Cancer Stem Cell Marker CD147 Expression in Erosive Oral Lichen Planus Compared to Moderately and Severely Dysplastic Leukoplakia
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissues
- A: lichen planus group
- B: leukoplakia group
- D: normal oral epithelium group
- A1: reticular lichen planus subgroup
- A2: erosive lichen planus subgroup
- B1: moderately and severely dysplastic leukoplakia subgroup
- B2: mildly dysplastic and non-dysplastic leukoplakia subgroup
- 10 samples of the reticular lichen planus subgroup (A1) that were coded as A1.1–A1.10
- 14 samples of the erosive lichen planus subgroup (A2) that were coded as A2.1–A2.14
- 16 samples of the moderately and severely dysplastic leukoplakia subgroup (B1) that were coded as B1.1–B1.16
- 14 samples of the mildly dysplastic and non-dysplastic leukoplakia subgroup (B2) that were coded as B2.1–B2.14
- 5 samples of the normal oral epithelium group that were coded as D.1–D.5
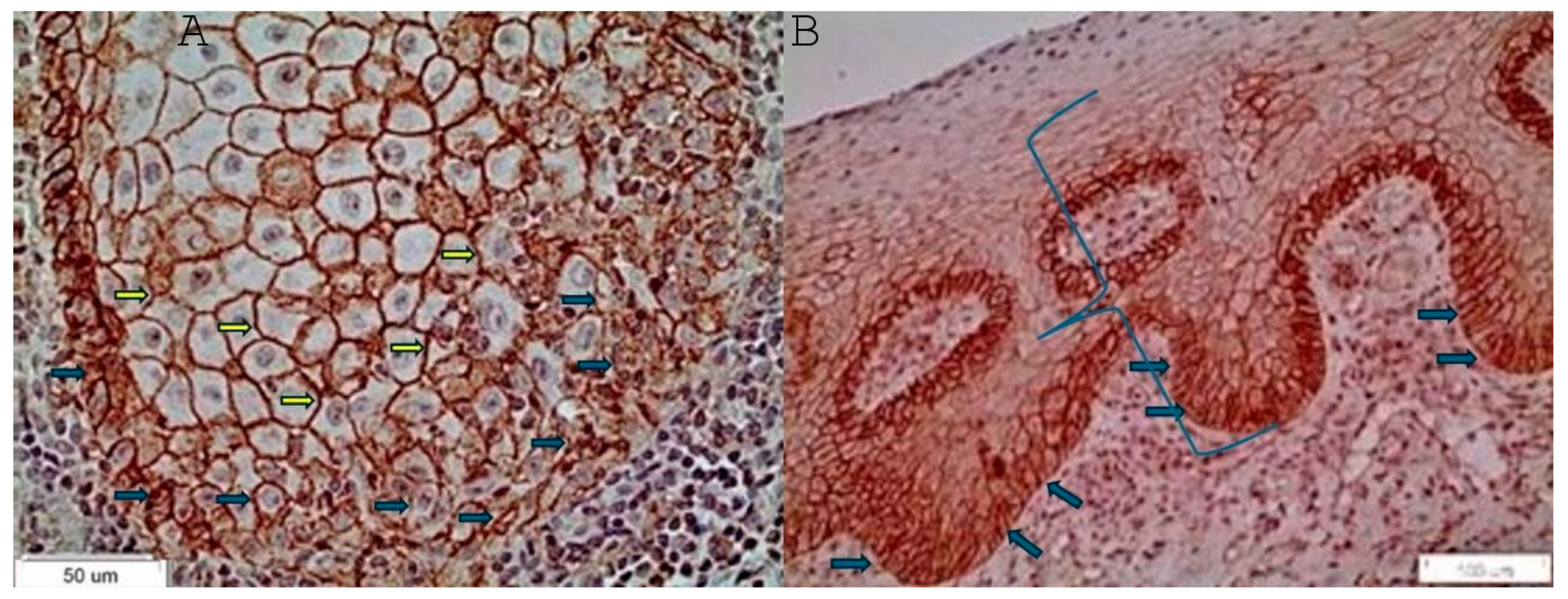
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
- Statistically significantly higher expression of CD147 in the lichen planus group than in the leukoplakia group (Pearson’s chi-square, p = 0.009) (Figure 2A).
- Statistically significantly higher expression of CD147 in the lichen planus group than in the normal oral epithelium group (Pearson’s chi-square, p = 0.036) (Figure 2B).
- Statistically significantly higher expression of CD147 in the erosive lichen planus subgroup than in the reticular lichen planus subgroup (Fisher’s exact test, p = 0.006) (Figure 2C).
- Statistically significantly higher expression of CD147 in the reticular lichen planus subgroup than in the moderately and severely dysplastic leukoplakia subgroup (Fisher’s exact test, p = 0.014) (Figure 2D).
