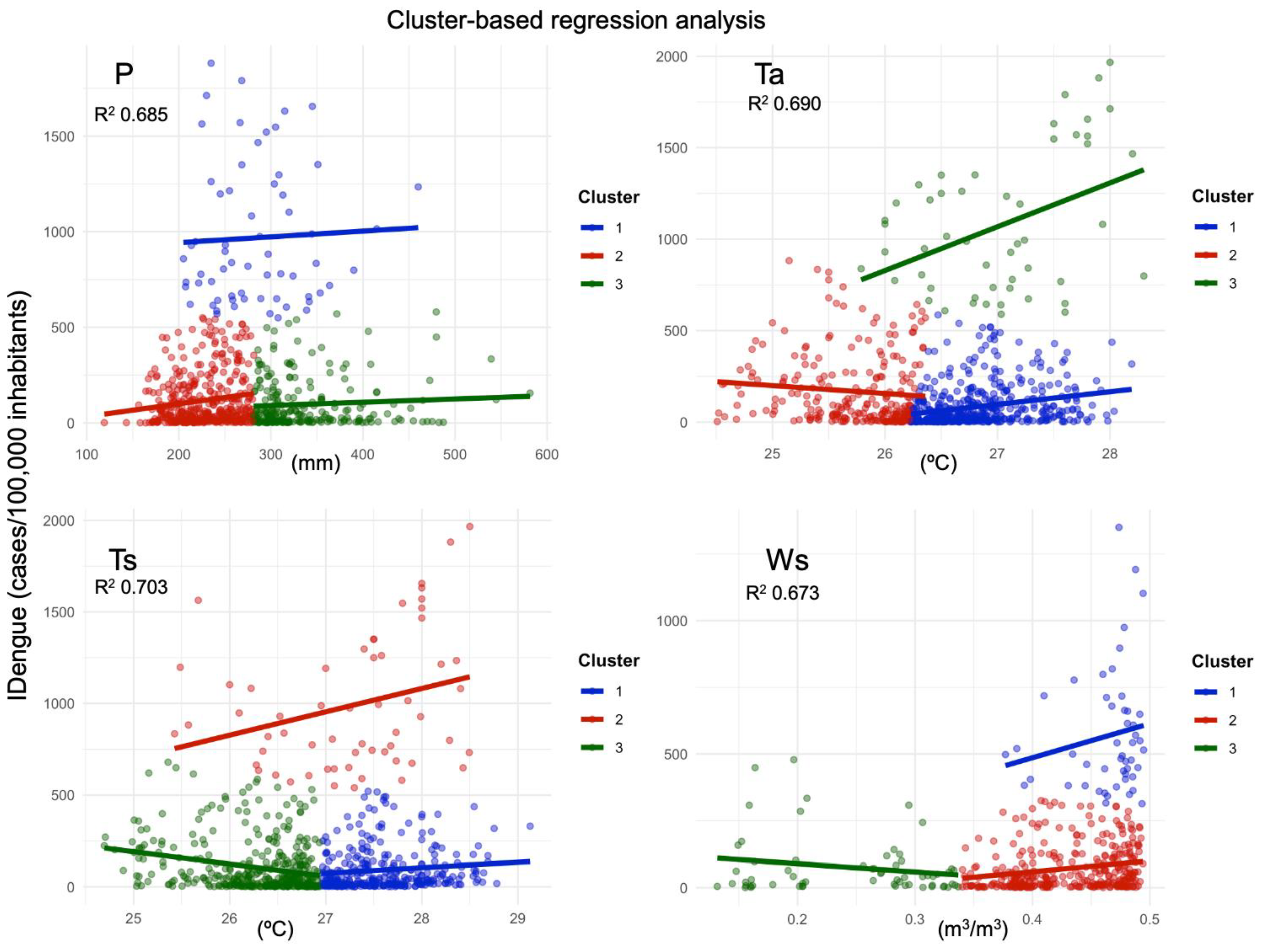
| Variables | R2 | Adjusted R2 | Residual Std Error | F-Statistic | Intercept | ||
|---|---|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | |||||
| P | 0.685 | 0.682 | 167.1 | 304.7 * | 1.30 | 2.67 | 0.97 |
| Ws | 0.673 | 0.669 | 130.4 | 279.9 * | 979.5 | 406.1 | −306.1 |
| Ts | 0.703 | 0.701 | 154.9 | 363.6 ** | 29.7 | 127.5 | −66.6 |
| Ta | 0.690 | 0.688 | 163.7 | 351.4 ** | 69.1 | −43.2 | 239.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, E.M.F.; Ferreira, D.B.d.S.; Silva Jr, J.d.A.; Moraes, B.C.d.; Lima, A.M.M.d.; Silva, B.C.S.d.; Godoy, B.S.; Coutinho, E.d.C.; Parente, A.T.; Cohen, J.C.P.; et al. Mapping the Incidence of Dengue Fever in the State of Pará, Eastern Amazon: Epidemiology and Relationships with Climate. Reports 2025, 8, 61. https://doi.org/10.3390/reports8020061
Serra EMF, Ferreira DBdS, Silva Jr JdA, Moraes BCd, Lima AMMd, Silva BCSd, Godoy BS, Coutinho EdC, Parente AT, Cohen JCP, et al. Mapping the Incidence of Dengue Fever in the State of Pará, Eastern Amazon: Epidemiology and Relationships with Climate. Reports. 2025; 8(2):61. https://doi.org/10.3390/reports8020061
Chicago/Turabian StyleSerra, Emilene Monteiro Furtado, Douglas Batista da Silva Ferreira, João de Athaydes Silva Jr, Bergson Cavalcanti de Moraes, Aline Maria Meiguins de Lima, Brenda Caroline Sampaio da Silva, Bruno Spacek Godoy, Eliane de Castro Coutinho, Andressa Tavares Parente, Julia Clarinda Paiva Cohen, and et al. 2025. "Mapping the Incidence of Dengue Fever in the State of Pará, Eastern Amazon: Epidemiology and Relationships with Climate" Reports 8, no. 2: 61. https://doi.org/10.3390/reports8020061
APA StyleSerra, E. M. F., Ferreira, D. B. d. S., Silva Jr, J. d. A., Moraes, B. C. d., Lima, A. M. M. d., Silva, B. C. S. d., Godoy, B. S., Coutinho, E. d. C., Parente, A. T., Cohen, J. C. P., Cunha, A. C. d., & Souza, E. B. d. (2025). Mapping the Incidence of Dengue Fever in the State of Pará, Eastern Amazon: Epidemiology and Relationships with Climate. Reports, 8(2), 61. https://doi.org/10.3390/reports8020061







