Endoscopic Guided Dilations without Intralesional Corticosteroid Injections: Pediatric Crohn’s Patients Case Series
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feuerstein, J.D.; Cheifetz, A.S. Crohn disease: Epidemiology, diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Von Allmen, D. Pediatric Crohn’s Disease. Clin. Colon Rectal. Surg. 2018, 31, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lahad, A.; Batia, W. Current therapy of pediatric crohn’s disease. World J. Gastrointest. Pathophysiol. 2015, 6, 33–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klag, T.; Wehkamp, J.; Goetz, M. Endoscopic balloon dilation for crohn’s disease-associated strictures. Clin. Endosc. 2017, 50, 429–436. [Google Scholar] [CrossRef] [PubMed]
- McSorley, B.; Cina, R.A.; Jump, C.; Palmadottir, J.; Quiros, J.A. Endoscopic balloon dilation for management of stricturing crohn’s disease in children. World J. Gastrointest. Endosc. 2021, 13, 382–390. [Google Scholar] [CrossRef] [PubMed]
- East, J.E.; Brooker, J.C.; Rutter, M.D.; Saunders, B.P. A pilot study of intrastricture steroid versus placebo injection after balloon dilatation of crohn’s strictures. Clin. Gastroenterol. Hepatol. 2007, 5, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Oliva, S.; Passariello, M.; Pallotta, N.; Civitelli, F.; Frediani, S.; Gualdi, G.; Gandullia, P.; Mallardo, S.; Cucchiara, S. Intralesional steroid injection after endoscopic balloon dilation in pediatric Crohn’s disease with stricture: A prospective, randomized, double-blind, controlled trial. Gastrointest. Endosc. 2010, 72, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, R.; Poornachandra, K.S. Intralesional steroid injection therapy in the management of resistant gastrointestinal strictures. World J. Gastrointest. Endosc. 2010, 2, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.; Rees, C.J.; Rutter, M.D.; Macafee, D.A. Review of the use of intralesional steroid injections in the management of ileocolonic crohn’s strictures. Frontline Gastroenterol. 2013, 4, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, S.J.; Jeon, S.R.; Ye, B.D.; Park, J.J.; Cheon, J.H.; Kim, T.I.; Kim, W.H. Long-Term Outcomes of Endoscopic Balloon Dilation for Benign Strictures in Patients with Inflammatory Bowel Disease. Gut Liver 2018, 12, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Hirdes, M.M.; van Hooft, J.E.; Koornstra, J.J.; Timmer, R.; Leenders, M.; Weersma, R.K.; Weusten, B.L.; van Hillegersberg, R.; van Berge Henegouwen, M.I.; Plukker, J.T.; et al. Endoscopic Corticosteroid Injections Do Not Reduce Dysphagia after Endoscopic Dilation Therapy in Patients with Benign Esophagogastric Anastomotic Strictures. Clin. Gastroenterol. Hepatol. 2013, 11, 795–801.e1. [Google Scholar] [CrossRef] [PubMed]