- Statistically significantly higher expression of CD147 in the erosive lichen planus subgroup than in the moderately and severely dysplastic leukoplakia subgroup (Pearson’s chi-square test, p = 0.01) (Figure 2E).
- Statistically significantly higher expression of CD147 in the erosive lichen planus subgroup than in the mildly and non-dysplastic leukoplakia subgroup (Fisher’s exact test, p = 0.002) (Figure 2F).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, I. Oral potentially malignant disorders: Is malignant transformation predictable and preventable? Med. Oral Patol. Oral Y Cirugía Bucal 2014, 19, e386. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Westhausen, A.M. Oral lichen planus and lichenoid lesions: What’s new? Quintessence Int. 2020, 51, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jaafari-Ashkavandi, Z.; Mardani, M.; Pardis, S.; Amanpour, S. Oral mucocutaneous diseases: Clinicopathologic analysis and malignant transformation. J. Craniofacial Surg. 2011, 22, 949–951. [Google Scholar] [CrossRef]
- Rimkevičius, A.; Aleksejūnienė, J.; Pūrienė, A.; Šeinin, D.; Rastenienė, R. Oral lichen planus: A 4-year clinical follow-up study. Turk. J. Med. Sci. 2017, 47, 514–522. [Google Scholar] [CrossRef]
- Dudhia, B.; Dudhia, S.; Patel, P.; Jani, Y. Oral lichen planus to oral lichenoid lesions: Evolution or revolution. J. Oral Maxillofac. Pathol. 2015, 19, 364. [Google Scholar] [CrossRef]
- Laeijendecker, R.; van Joost, T.; Tank, B.; Oranje, A.P.; Neumann, H.A.M. Oral lichen planus in childhood. Pediatr. Dermatol. 2005, 22, 299–304. [Google Scholar] [CrossRef]
- Patel, S.; Yeoman, C.M.; Murphy, R. Oral lichen planus in childhood: A report of three cases. Int. J. Paediatr. Dent. 2005, 15, 118–122. [Google Scholar] [CrossRef]
- Gümrü, B. A retrospective study of 370 patients with oral lichen planus in Turkey. Med. Oral Patol. Oral Y Cirugía Bucal 2013, 18, e427. [Google Scholar] [CrossRef]
- Gupta, S.; Jawanda, M.K. Oral lichen planus: An update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J. Dermatol. 2015, 60, 222–229. [Google Scholar] [CrossRef]
- de Sousa, F.A.C.G.; Rosa, L.E.B. Oral lichen planus: Clinical and histopathological considerations. Rev. Bras. De Otorrinolaringol. 2008, 74, 284–292. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Scully, C.; Gil-Montoya, J.A. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, U.; Jontell, M.; Holmstrup, P. Oral lichen planus and malignant transformation: Is a recall of patients justified? Crit. Rev. Oral Biol. Med. 2002, 13, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.; Pagano, M.; Carrozzo, M.; Carbone, M.; Bobba, V.; Palestro, G.; Gandolfo, S. Sequential immunohistochemical p53 expression in biopsies of oral lichen planus undergoing malignant evolution. J. Oral Pathol. Med. 2001, 30, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.L.; Mignogna, M.D.; Favia, G.; Procaccini, M.; Testa, N.F.; Bucci, E. The possible association between oral lichen planus and oral squamous cell carcinoma: A clinical evaluation on 14 cases and a review of the literature. Oral Oncol. 1998, 34, 239–246. [Google Scholar] [CrossRef]
- van der Meij, E.H.; Schepman, K.P.; Smeele, L.E.; van der Wal, J.E.; Bezemer, P.D.; van der Waal, I. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 307–310. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Muzio, L.L.; Russo, L.L.; Fedele, S.; Ruoppo, E.; Bucci, E. Clinical guidelines in early detection of oral squamous cell carcinoma arising in oral lichen planus: A 5-year experience. Oral Oncol. 2001, 37, 262–267. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Russo, L.L.; Fedele, S.; Ruoppo, E.; Califano, L.; Muzio, L.L. Clinical behaviour of malignant transforming oral lichen planus. Eur. J. Surg. Oncol. 2002, 28, 838–843. [Google Scholar] [CrossRef]
- Abbate, G.; Foscolo, A.M.; Gallotti, M.; Lancella, A.; Mingo, F. Neoplastic transformation of oral lichen: Case report and review of the literature. Acta Otorhinolaryngol. Ital. 2006, 26, 47. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2639954/ (accessed on 22 November 2020).
