Prolonged Neurological and Musculoskeletal Symptoms Following Shingrix Vaccination
Abstract
1. Introduction and Clinical Significance
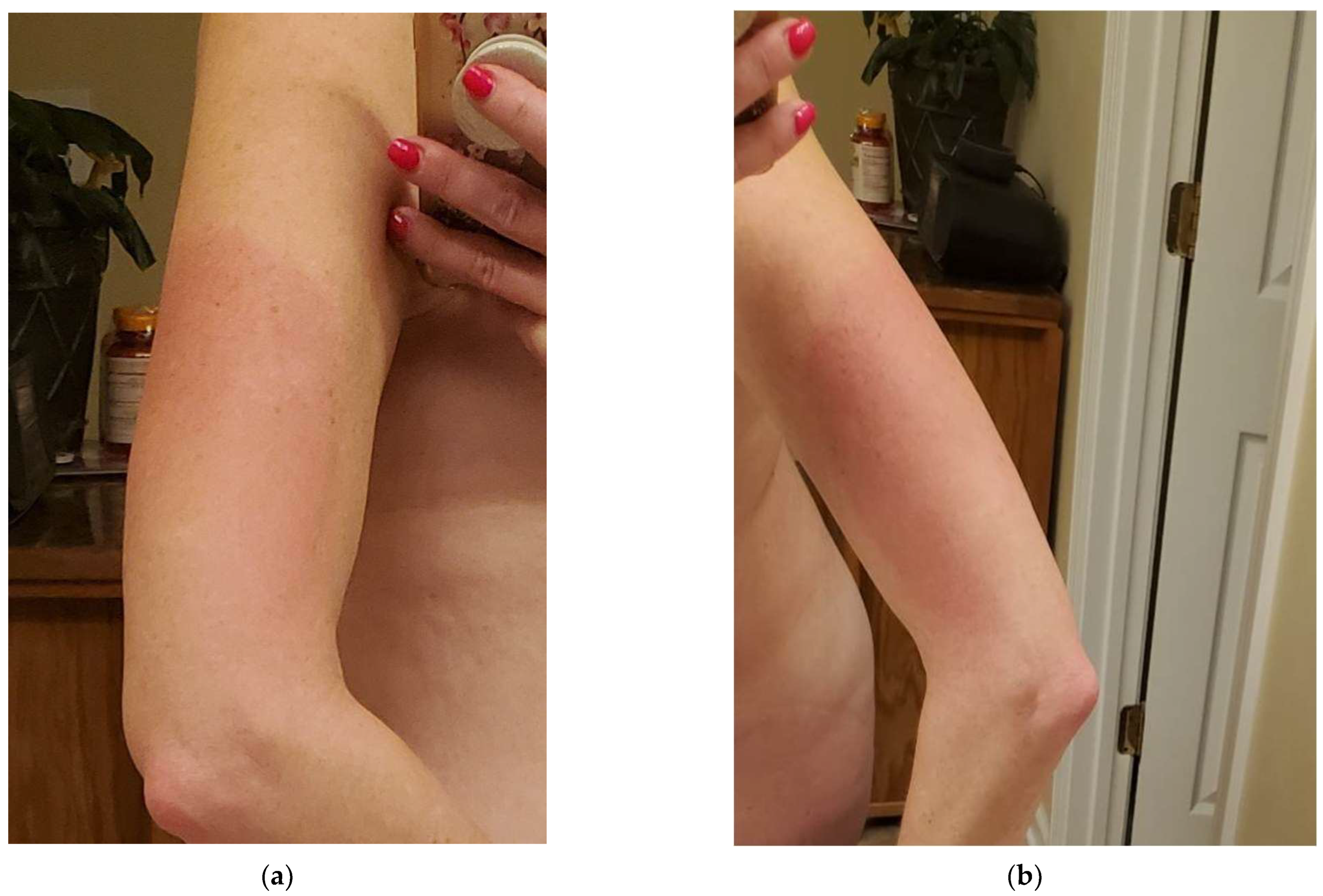
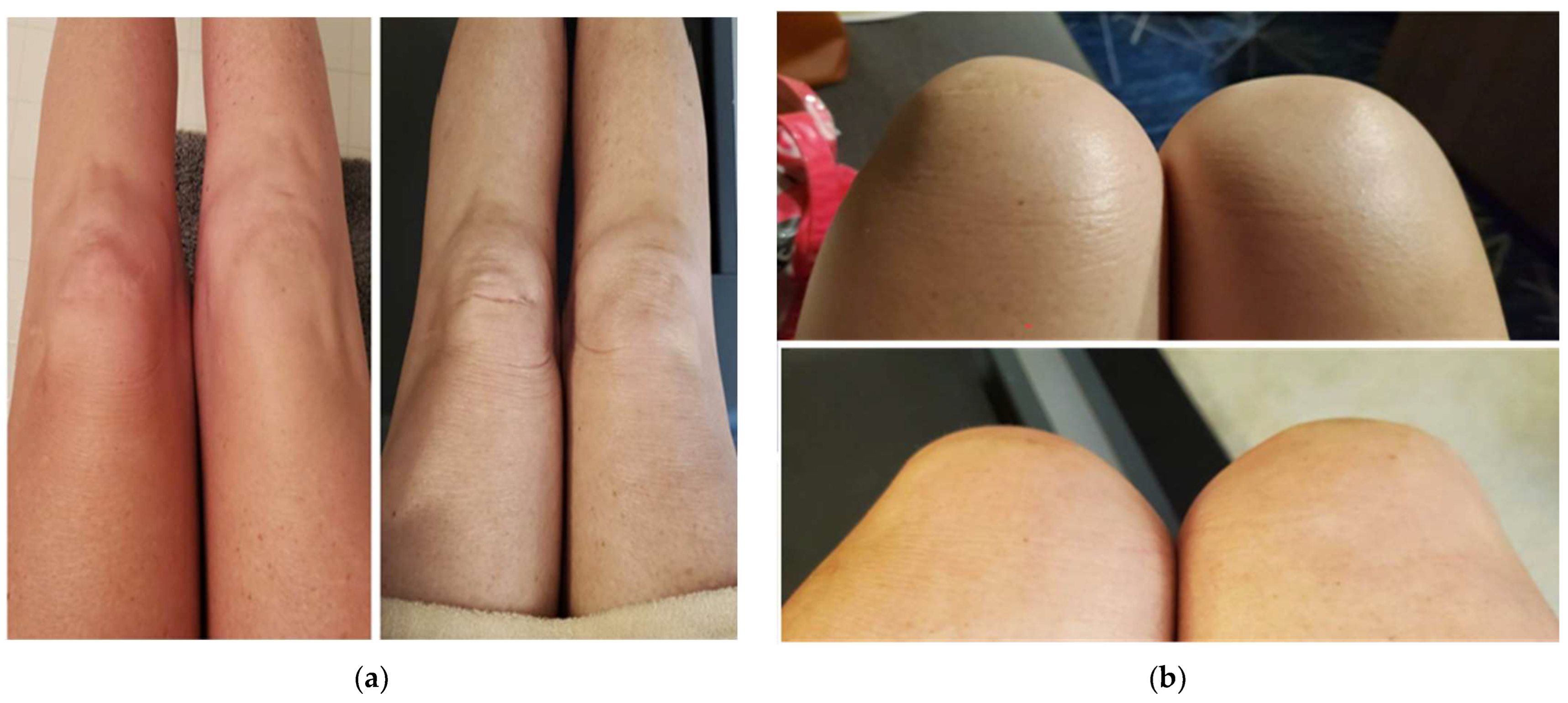
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levin, M.J.; Weinberg, A. Adjuvanted Recombinant Glycoprotein E Herpes Zoster Vaccine. Clin. Infect. Dis. 2019, 70, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Hong, S.J.; Lee, A.; Kim, J.; Lee, S.; Casper, C.; Carter, D.; Reed, S.G.; Simeon, G.; Shin, E.-C. An adjuvanted zoster vaccine elicits potent cellular immune responses in mice without QS21. NPJ Vaccines 2022, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- JohnJohnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.-P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes Zoster Epidemiology, Management, and Disease and Economic Burden in Europe: A Multidiscipli-nary Perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [PubMed]
- James, S.F.; Chahine, E.B.; Sucher, A.J.; Hanna, C. Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. Ann. Pharmacother. 2018, 52, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Dooling, K.L.; Guo, A.; Patel, M.; Lee, G.M.; Moore, K.; Belongia, E.A.; Harpaz, R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Goud, R.; Lufkin, B.; Duffy, J.; Whitaker, B.; Wong, H.-L.; Liao, J.; Lo, A.