Transoral Videolaryngoscopic Surgery for an Undifferentiated Pleomorphic Sarcoma of the Tongue Base: A Case Report
Abstract
1. Introduction and Clinical Significance
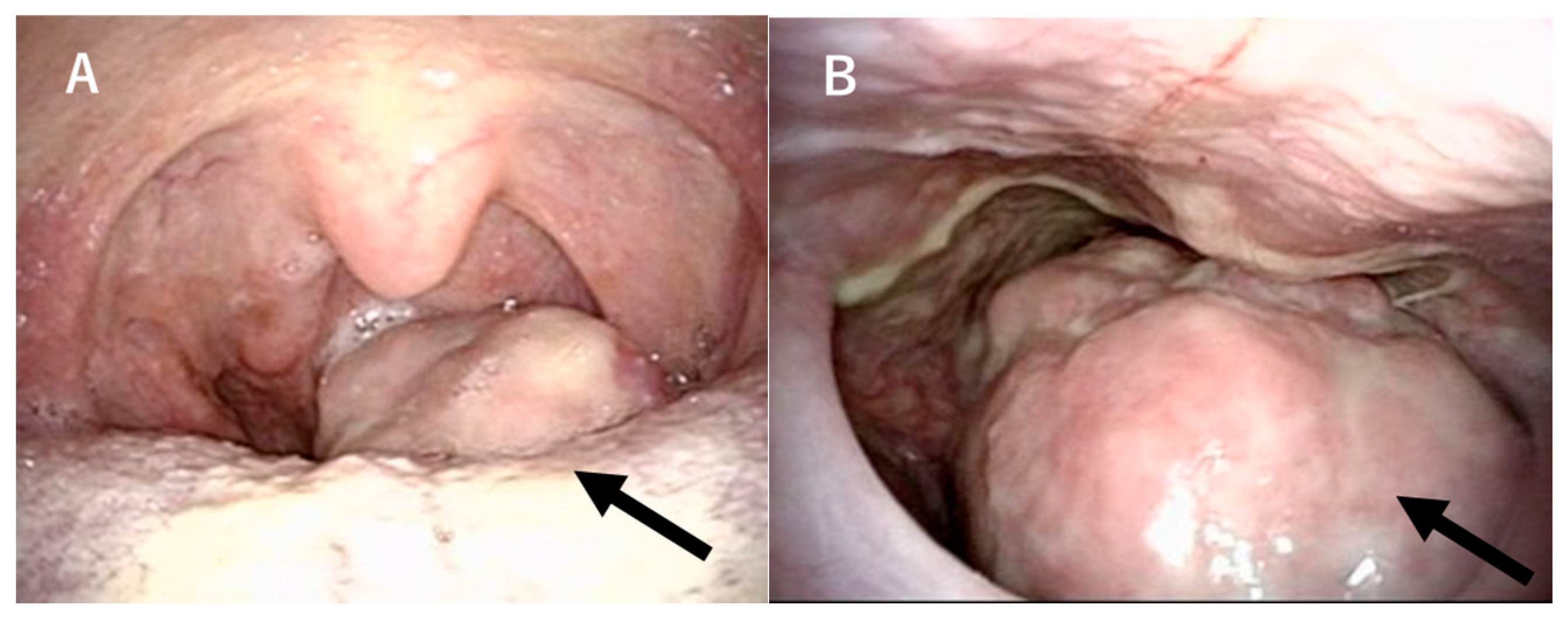
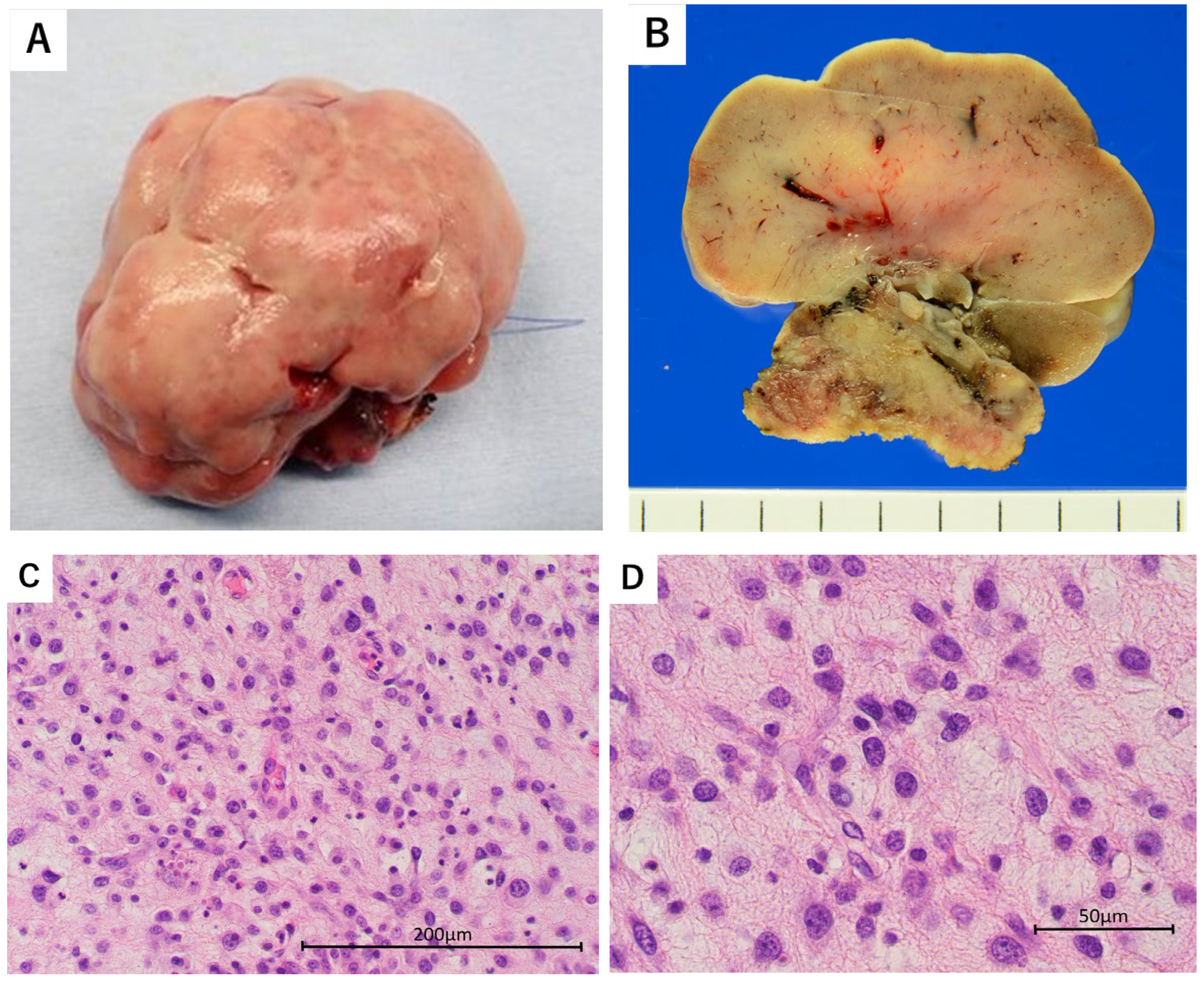
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UPS | Undifferentiated pleomorphic sarcoma |
| MFH | Malignant fibrous histiocytoma |
| TOVS | Transoral videolaryngoscopic surgery |
| TORS | Transoral robotic surgery |
References
- The World Health Organization. Soft Tissue and Bone Tumours—WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Weiss, S.W.; Enzinger, F.M. Malignant fibrous histiocytoma: An analysis of 200 cases. Cancer 1978, 41, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.W.; Moore, B.A.; Patel, S.R.; Guadagnolo, B.A.; Roberts, D.B.; Sturgis, E.M. Malignant fibrous histiocytoma of the head and neck region. Head Neck 2011, 33, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sadati, K.S.; Haber, M.; Sataloff, R.T. Malignant fibrous histiocytoma of the head and neck after radiation for squamous cell carcinoma. Ear. Nose Throat J. 2004, 83, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Dekanić, A.; Velepič, M.; Gobić, M.B.; Hadžisejdić, I.; Jonjić, N. Undifferentiated pleomorphic sarcoma in oropharyngeal mucosa of patients with multiple basal cell carcinomas. Rare Tumors 2021, 13, 20363613211026483. [Google Scholar] [CrossRef]
- Sabesan, T.; Xuexi, W.; Yongfa, Q.; Pingzhang, T.; Ilankovan, V. Malignant fibrous histiocytoma: Outcome of tumours in the head and neck compared with those in the trunk and extremities. Br. J. Oral Maxillofac. Surg. 2006, 44, 209–212. [Google Scholar] [CrossRef]
- Boccalatte, L.-A.; Gomez, N.-L.; Yanzon, A.; Mazzaro, E.-L.; Cayol, F.; Figari, M.-F. Head and Neck Tumors: Management of Primary Undifferentiated Pleomorphic Sarcoma. Iran. J. Otorhinolaryngol. 2019, 31, 335–342. [Google Scholar]
- Crago, A.M.; Cardona, K.; Koseła-Paterczyk, H.; Rutkowski, P. Management of Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma. Surg. Oncol. Clin. N. Am. 2022, 31, 419–430. [Google Scholar] [CrossRef]
- Sayin, I.; Fakhoury, R.; Prasad, V.M.N.; Remacle, M.; Lawson, G. Transoral robotic surgery for base of tongue neoplasms. B-ENT 2015, 11 (Suppl. S24), 45–50. [Google Scholar]