- Georgakopoulou, E.A.; Achtari, M.D.; Achtaris, M.; Foukas, P.G.; Kotsinas, A. Oral lichen planus as a preneoplastic inflammatory model. BioMed Res. Int. 2012, 2012, 759626. [Google Scholar] [CrossRef]
- Scully, C.; Beyli, M.; Ferreiro, M.C.; Ficarra, G.; Gill, Y.; Griffiths, M.; Holmstrup, P.; Mutlu, S.; Porter, S.; Wray, D. Update on oral lichen planus: Etiopathogenesis and management. Crit. Rev. Oral Biol. Med. 1998, 9, 86–122. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Bánóczy, J.; Dinya, E.; Tamás, G. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J. Oral Pathol. Med. 1992, 21, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Lundström, I.M.C. Incidence of diabetes mellitus in patients with oral lichen planus. Int. J. Oral Surg. 1983, 12, 147–152. [Google Scholar] [CrossRef]
- Torrente-Castells, E.; Figueiredo, R.; Berini-Aytés, L.; Gay-Escoda, C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med. Oral Patol. Oral Y Cir. Bucal 2010, 15, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lamey, P.J.; Gibson, J.; Barclay, S.C.; Miller, S. Grinspan’s syndrome: A drug-induced phenomenon? Oral Surg. Oral Med. Oral Pathol. 1990, 70, 184–185. [Google Scholar] [CrossRef]
- Sharma, N.; Malhotra, S.K.; Kuthial, M.; Chahal, K.S. Vulvo-vaginal ano-gingival syndrome: Another variant of mucosal lichen planus. Indian J. Sex. Transm. Dis. AIDS 2017, 38, 86. [Google Scholar] [CrossRef]
- Birkenfeld, S.; Dreiher, J.; Weitzman, D.; Cohen, A.D. A study on the association with hepatitis B and hepatitis C in 1557 patients with lichen planus. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Giovannelli, L.; Aricò, P.; Lama, A.; Di Liberto, C.; Ammatuna, P.; D’Angelo, M. HPV DNA in clinically different variants of oral leukoplakia and lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 705–711. [Google Scholar] [CrossRef]
- Gorsky, M.; Epstein, J.B. Oral lichen planus: Malignant transformation and human papilloma virus: A review of potential clinical implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 461–464. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, N.; Thappa, D.M. Hypertrophic lichen planus as a presenting feature of human immunodeficiency virus infection. Indian J. Dermatol. 2009, 54, 8. Available online: https://www.e-ijd.org/article.asp?issn=0019-5154;year=2009;volume=54;issue=5;spage=8;epage=10;aulast=Kumari (accessed on 22 November 2020).
- Sand, L.P.; Jalouli, J.; Larsson, P.A.; Hirsch, J.M. Prevalence of Epstein-Barr virus in oral squamous cell carcinoma, oral lichen planus, and normal oral mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, B.; Sengüven, B.; Demir, C. Prevalence of herpes simplex, Epstein Barr and human Papilloma viruses in oral lichen planus. Med. Oral Patol Oral Cir Bucal 2011, 16, e170. [Google Scholar] [CrossRef] [PubMed]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef]
- Monteiro, L.S.; Delgado, M.L.; Ricardo, S.; Garcez, F.; Amaral, B.D.; Pacheco, J.J.; Bousbaa, H. EMMPRIN expression in oral squamous cell carcinomas: Correlation with tumor proliferation and patient survival. BioMed Res. Int. 2014, 2014, 905680. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J. Oral Pathol. Med. 2008, 37, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.Y.; Gatter, K.C. The role of immunocytochemistry in diagnostic pathology. J. Clin. Pathol. 1987, 40, 1042–1054. [Google Scholar] [CrossRef]
- Krenacs, L.; Krenacs, T.; Stelkovics, E.; Raffeld, M. Heat-Induced Antigen Retrieval for Immunohistochemical Reactions in Routinely Processed Paraffin Sections. Immunocytochemical Methods Protoc. 2010, 588, 103–119. [Google Scholar] [CrossRef]
- Battifora, H.; Kopinski, M. The influence of protease digestion and duration of fixation on the immunostaining of keratins. A comparison of formalin and ethanol fixation. J. Histochem. Cytochem. 1986, 34, 1095–1100. [Google Scholar] [CrossRef]
- Cattoretti, G.; Pileri, S.; Parravicini, C.; Becker, M.H.; Poggi, S.; Bifulco, C.; Rilke, F. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J. Pathol. 1993, 171, 83–98. [Google Scholar] [CrossRef]
- Norton, A.J.; Jordan, S.; Yeomans, P. Brief, high-temperature heat denaturation (pressure cooking): A simple and effective method of antigen retrieval for routinely processed tissues. J. Pathol. 1994, 173, 371–379. [Google Scholar] [CrossRef]