- Atreja, A.; Aggarwal, A.; Dwivedi, S.; Rieder, F.; Lopez, R.; Lashner, B.A.; Brzezinski, A.; Vargo, J.J.; Shen, B. Safety and efficacy of endoscopic dilation for primary and anastomotic Crohn’s disease strictures. J. Crohn’s Colitis 2014, 8, 392–400. [Google Scholar] [CrossRef] [PubMed]
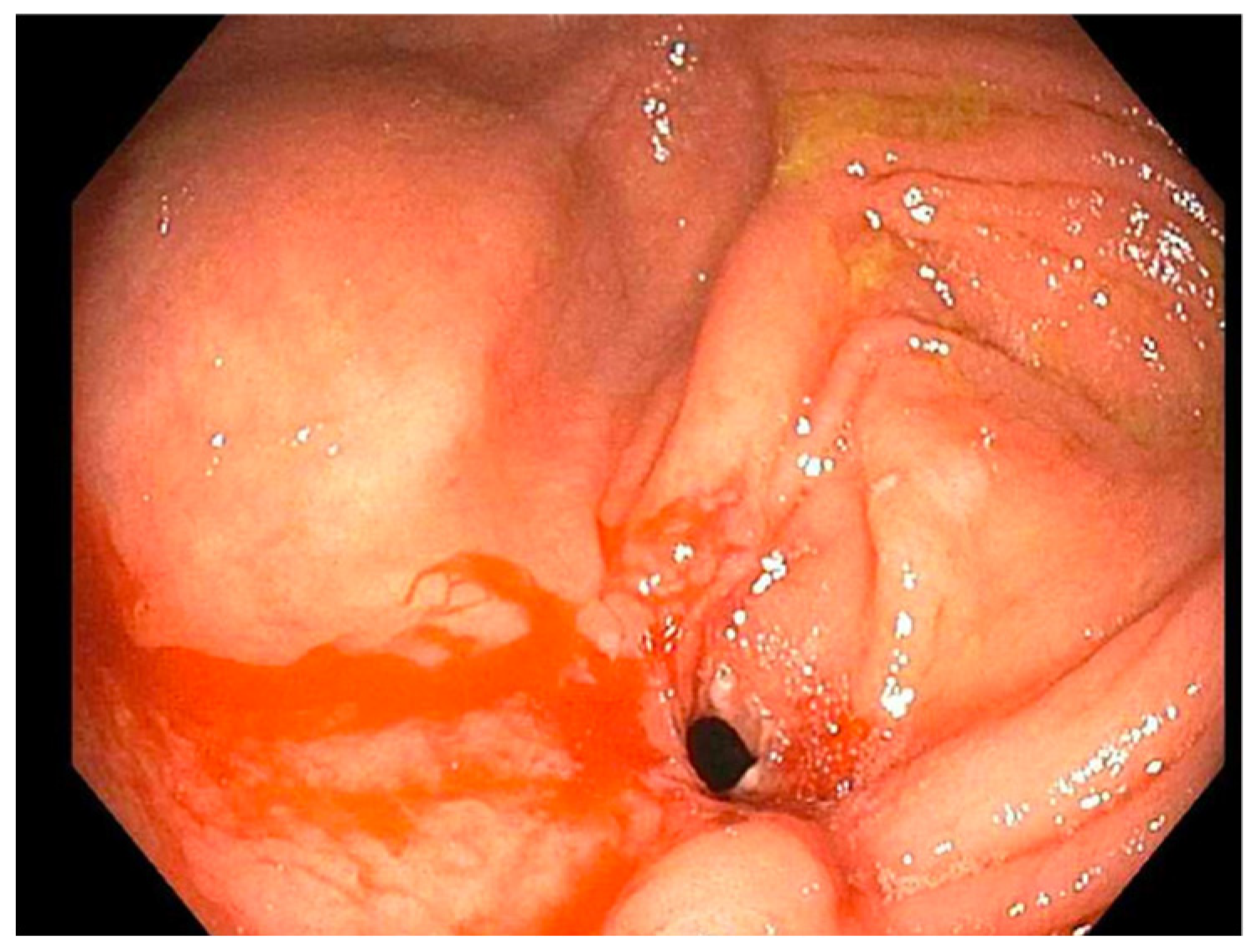

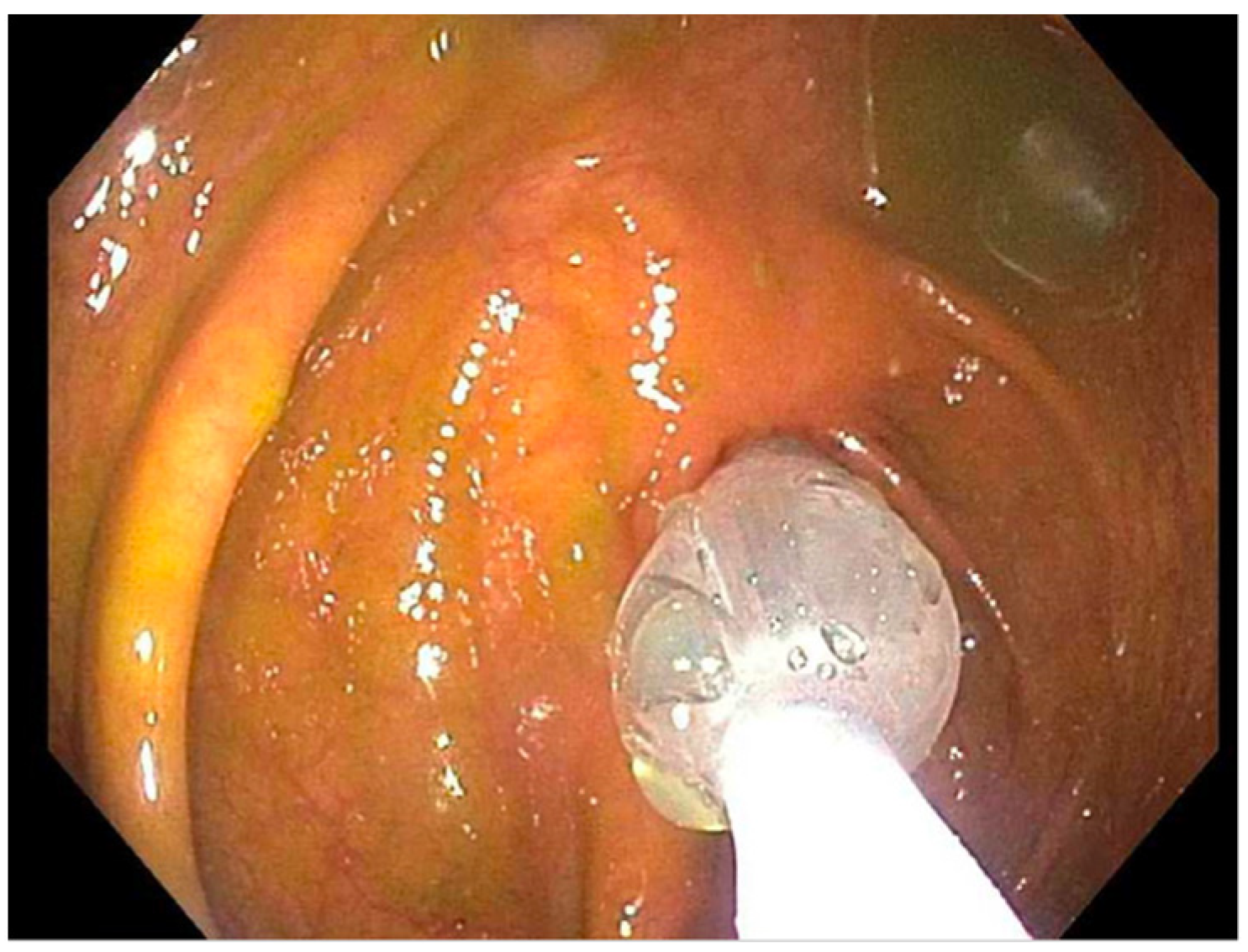
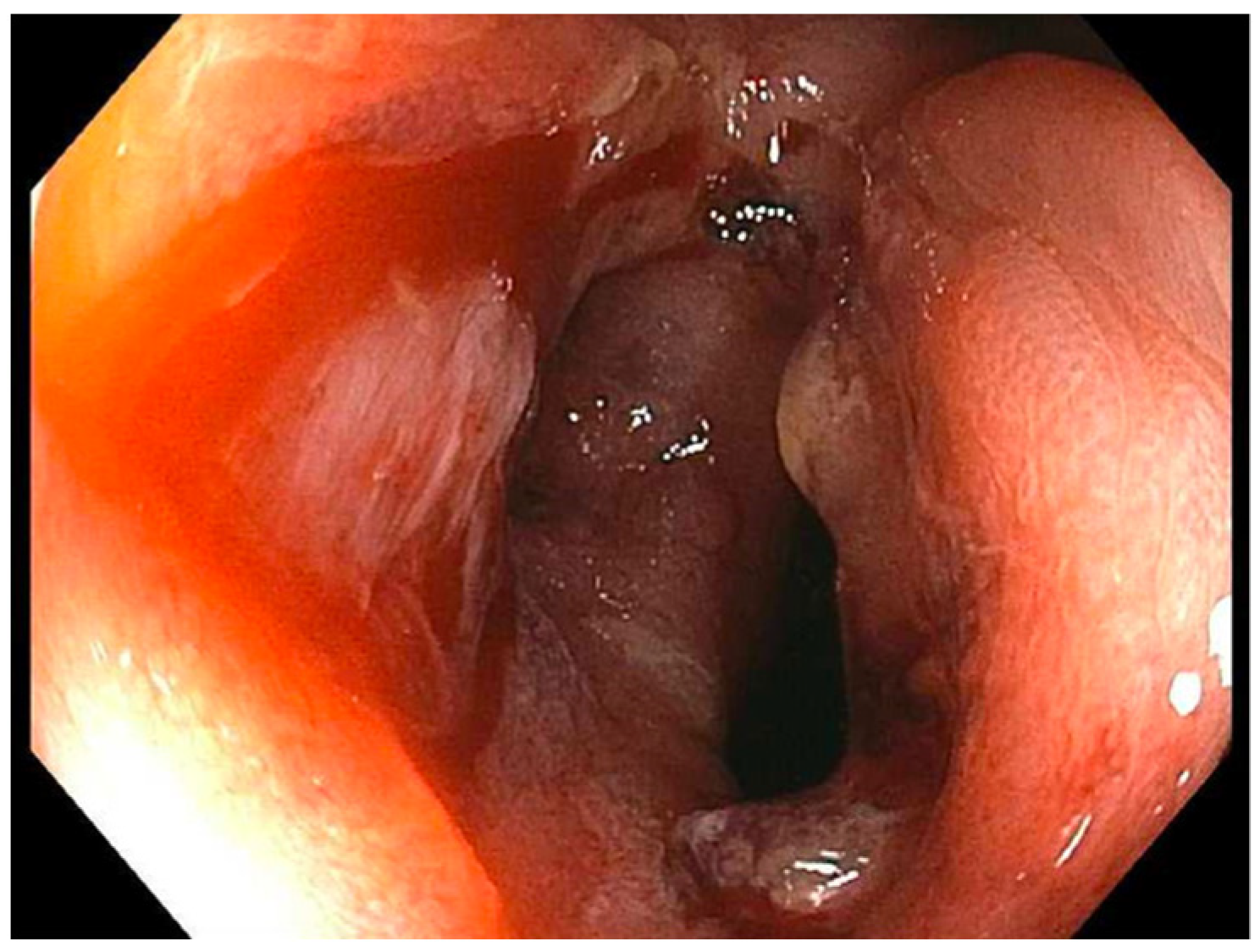

| Case | Treatment | Dilation 1 | Dilation 2 | Dilation 3 |
|---|---|---|---|---|
| 1 | (2019: Remicade 10 mg) (2019: Humira 40 mg) (2020: Humira 80 mg subcutaneous) | November 2019, dilated (b) TI from (12 to 18 mm) (a) TI stricture dilated to (16.5 mm) | November 2020, dilated TI to (20 mm) | March 2023, laparoscopic assisted ileocecectomy |
| 2 | (2019: prednisone 40 mg) (2019: Remicade 5 mg) (Remicade 10 mg) | September 2019, ICV dilated from (8 to 11 mm) | December 2020 and August 2021 ICV dilated from (9 to 18 mm) then (12 to 18 mm) | June 2022, ICV dilated from (13.5 to 20 mm) |
| 3 | (2019: prednisone 20 mg BID w/mesalamine 500 mg TID) (2020: 6-MP 50 mg) (2021: Humira 40 mg/MTX 25 mg) | February 2021, PAC dilated from (7 to 15 mm) | August 2021, PAC dilated from (15 to 20 mm) | August 2022, mucosal narrowing stretched to 20 mm without noted renting |