- Shiotani, A.; Tomifuji, M.; Araki, K.; Yamashita, T.; Saito, K. Videolaryngoscopic transoral en bloc resection of supraglottic and hypopharyngeal cancers using laparoscopic surgical instruments. Ann. Otol. Rhinol. Laryngol. 2010, 119, 225–232. [Google Scholar] [CrossRef]
- Tomifuji, M.; Araki, K.; Yamashita, T.; Shiotani, A. Transoral videolaryngoscopic surgery for oropharyngeal, hypopharyngeal, and supraglottic cancer. Eur. Arch. Otorhinolaryngol. 2014, 271, 589–597. [Google Scholar] [CrossRef]
- Tirelli, G.; Nata, F.B.; Piovesana, M.; Quatela, E.; Gardenal, N.; Hayden, R.E. Transoral surgery (TOS) in oropharyngeal cancer: Different tools, a single mini-invasive philosophy. Surg. Oncol. 2018, 27, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, A.; Iyer, N.G. Radiation-induced sarcomas of the head and neck. World J. Clin. Oncol. 2014, 5, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.A.; Davis, P.L., 3rd; Browne, J.D. Malignant fibrous histiocytoma of the head and neck: A case series. Am. J. Otolaryngol. 2013, 34, 10–15. [Google Scholar] [CrossRef]
- Fujiwara, T.; Stevenson, J.; Parry, M.; Tsuda, Y.; Tsoi, K.; Jeys, L. What is an adequate margin for infiltrative soft-tissue sarcomas? Eur. J. Surg. Oncol. 2020, 46, 277–281. [Google Scholar] [CrossRef]
- Van Abel, K.M.; Moore, E.J. Surgical management of the base of tongue. Oper. Tech. Otolaryngol.-Head Neck Surg. 2013, 24, 74–85. [Google Scholar] [CrossRef]
- Richmon, J.D.; Quon, H.; Gourin, C.G. The effect of transoral robotic surgery on short-term outcomes and cost of care after oropharyngeal cancer surgery. Laryngoscope 2014, 124, 165–171. [Google Scholar] [CrossRef]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; Delaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef]
- Belal, A.; Kandil, A.; Allam, A.; Khafaga, Y.; El-Husseiny, G.; El-Enbaby, A.; Memon, M.; Younge, D.; Moreau, P.; Gray, A.; et al. Malignant fibrous histiocytoma: A retrospective study of 109 cases. Am. J. Clin. Oncol. 2002, 25, 16–22. [Google Scholar] [CrossRef]
- Baldini, E.H.; Goldberg, J.; Jenner, C.; Manola, J.B.; Demetri, G.D.; Fletcher, C.D.; Singer, S. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J. Clin. Oncol. 1999, 17, 3252–3259. [Google Scholar] [CrossRef]
