Prolonged Survival Outcome in a Patient with Refractory Metastatic Colorectal Cancer Treated with Regorafenib Plus 5-Fluorouracil: A Case Report and Literature Review
Abstract
1. Introduction and Clinical Significance
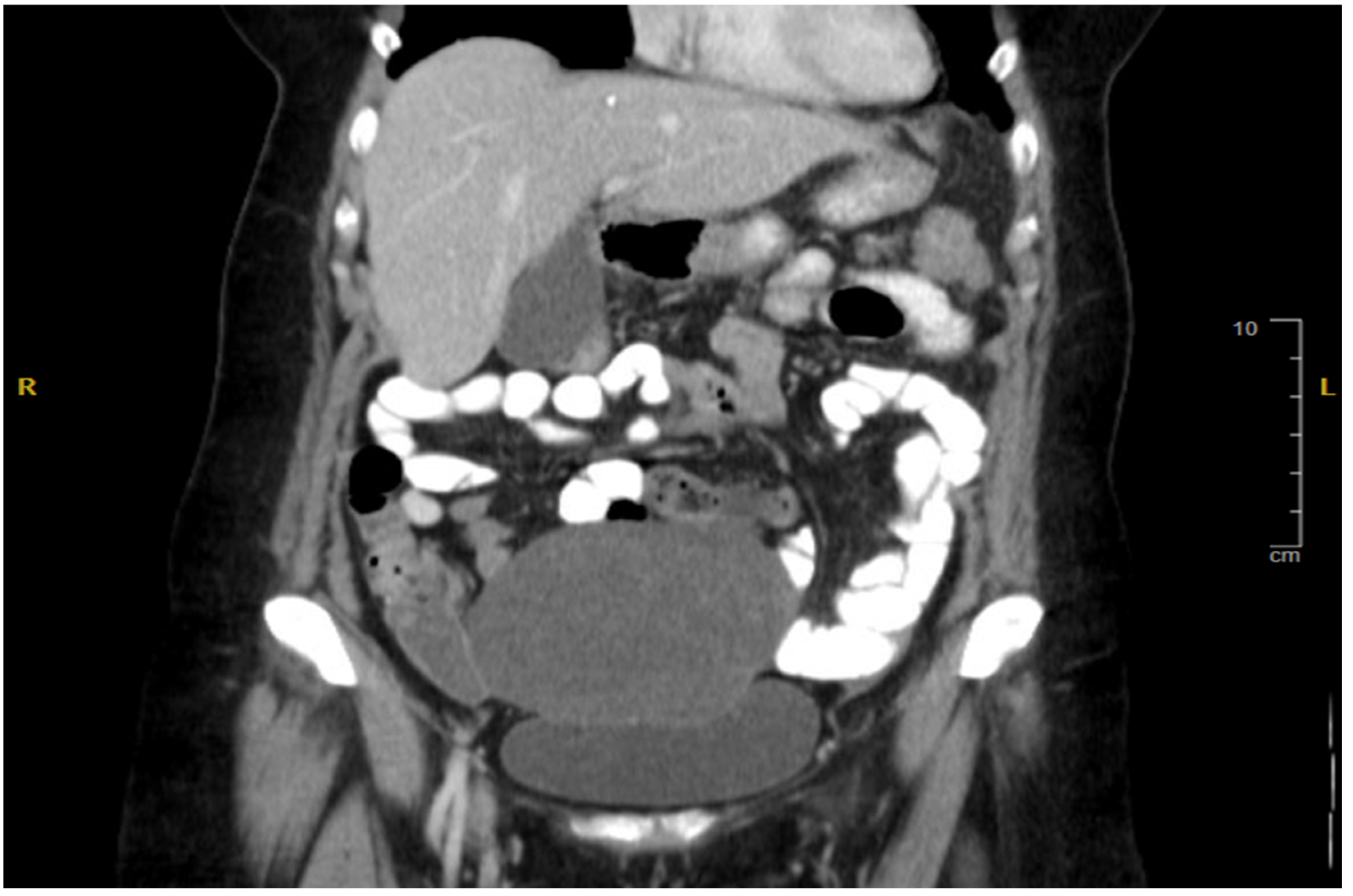
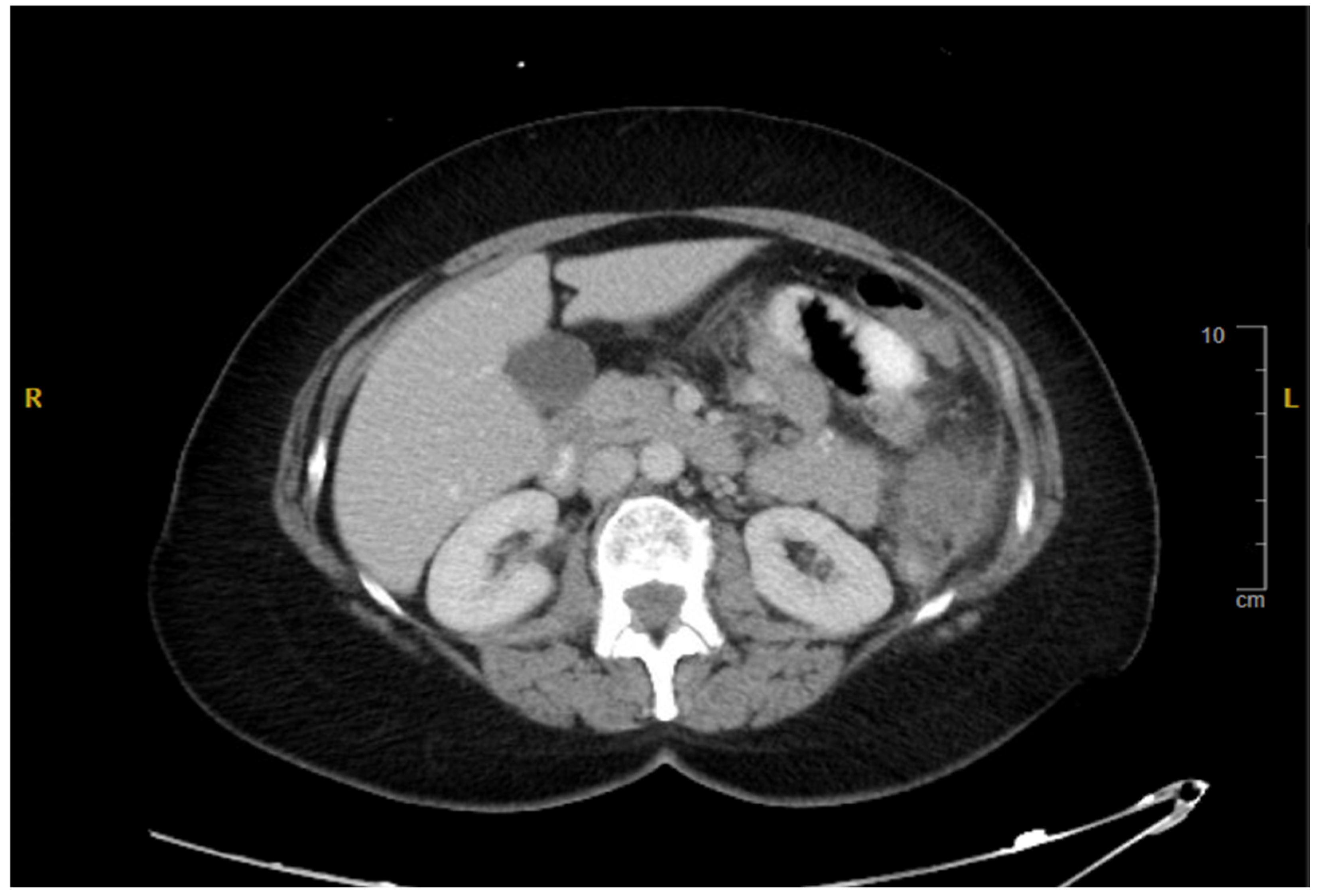
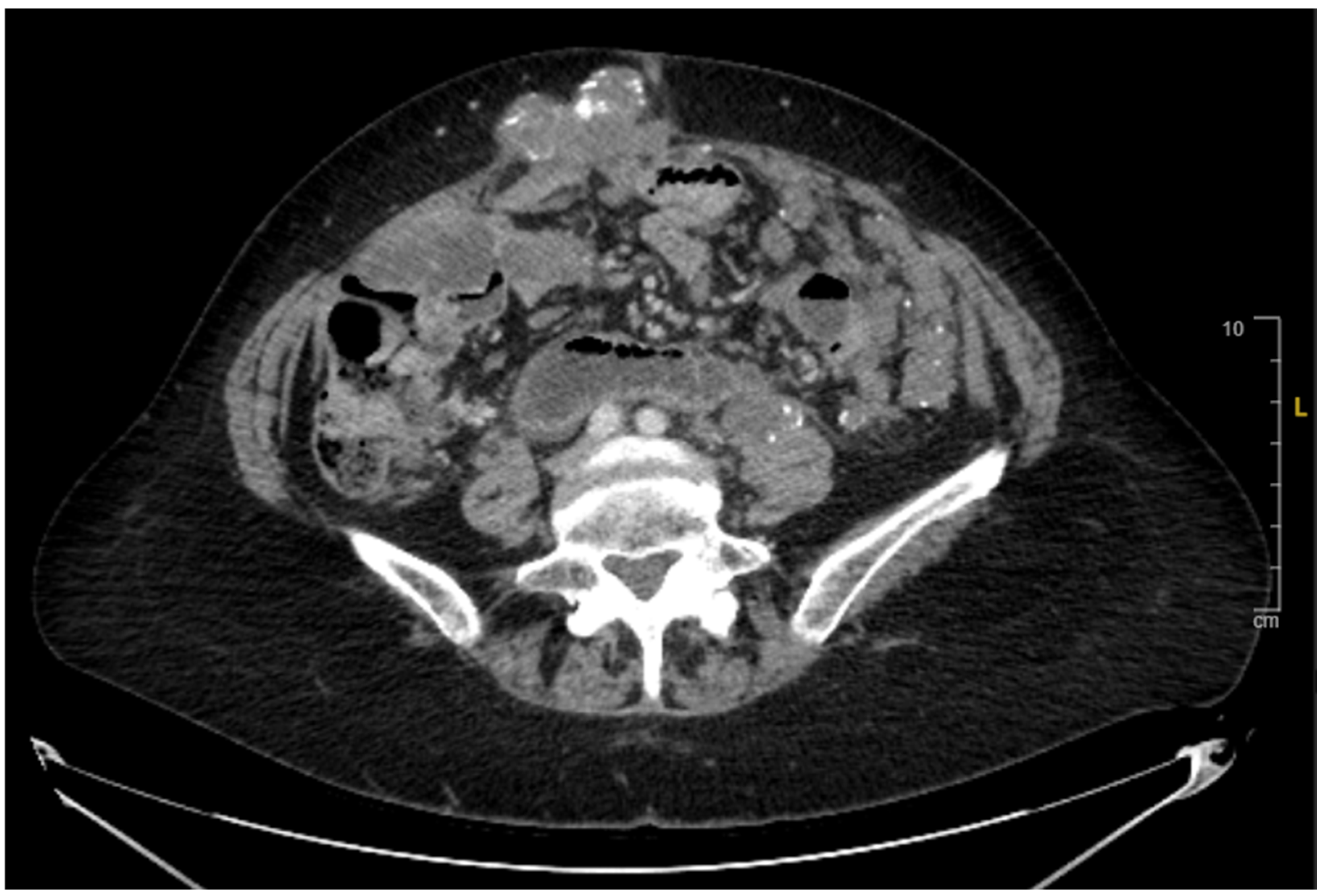
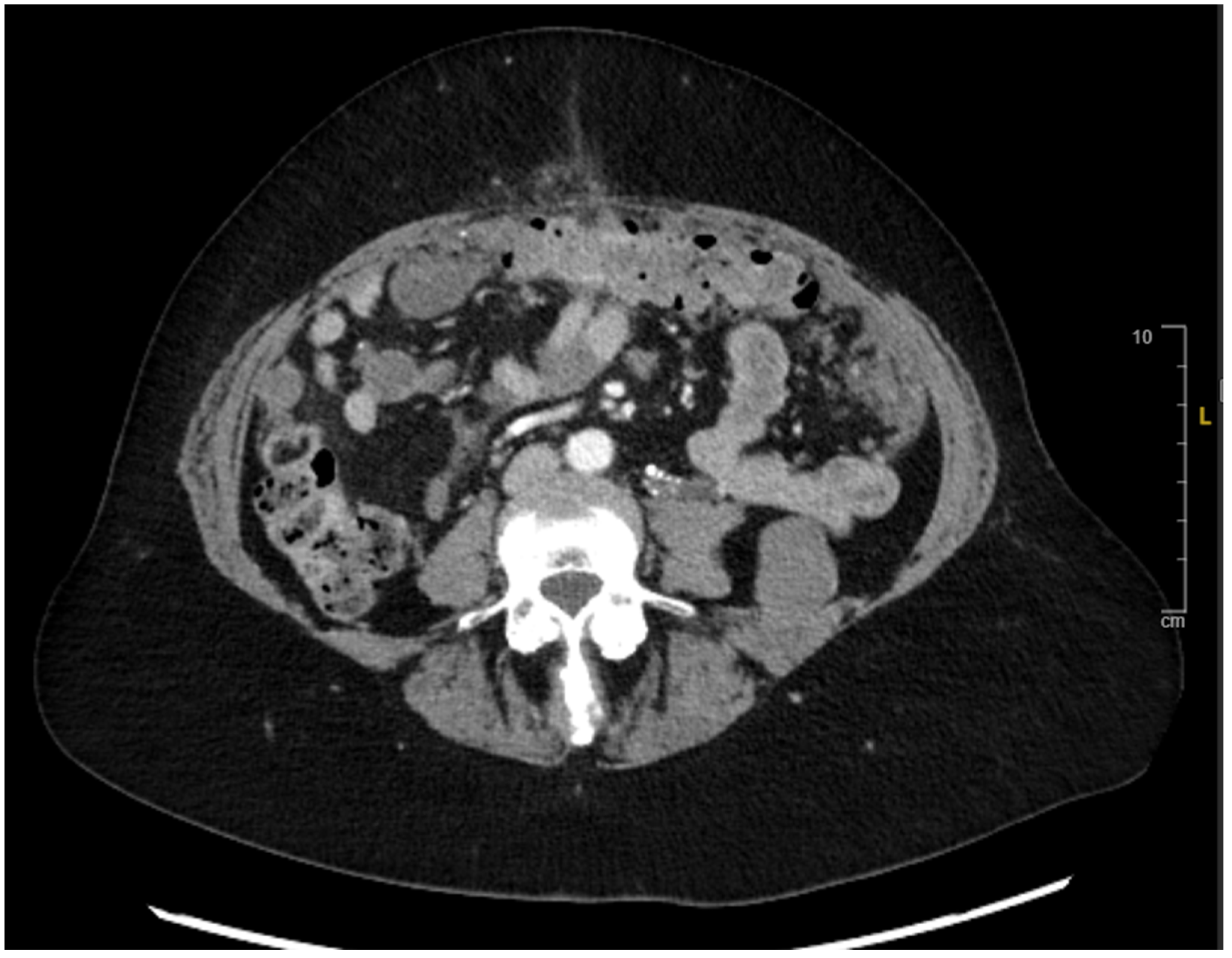
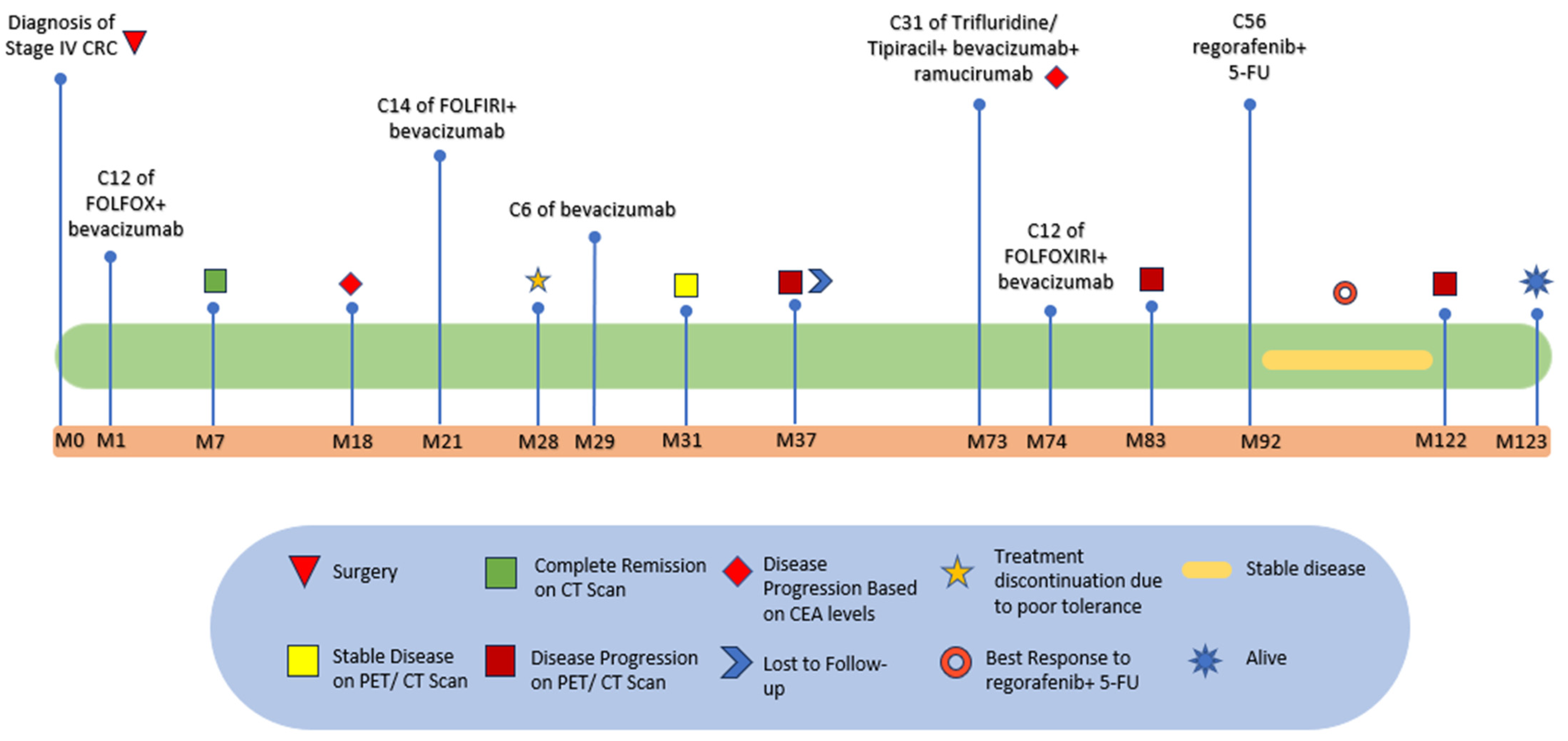
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcellinaro, R.; Spoletini, D.; Grieco, M.; Avella, P.; Cappuccio, M.; Troiano, R.; Lisi, G.; Garbarino, G.M.; Carlini, M. Colorectal Cancer: Current Updates and Future Perspectives. J. Clin. Med. 2023, 13, 40. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Zhao, L.; Fu, X.; Shang, Y.; Gao, Q. Long-term survival of a patient with microsatellite-stable refractory colorectal cancer with regorafenib and PD-1 inhibitor sintilimab: A case report and review of literature. BMC Gastroenterol. 