| 4 | (2021: Humira 40 mg every other week/steroid 40 mg daily, escalated to Humira 40 mg weekly) (2022: MTX 25 mg) | September 2022, dilation of the ICV from (4 to 13.5 mm) (food bezoar noted) | November 2022, ICV dilated from (8 to 15 mm) | December 2022, ICV dilated (12 to 15 mm) |
| Case | Sex/Age | BMI | IBD/Dx | Stricture Site |
|---|---|---|---|---|
| 1 | F/17 | 60% | 4/5/18 | TI (2 strictures (a)/(b)) |
| 2 | M/16 | 20% | 8/30/19 | ICV |
| 3 | M/18 | 86% | 2/4/15 | PAC |
| 4 | M/15 | 1.5% | 12/22/21 | ICV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawaz, L.; Slim, Y.; Freswick, P.N. Endoscopic Guided Dilations without Intralesional Corticosteroid Injections: Pediatric Crohn’s Patients Case Series. Reports 2024, 7, 81. https://doi.org/10.3390/reports7040081
Fawaz L, Slim Y, Freswick PN. Endoscopic Guided Dilations without Intralesional Corticosteroid Injections: Pediatric Crohn’s Patients Case Series. Reports. 2024; 7(4):81. https://doi.org/10.3390/reports7040081
Chicago/Turabian StyleFawaz, Leo, Yousif Slim, and Peter N. Freswick. 2024. "Endoscopic Guided Dilations without Intralesional Corticosteroid Injections: Pediatric Crohn’s Patients Case Series" Reports 7, no. 4: 81. https://doi.org/10.3390/reports7040081
APA StyleFawaz, L., Slim, Y., & Freswick, P. N. (2024). Endoscopic Guided Dilations without Intralesional Corticosteroid Injections: Pediatric Crohn’s Patients Case Series. Reports, 7(4), 81. https://doi.org/10.3390/reports7040081






