Intraoral Immature Malignant Teratoma with No Evidence of Other Sites of Involvement in a 6-Year-Old Patient: A Case Report
Abstract
1. Introduction and Clinical Significance
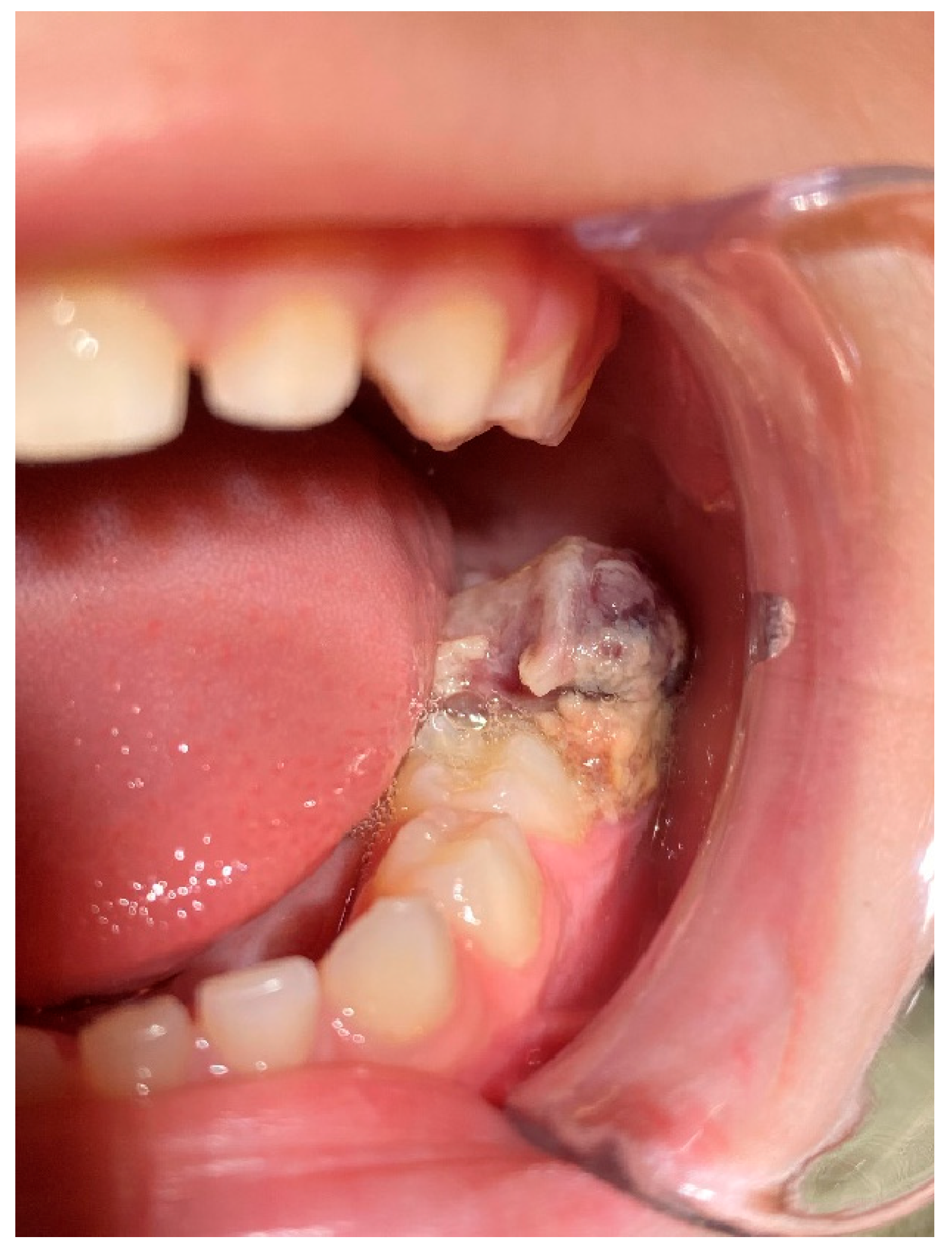
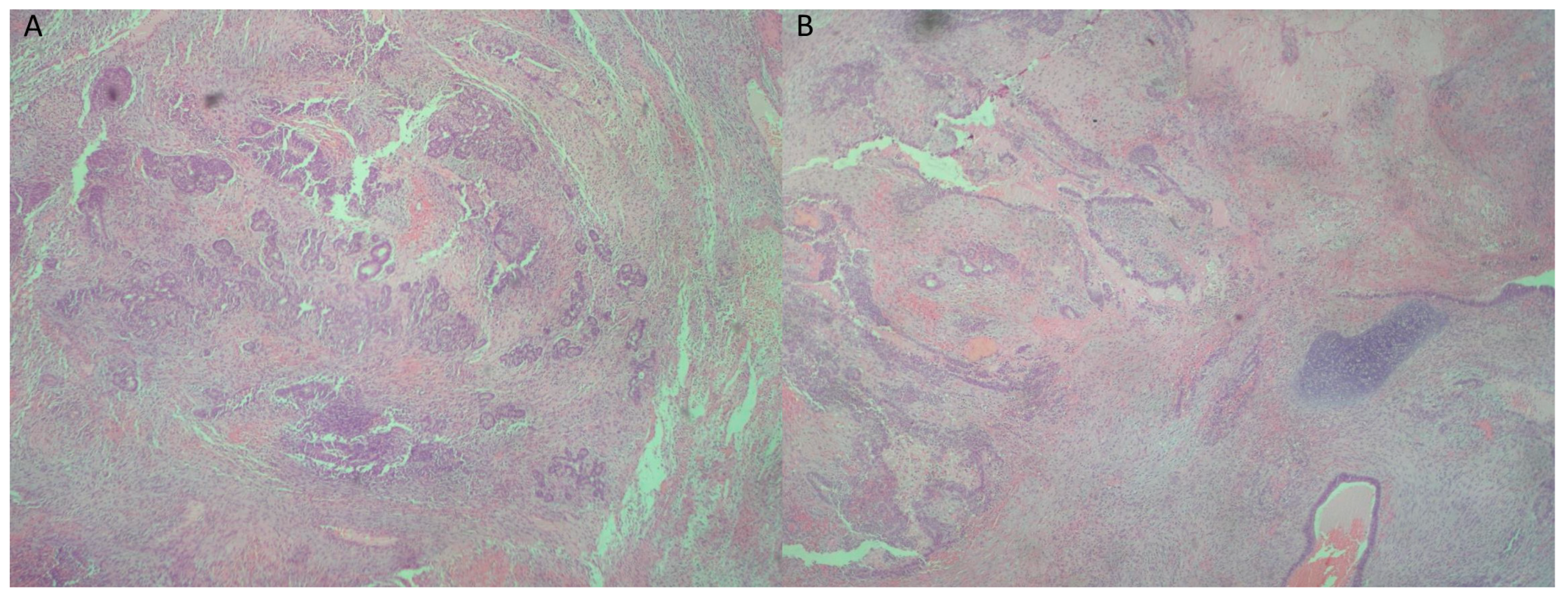
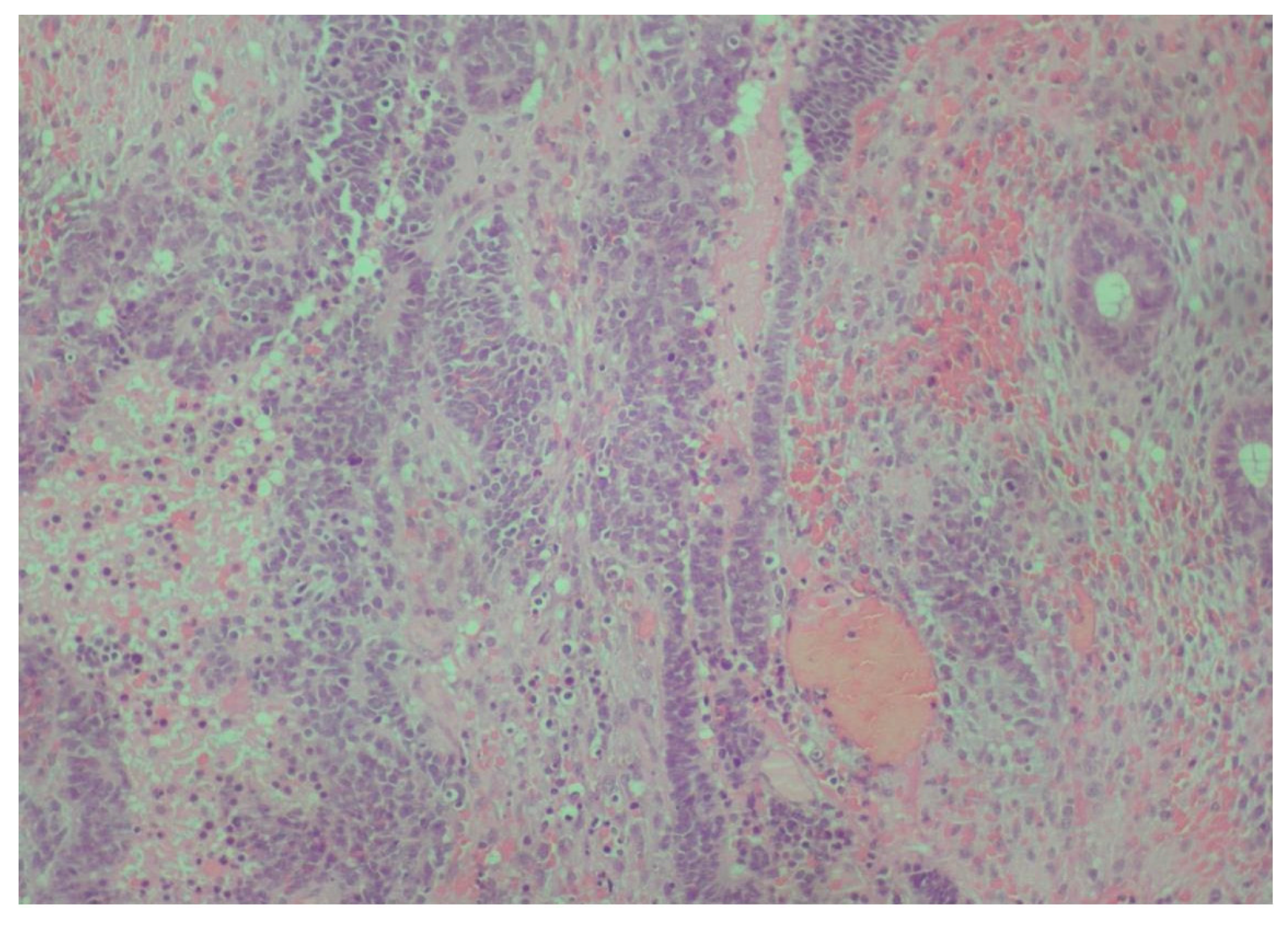
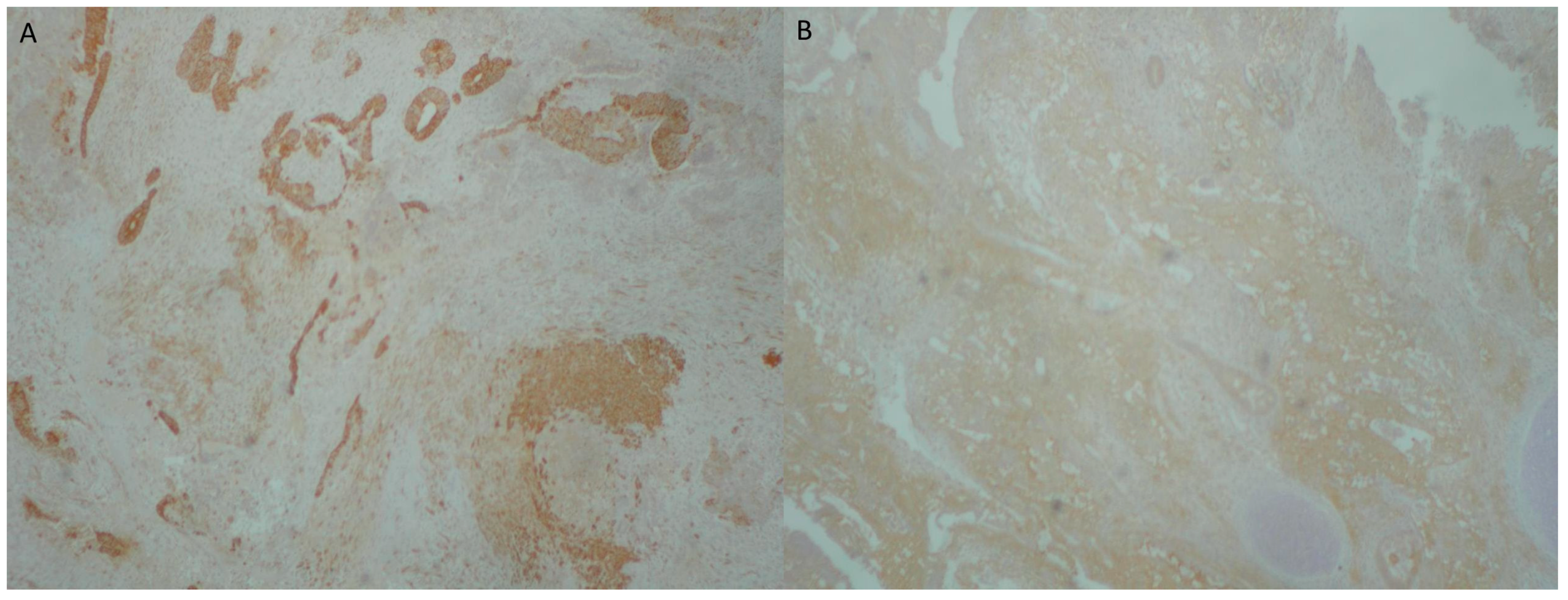
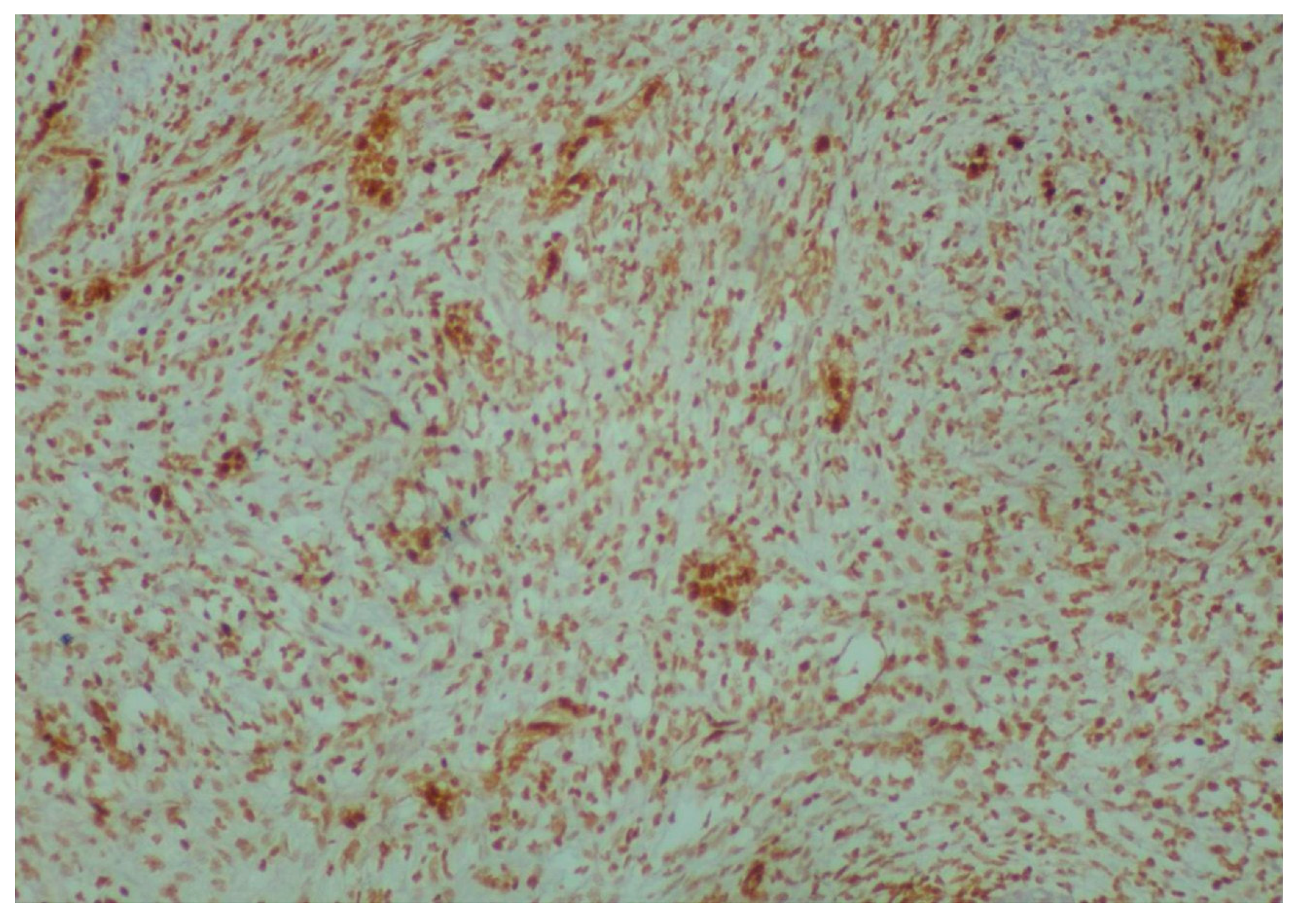
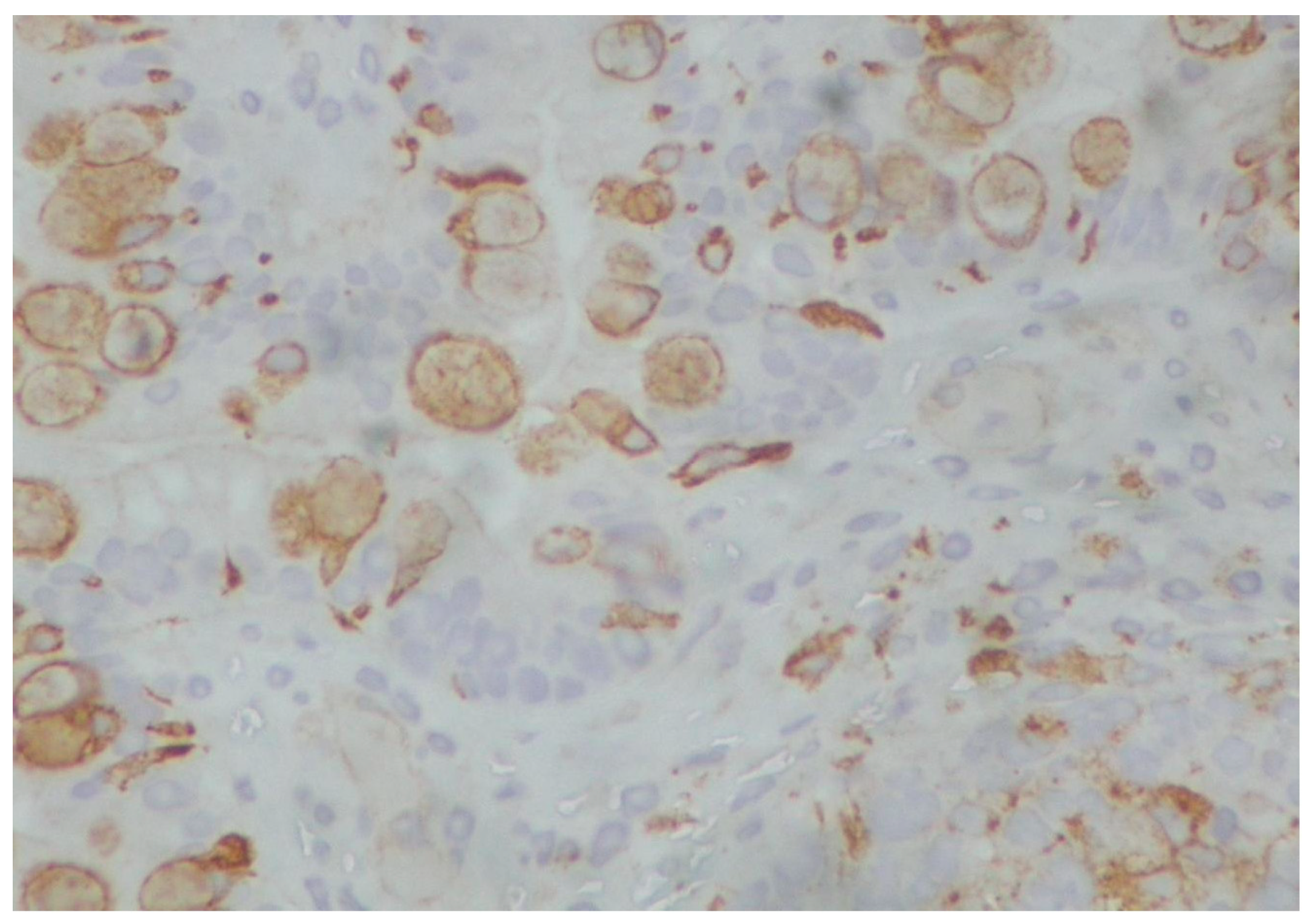
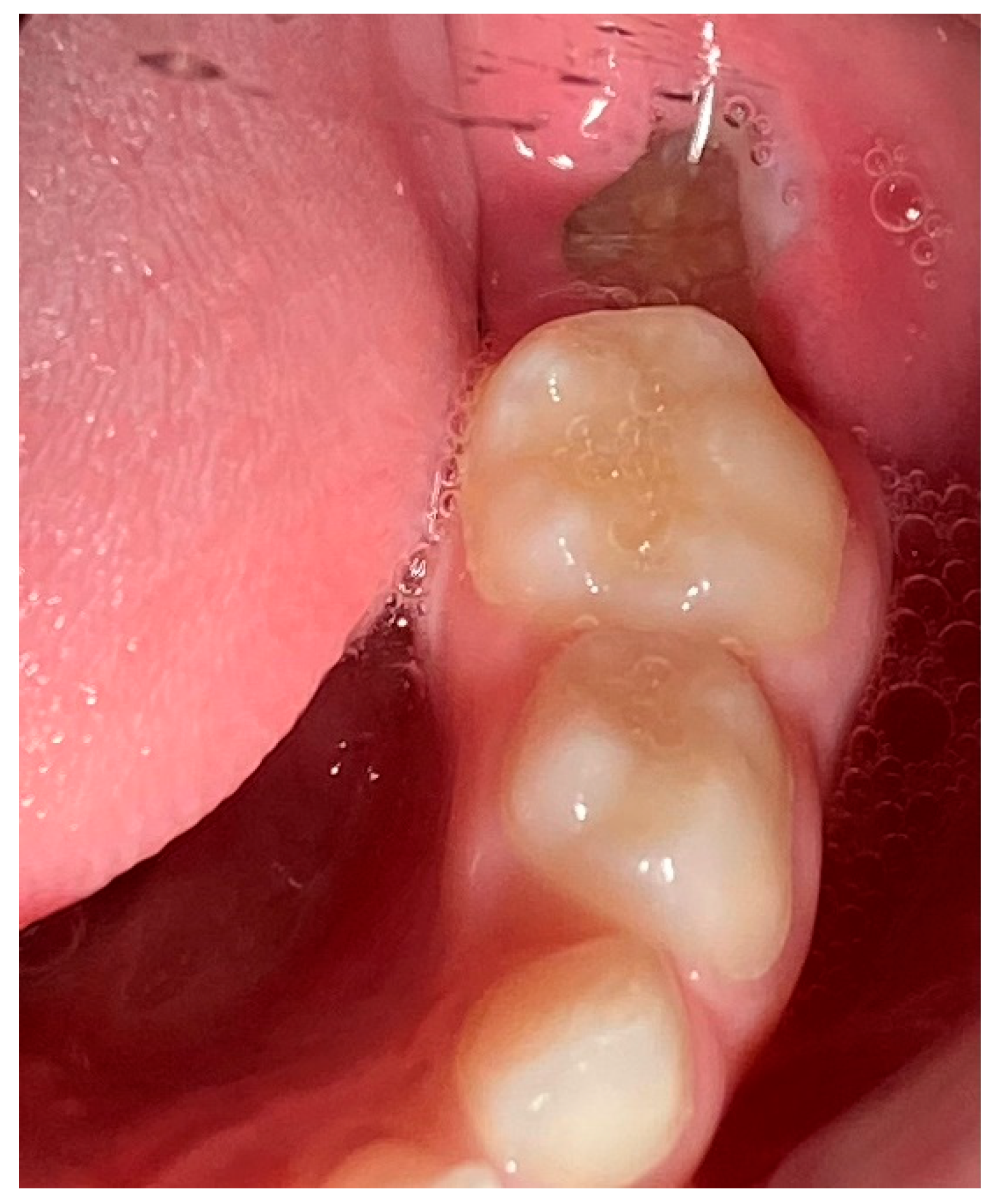
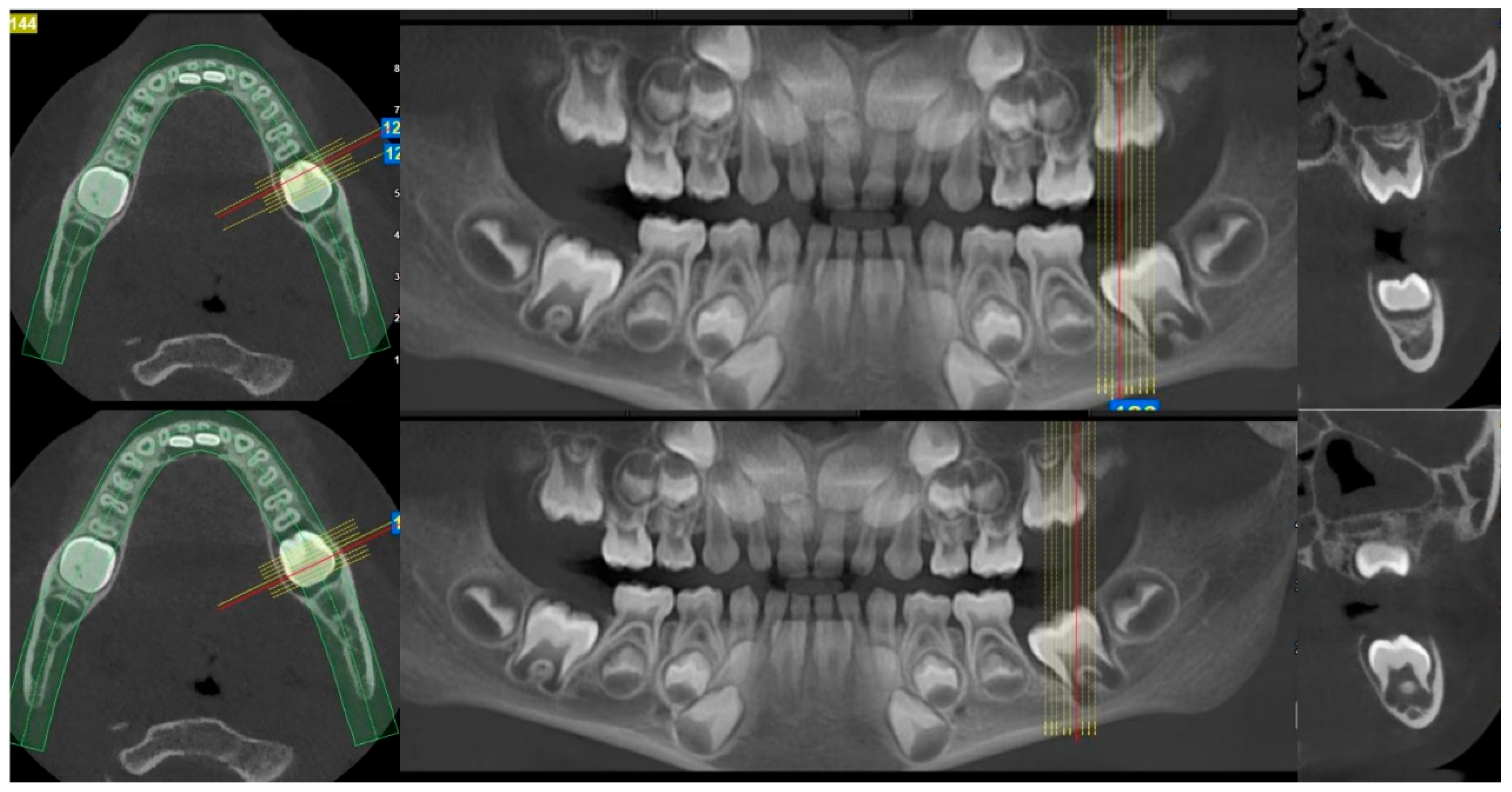
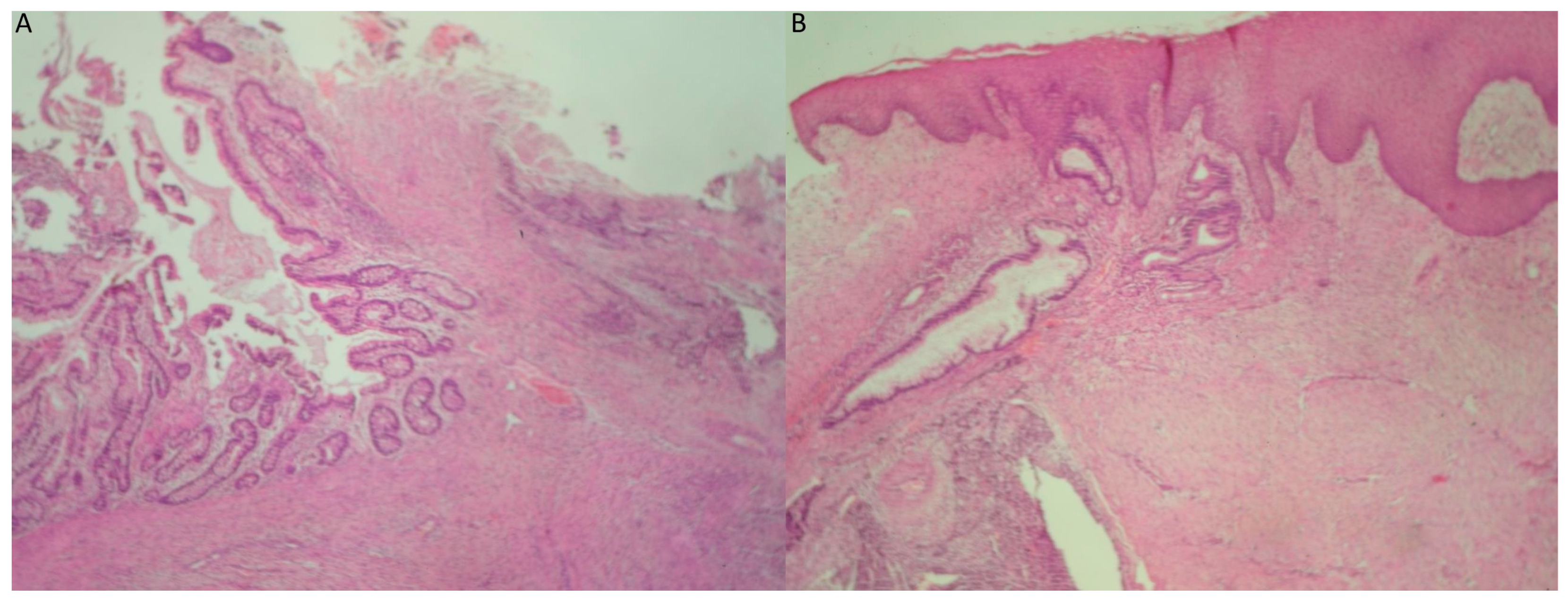
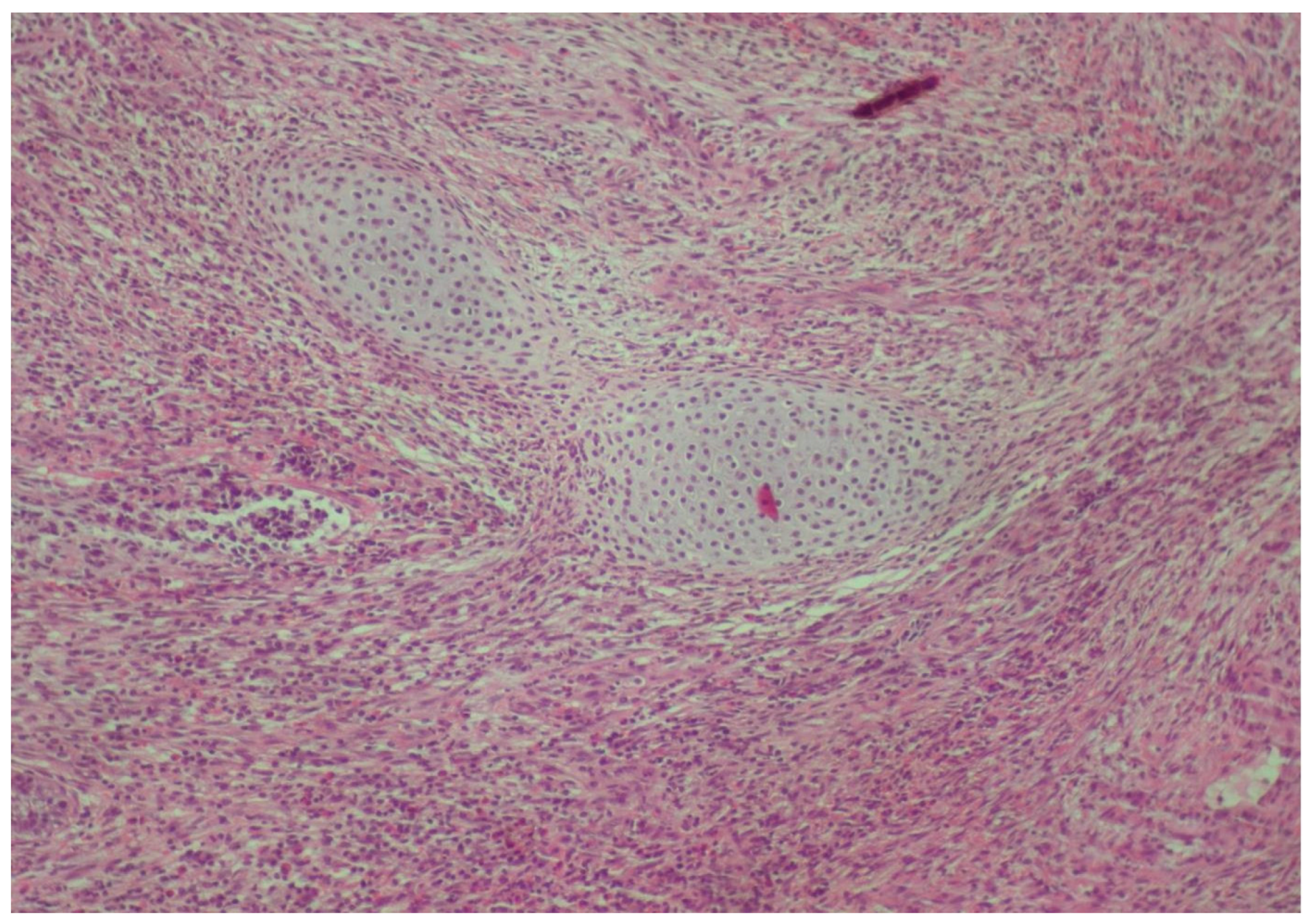
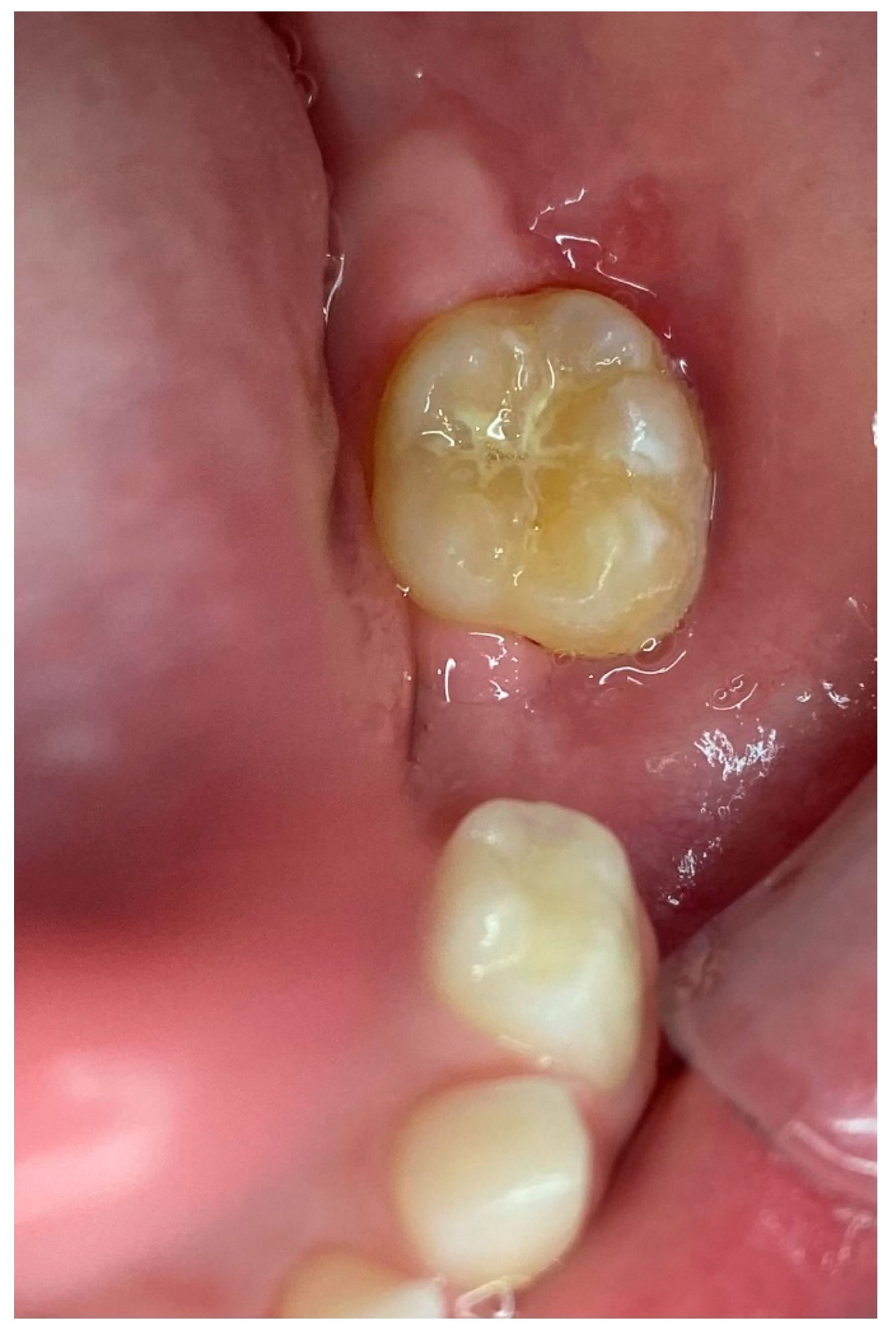
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakravarti, A.; Shashidhar, T.B.; Naglot, S.; Sahni, J.K. Head and Neck Teratomas in Children: A Case Series. Indian. J. Otolaryngol. Head. Neck Surg. 2011, 63, 193. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kunnath, A.J.; Gallant, J.; Belcher, R.H. Surgical Management and Outcomes of Pediatric Congenital Head and Neck Teratomas: A Scoping Review. OTO Open 2023, 7, e66. [Google Scholar] [CrossRef] [PubMed]