2021, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Kunst, N.; Alarid-Escudero, F.; Aas, E.; Coupé, V.M.; Schrag, D.; Kuntz, K.M. Estimating population-based recurrence rates of colorectal cancer over time in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Esmail, A.; Abdelrahim, M. Triple-Regimen of Vemurafenib, Irinotecan, and Cetuximab for the Treatment of BRAFV600E-Mutant.CRC: A Case Report and Review. Front. Pharmacol. 2021, 12, 795381. [Google Scholar] [CrossRef]
- Schuell, B.; Gruenberger, T.; Kornek, G.; Dworan, N.; Depisch, D.; Lang, F.; Schneeweiss, B.; Scheithauer, W. Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br. J. Cancer 2005, 93, 744–748. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: Underlying mechanisms and reversal strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Cutsem, E.V.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine–Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef]
- FDA approves regorafenib (Stivarga) for metastatic colorectal cancer. Oncology 2012, 26, 896. [PubMed]
- Fondevila, F.; Méndez-Blanco, C.; Fernández-Palanca, P.; González-Gallego, J.; Mauriz, J.L. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Martinelli, E.; Cascinu, S.; Sobrero, A.; Banzi, M.; Seitz, J.F.; Barone, C.; Ychou, M.; Peeters, M.; Brenner, B. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: Results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncology 2019, 24, 185–192. [Google Scholar] [CrossRef]
- Prince, G.T.; Cameron, M.C.; Fathi, R.; Alkousakis, T. Topical 5-fluorouracil in dermatologic disease. Int. J. Dermatol. 2018, 57, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Afzal, S.; Jensen, S.; Vainer, B.; Vogel, U.; Matsen, J.; Sørensen, J.; Andersen, P.K.; Poulsen, H. MTHFR polymorphisms and 5-FU-based adjuvant chemotherapy in colorectal cancer. Ann. Oncol. 2009, 20, 1660–1666. [Google Scholar] [CrossRef]