-C.; Parulekar, S.; Agger, P.; Anderson, S.A.; et al. Risk of Guillain-Barré Syndrome Following Recombinant Zoster Vaccine in Medicare Beneficiaries. JAMA Intern. Med. 2021, 181, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.M.; Shimabukuro, T.T.; Su, J.R.; Hibbs, B.F.; Dooling, K.L.; Goud, R.; Lewis, P.; Ng, C.S.; Cano, M.V. Postlicensure Safety Surveillance of Recombinant Zoster Vaccine (Shingrix)—United States, October 2017–June 2018. Morb. Mortal. Wkly. Rep. 2019, 68, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-Induced Autoimmunity: The Role of Molecular Mimicry and Immune Crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.B.; Kikuchi, S.; Rejzek, M.; Owen, C.; Reed, J.; Orme, A.; Misra, R.C.; El-Demerdash, A.; Hill, L.; Hodgson, H.; et al. Complete biosynthesis of the potent vaccine adjuvant QS-21. Nat. Chem. Biol. 2024, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Famuyiro, T.; Smith, S.T.; Raji, M. Making the case for universal herpes zoster vaccination in older adults. Ann. Long-Term Care 2018, 26, 27–31. [Google Scholar] [CrossRef]

| Labs | Values | Reference Ranges |
|---|---|---|
| HgbA1c | 5.0% | <5.7% |
| T3, Reverse | 21 | 8–25 ng/mL |
| T3, free | 4.9 (H) | 2.3–4.2 ng/mL |
| T3 (TT3) | 179 (H) | 60–170 ng/dL |
| Free T4 | 1.1 | 0.8–1.8 |
| Vitamin B-12 | >2000 (H) | 180–914 pg/mL |
| Methylmalonic acid | 166 | 87–318 nmol/L |
| Homocysteine | 7.7 | <10.4 μmol/L |