- Lin, S.K.; How, S.W.; Wang, J.T.; Liu, B.Y.; Chiang, C.P. Oral post-radiation malignant fibrous histiocytoma: A clinicopathological study. J. Oral Pathol. Med. 1994, 23, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Zapater, E.; Garcia, M.; Perez, J.; Lopez, R.; Alvarez, S.; Martinez, A.; Gomez, P.; Sanchez, L.; Ruiz, F.; Torres, M. Malignant fibrous histiocytoma of the head and neck. Bull Group Int. Rech Sci. Stomatol. Odontol. 1995, 38, 121–124. [Google Scholar] [PubMed]
- Chen, Y.K.; Lin, L.M.; Lin, C.C. Malignant fibrous histiocytoma of the tongue. J. Laryngol. Otol. 2001, 115, 763–765. [Google Scholar] [CrossRef]
- Wiesmiller, K.; Barth, T.F.; Gronau, S. Early radiation-induced malignant fibrous histiocytoma of the oral cavity. J. Laryngol. Otol. 2003, 117, 224–226. [Google Scholar] [CrossRef]
- Rapidis, A.D.; Andressakis, D.D.; Lagogiannis, G.A.; Douzinas, E.E. Malignant fibrous histiocytoma of the tongue: Review of the literature and report of a case. J. Oral Maxillofac. Surg. 2005, 63, 546–550. [Google Scholar] [CrossRef]
- Alfredo, E.; de Pádua, J.M.; Vicentini, E.L.; Marchesan, M.A.; Lia, R.C.C.; da Cruz Perez, D.E.; Silva-Sousa, Y.T.C. Oral undifferentiated high-grade pleomorphic sarcoma: Report of a case. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, e37–e40. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Lv, Y.; Li, Z. Malignant fibrous histiocytoma of the floor of mouth: A case report and review of the literature. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e106–e111. [Google Scholar] [CrossRef]
- Sharma, A.; Vyas, P.; Agarwal, D. Undifferentiated pleomorphic sarcoma of the floor of mouth: A rare case. J. Oral Maxillofac. Pathol. 2023, 27 (Suppl. S1), S33–S37. [Google Scholar]
- Tanaka, K.; Kawano, M.; Iwasaki, T.; Itonaga, I.; Tsumura, H. A meta-analysis of randomized controlled trials that compare standard doxorubicin with other first-line chemotherapies for advanced/metastatic soft tissue sarcomas. PLoS ONE 2019, 14, e0210671. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]


| Article | Age; Gender | Tumor Location | Long Diameter (cm) | Prior Radiation | Primary Treatment | Resection Margin | Adjuvant Therapy | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Lin et al., 1994 [22] | 57; Male | Tongue | ND | 2.5 y ago | Surgical excision | ND | No | 6 m rec; 26 m DOD |