- Sahai, A.; Narkhede, K.; Shriwastav, P.; Gaikwad, A.; Marfatia, H. Mature Cystic Oral Teratoma in a Neonate- A Case Report. Indian. J. Otolaryngol. Head. Neck Surg. 2024, 76, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Morency, E.G.; Lerner, D.; Garcia, R.; Kalir, T. High-grade sarcoma masquerading as growing teratoma syndrome after resection of ovarian immature teratoma: Report of a case. Int. J. Gynecol. Pathol. 2012, 31, 276–279. [Google Scholar] [CrossRef]
- Narayanan, G.; Kamala, L.H.; Nair, S.G.; Purushothaman, P.N.; Kumar, A.; Kattoor, J. Ewing’s sarcoma in adolescents and adults—10-year experience from a tertiary cancer center in India. J. Cancer Res. Ther. 2024, 20, 79–84. [Google Scholar] [CrossRef]
- Livellara, V.; Bergamaschi, L.; Puma, N.; Chiaravalli, S.; Podda, M.; Casanova, M.; Gasparini, P.; Pecori, E.; Alessandro, O.; Nigro, O.; et al. Extraosseous Ewing sarcoma in children and adolescents: A retrospective series from a referral pediatric oncology center. Pediatr. Blood Cancer 2022, 69, e29512. [Google Scholar] [CrossRef]
- Davido, N.; Rigolet, A.; Kerner, S.; Gruffaz, F.; Boucher, Y. Case of Ewing’s sarcoma misdiagnosed as a periapical lesion of maxillary incisor. J. Endod. 2011, 37, 259–264. [Google Scholar] [CrossRef]
- Chavan, M.; Dhakal, S.; Singh, A.; Rai, V.; Arora, S.; Mallipeddi, M.C.; Das, A. Ewing sarcoma genomics and recent therapeutic advancements. Pediatr. Hematol. Oncol. J. 2023, 8, 50–65. [Google Scholar] [CrossRef]
- Khan, S.; Abid, Z.; Haider, G.; Bukhari, N.; Zehra, D.; Hashmi, M.; Abid, M.; Ibrahim, U. Incidence of Ewing’s Sarcoma in Different Age Groups, Their Associated Features, and Its Correlation With Primary Care Interval. Cureus 2021, 13, e13986. [Google Scholar] [CrossRef]
- Brazão-Silva, M.T.; Fernandes, A.V.; de Faria, P.R.; Cardoso, S.V.; Loyola, A.M. Ewing’s sarcoma of the mandible in a young child. Braz. Dent. J. 2010, 21, 74–79. [Google Scholar] [CrossRef]