| Magnesium | 2.0 | 1.7–2.8 mg/dL |
| Magnesium, RBC | 5.4 | 4.0–6.4 mg/dL |
| C-reactive protein | <0.40 | Normal < 1.0 |
| Anachoice(R) screen | Negative | Negative |
| Iron | 139 | 26–154 UG/DL |
| UIBC | 201 | 162–408 UG/DL |
| Tibc | 340 | 259–492 UG/DL |
| Transferrin saturation | 41% (H) | 8.9–40.5% |
| Ferritin | 156.9 (H) | 13–150 ng/mL |
| Vitamin D, hydroxy | 107 (H) | 30–100 ng/mL |
| Rheumatoid Factor | <14 | <14 IU/mL |
| CCP Ab IgG | <16 | Negative: <20 Weak positive: 21–39 Moderate positive: 40–59 Strong positive: >59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollar, S.; Khalid, A.; Brooks, B.D.; Wons, M. Prolonged Neurological and Musculoskeletal Symptoms Following Shingrix Vaccination. Reports 2024, 7, 83. https://doi.org/10.3390/reports7040083
Hollar S, Khalid A, Brooks BD, Wons M. Prolonged Neurological and Musculoskeletal Symptoms Following Shingrix Vaccination. Reports. 2024; 7(4):83. https://doi.org/10.3390/reports7040083
Chicago/Turabian StyleHollar, Sabrina, Amna Khalid, Benjamin D. Brooks, and Michael Wons. 2024. "Prolonged Neurological and Musculoskeletal Symptoms Following Shingrix Vaccination" Reports 7, no. 4: 83. https://doi.org/10.3390/reports7040083
APA StyleHollar, S., Khalid, A., Brooks, B. D., & Wons, M. (2024). Prolonged Neurological and Musculoskeletal Symptoms Following Shingrix Vaccination. Reports, 7(4), 83. https://doi.org/10.3390/reports7040083