- Canman, C.E.; Tang, H.-Y.; Normolle, D.P.; Lawrence, T.S.; Maybaum, J. Variations in patterns of DNA damage induced in human colorectal tumor cells by 5-fluorodeoxyuridine: Implications for mechanisms of resistance and cytotoxicity. Proc. Natl. Acad. Sci. USA 1992, 89, 10474–10478. [Google Scholar] [CrossRef]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Zhu, D.; Yu, Q.; Zhang, Z.; Sun, M.; Cai, S.; Zhang, W. Association of single nucleotide polymorphisms in MTHFR and ABCG2 with the different efficacy of first-line chemotherapy in metastatic colorectal cancer. Med. Oncol. 2014, 31, 802. [Google Scholar] [CrossRef] [PubMed]
- Marks, E.I.; Tan, C.; Zhang, J.; Zhou, L.; Yang, Z.; Scicchitano, A.; El-Deiry, W.S. Regorafenib with a fluoropyrimidine for metastatic colorectal cancer after progression on multiple 5-FU-containing combination therapies and regorafenib monotherapy. Cancer Biol. Ther. 2015, 16, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-H.; Xu, X.-G.; Yan, S.-L.; Sun, Z.; Ying, Y.; Wang, B.-K.; Tu, Y.-X. Regorafenib suppresses colon tumorigenesis and the generation of drug resistant cancer stem-like cells via modulation of miR-34a associated signaling. J. Exp. Clin. Cancer Res. 2018, 37, 151. [Google Scholar] [CrossRef] [PubMed]
- Baik, H.; Lee, H.J.; Park, J.; Park, H.Y.; Park, J.; Lee, S.; Bae, K.B. Complete response of MSI-high metastatic colon cancer following treatment with regorafenib: A case report. Mol. Clin. Oncol. 2021, 15, 243. [Google Scholar] [CrossRef]
- Calcagno, F.; Lenoble, S.; Lakkis, Z.; Nguyen, T.; Limat, S.; Borg, C.; Jary, M.; Kim, S.; Nerich, V. Efficacy, safety and cost of regorafenib in patients with metastatic colorectal cancer in French clinical practice. Clin. Med. Insights Oncol. 2016, 10, CMO–S38335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmail, A.; Khasawneh, B.; Al-Najjar, E.; Zaidan, R.; Abdelrahim, M. Prolonged Survival Outcome in a Patient with Refractory Metastatic Colorectal Cancer Treated with Regorafenib Plus 5-Fluorouracil: A Case Report and Literature Review. Reports 2025, 8, 59. https://doi.org/10.3390/reports8020059
Esmail A, Khasawneh B, Al-Najjar E, Zaidan R, Abdelrahim M. Prolonged Survival Outcome in a Patient with Refractory Metastatic Colorectal Cancer Treated with Regorafenib Plus 5-Fluorouracil: A Case Report and Literature Review. Reports. 2025; 8(2):59. https://doi.org/10.3390/reports8020059
Chicago/Turabian StyleEsmail, Abdullah, Bayan Khasawneh, Ebtesam Al-Najjar, Raed Zaidan, and Maen Abdelrahim. 2025. "Prolonged Survival Outcome in a Patient with Refractory Metastatic Colorectal Cancer Treated with Regorafenib Plus 5-Fluorouracil: A Case Report and Literature Review" Reports 8, no. 2: 59. https://doi.org/10.3390/reports8020059
APA StyleEsmail, A., Khasawneh, B., Al-Najjar, E., Zaidan, R., & Abdelrahim, M. (2025). Prolonged Survival Outcome in a Patient with Refractory Metastatic Colorectal Cancer Treated with Regorafenib Plus 5-Fluorouracil: A Case Report and Literature Review. Reports, 8(2), 59. https://doi.org/10.3390/reports8020059