- Ko, E.; Brouns, E.R.; Korones, D.N.; Pochal, W.F.; Philipone, E.M.; Zegarelli, D.J.; Yoon, A.J. Primary Ewing sarcoma of the anterior mandible localized to the midline. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e46–e50. [Google Scholar] [CrossRef] [PubMed]
- Margaix-Muñoz, M.; Bagán, J.; Poveda-Roda, R. Ewing sarcoma of the oral cavity. A review. J. Clin. Exp. Dent. 2017, 9, e294. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Bishop, J.A. Soft Tissue Special Issue: Adamantinoma-Like Ewing Sarcoma of the Head and Neck: A Practical Review of a Challenging Emerging Entity. Head. Neck Pathol. 2020, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Fletcher, C.D.M. p63 immunohistochemical staining is limited in soft tissue tumors. Am. J. Clin. Pathol. 2011, 136, 762–766. [Google Scholar] [CrossRef]
- Bishop, J.A.; Montgomery, E.A.; Westra, W.H. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and malignant mesenchymal processes. Am. J. Surg. Pathol. 2014, 38, 257–264. [Google Scholar] [CrossRef]
- Rooper, L.M.; Jo, V.Y.; Antonescu, C.R.; Nose, V.; Westra, W.H.; Seethala, R.R.; Bishop, J.A. Adamantinoma-like Ewing Sarcoma of the Salivary Glands: A Newly Recognized Mimicker of Basaloid Salivary Carcinomas. Am. J. Surg. Pathol. 2019, 43, 187–194. [Google Scholar] [CrossRef]
- Donohoe, E.; Martin, D.; Faul, P.; O’Sullivan, M.J.; Barry, T. Molecular confirmation of primary monophasic synovial sarcoma of the mandible: Diagnosis and management. Br. J. Oral. Maxillofac. Surg. 2022, 60, 994–996. [Google Scholar] [CrossRef]
- Khalili, M.; Eshghyar, N.; Ensani, F.; Shakib, P.A. Synovial sarcoma of the mandible. J. Res. Med. Sci. 2012, 17, 1082. [Google Scholar]
- Gazendam, A.M.; Popovic, S.; Munir, S.; Parasu, N.; Wilson, D.; Ghert, M. Synovial Sarcoma: A Clinical Review. Curr. Oncol. 2021, 28, 1909–1920. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, S.; Fu, S.; Hu, Y.; He, Y. Management of the primary intraosseous synovial sarcoma of the jaws: Be careful of the surgical margin. J. Oral Maxillofac. Surg. 2015, 73, 550–563. [Google Scholar] [CrossRef]