| Zapater et al., 1995 [23] | 71; Male | Tongue | 4 | No | RT | NA | No | 8 m rec; 9 m DOD |
| Chen et al., 2001 [24] | 16; Female | Tongue | 2.5 | No | Surgical excision | Negative | No | 18 m NER |
| Wiesmiller et al., 2003 [25] | 79; Male | Tongue | 3 | 5 y ago | Surgical excision | ND | No | 6 m NER |
| Rapidis et al., 2005 [26] | 24; Male | Tongue | 2.6 | No | Surgical excision | 1 cm | No | 18 m NER |
| Alfredo et al., 2008 [27] | 56; Male | Oral floor | 4.5 | No | Surgical excision | Wide margin | RT | 25 m NER |
| Wu et al., 2022 [28] | 49; Male | Oral floor | 5 | No | Surgical excision | 2 cm | RT CT | 26 m NER |
| Sharma et al., 2023 [29] | 50; Female | Oral floor | 4.5 | No | Surgical excision | Wide margin | No | ND |
| Sadati et al., 2004 [4] | 60; Male | Lateral oropharyngeal wall | ND | 10 y ago | No (best supportive care) | NA | No | 2 m DOD |
| Dekanić et al., 2021 [5] | 83; Male | Posterior oropharyngeal wall | 2.5 | No | Surgical excision | ND | No | ND |
| Taruya et al., 2025 | 68; Male | Tongue base | 5 | No | Surgical excision | 1 cm | No | 50 m NER |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taruya, T.; Hamamoto, T.; Ueda, T.; Chikuie, N.; Takeno, S. Transoral Videolaryngoscopic Surgery for an Undifferentiated Pleomorphic Sarcoma of the Tongue Base: A Case Report. Reports 2025, 8, 58. https://doi.org/10.3390/reports8020058
Taruya T, Hamamoto T, Ueda T, Chikuie N, Takeno S. Transoral Videolaryngoscopic Surgery for an Undifferentiated Pleomorphic Sarcoma of the Tongue Base: A Case Report. Reports. 2025; 8(2):58. https://doi.org/10.3390/reports8020058
Chicago/Turabian StyleTaruya, Takayuki, Takao Hamamoto, Tsutomu Ueda, Nobuyuki Chikuie, and Sachio Takeno. 2025. "Transoral Videolaryngoscopic Surgery for an Undifferentiated Pleomorphic Sarcoma of the Tongue Base: A Case Report" Reports 8, no. 2: 58. https://doi.org/10.3390/reports8020058
APA StyleTaruya, T., Hamamoto, T., Ueda, T., Chikuie, N., & Takeno, S. (2025). Transoral Videolaryngoscopic Surgery for an Undifferentiated Pleomorphic Sarcoma of the Tongue Base: A Case Report. Reports, 8(2), 58. https://doi.org/10.3390/reports8020058