- Soltaninia, O.; Dehghan, A.; Fatehi, F.; Naderi, H. A rare intraosseous synovial sarcoma of the mandible: A case report. Int. J. Surg. Case Rep. 2024, 120, 109880. [Google Scholar] [CrossRef] [PubMed]
- Stanbouly, D.; Litman, E.; Lee, K.C.; Philipone, E. Synovial sarcoma of the head & neck: A review of reported cases in the literature. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Madabhavi, I.; Bhardawa, V.; Modi, M.; Patel, A.; Sarkar, M. Primary synovial sarcoma (SS) of larynx: An unusual site. Oral Oncol. 2018, 79, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Agrogiannis, G.; Toutouzas, K.G. Primary immature teratoma of the thigh: A review. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 773–778. [Google Scholar] [CrossRef]
- Zuquello, R.Á.; Tagliari, G.; Bagatini, R.; Camiña, R.H.; Caron, R.; Lorencette, N.A.; Baptistella, A.R.; Manfro, G. Immature teratoma presenting as a soft-tissue mass with no evidence of other sites of involvement: A case report. Diagn. Pathol. 2016, 11, 1–5. [Google Scholar] [CrossRef][Green Version]
- Allam-Nandyala, P.; Bui, M.M.; Caracciolo, J.T.; Hakam, A. Squamous cell carcinoma and osteosarcoma arising from a dermoid cyst-a case report and review of literature. Int. J. Clin. Exp. Pathol. 2010, 3, 313. [Google Scholar]
- Djordjevic, B.; Euscher, E.D.; Malpica, A. Growing teratoma syndrome of the ovary: Review of literature and first report of a carcinoid tumor arising in a growing teratoma of the ovary. Am. J. Surg. Pathol. 2007, 31, 1913–1918. [Google Scholar] [CrossRef]
- Wang, S.S.; Jiang, J.; Liang, X.H.; Tang, Y.L. Links between cancer stem cells and epithelial–mesenchymal transition. Onco. Targets Ther. 2015, 8, 2973. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zisis, V.; Charisi, C.; Poulopoulos, K.; Papadopoulos, P.; Poulopoulos, A. Intraoral Immature Malignant Teratoma with No Evidence of Other Sites of Involvement in a 6-Year-Old Patient: A Case Report. Reports 2025, 8, 3. https://doi.org/10.3390/reports8010003
Zisis V, Charisi C, Poulopoulos K, Papadopoulos P, Poulopoulos A. Intraoral Immature Malignant Teratoma with No Evidence of Other Sites of Involvement in a 6-Year-Old Patient: A Case Report. Reports. 2025; 8(1):3. https://doi.org/10.3390/reports8010003
Chicago/Turabian StyleZisis, Vasileios, Christina Charisi, Konstantinos Poulopoulos, Petros Papadopoulos, and Athanasios Poulopoulos. 2025. "Intraoral Immature Malignant Teratoma with No Evidence of Other Sites of Involvement in a 6-Year-Old Patient: A Case Report" Reports 8, no. 1: 3. https://doi.org/10.3390/reports8010003
APA StyleZisis, V., Charisi, C., Poulopoulos, K., Papadopoulos, P., & Poulopoulos, A. (2025). Intraoral Immature Malignant Teratoma with No Evidence of Other Sites of Involvement in a 6-Year-Old Patient: A Case Report. Reports, 8(1), 3. https://doi.org/10.3390/reports8010003