- Shi, S.R.; Key, M.E.; Kalra, K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: An enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991, 39, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Sarode, S.C.; Sarode, G.S.; Kalele, K. Oral Lichenoid Reaction: A Review. Int. J. Oral Maxillofac. Pathol. 2012, 3, 17–26. [Google Scholar]
- Coghlin, C.; Murray, G.I. The role of gene regulatory networks in promoting cancer progression and metastasis. Future Oncol. 2014, 10, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Curran, S.; Murray, G.I. Matrix metalloproteinasesmolecular aspects of their roles in tumour invasion and metastasis. Eur. J. Cancer 2000, 36, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Erdem, N.F.; Carlson, E.R.; Gerard, D.A.; Ichiki, A.T. Characterization of 3 Oral Squamous Cell Carcinoma Cell Lines With Different Invasion and/or Metastatic Potentials. J. Oral Maxillofac. Surg. 2007, 65, 1725–1733. [Google Scholar] [CrossRef]
- De Vicente, J.C.; Fresno, M.F.; Villalain, L.; Vega, J.A.; Vallejo, G.H. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol. 2005, 41, 283–293. [Google Scholar] [CrossRef]
- Papadimitropoulou, A.; Mamalaki, A. The glycosylated IgII Extracellular domain of EMMPRIN is implicated in the induction of MMP-2. Mol. Cell. Biochem. 2013, 379, 107–113. [Google Scholar] [CrossRef]
- Zisis, V.; Giannakopoulos, N.N.; Schmitter, M.; Poulopoulos, A.; Andreadis, D. Cancer Stem Cells’ Biomarker ALDH1&2 Increased Expression in Erosive Oral Lichen Planus Compared to Oral Leukoplakia. Cureus 2023, 15, e44278. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Sun, W.; Du, G.; Zhou, G. Inflammation-related cytokines in oral lichen planus: An overview. J. Oral Pathol. Med. 2015, 44, 1–14. [Google Scholar] [CrossRef]

| Patients | Group/Subgroup | Location | Gender | Age |
|---|---|---|---|---|
| A1.1 | RETICULAR OLP | TONGUE | MALE | 57 |
| A1.2 | RETICULAR OLP | TONGUE | MALE | 77 |
| A1.3 | RETICULAR OLP | TONGUE | FEMALE | 21 |
| A1.4 | RETICULAR OLP | TONGUE | FEMALE | 50 |
| A1.5 | RETICULAR OLP | TONGUE | FEMALE | 57 |
| A1.6 | RETICULAR OLP | BUCCAL MUCOSA | FEMALE | 72 |
| A1.7 | RETICULAR OLP | TONGUE | FEMALE | 38 |
| A1.8 | RETICULAR OLP | BUCCAL MUCOSA | MALE | 73 |
| A1.9 | RETICULAR OLP | BUCCAL MUCOSA | FEMALE | 49 |
| A1.10 | RETICULAR OLP | CHEEK | FEMALE | 42 |
| A2.1 | EROSIVE OLP | INTERDENTAL PAPILLA | FEMALE | 77 |
| A2.2 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 77 |
| A2.3 | EROSIVE OLP | TONGUE | FEMALE | 59 |
| A2.4 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 54 |
| A2.5 | EROSIVE OLP | PALATE | FEMALE | 55 |
| A2.6 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 49 |
| A2.7 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 72 |
| A2.8 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 76 |
| A2.9 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 58 |
| A2.10 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 64 |
| A2.11 | EROSIVE OLP | BUCCAL MUCOSA | MALE | 56 |
| A2.12 | EROSIVE OLP | BUCCAL MUCOSA | MALE | 56 |
| A2.13 | EROSIVE OLP | GINGIVA | FEMALE | 26 |
| A2.14 | EROSIVE OLP | BUCCAL MUCOSA | FEMALE | 37 |
| B1.1 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | FEMALE | 44 |
| B1.2 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | FEMALE | 60 |
| B1.3 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | MALE | 58 |
| B1.4 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | FEMALE | 67 |
| B1.5 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | FEMALE | 62 |
| B1.6 | MODERATELY AND SEVERELY DYSPLASTIC OL | BUCCAL MUCOSA | MALE | 66 |
| B1.7 | MODERATELY AND SEVERELY DYSPLASTIC OL | BUCCAL MUCOSA | MALE | 67 |
| B1.8 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | MALE | 43 |
| B1.9 | MODERATELY AND SEVERELY DYSPLASTIC OL | GINGIVOBUCCAL SULCUS | FEMALE | 75 |
| B1.10 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | MALE | 50 |
| B1.11 | MODERATELY AND SEVERELY DYSPLASTIC OL | GINGIVOBUCCAL SULCUS | MALE | 59 |
| B1.12 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | MALE | 75 |
| B1.13 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | MALE | 64 |
| B1.14 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | MALE | 45 |
| B1.15 | MODERATELY AND SEVERELY DYSPLASTIC OL | PALATE | MALE | 72 |
| B1.16 | MODERATELY AND SEVERELY DYSPLASTIC OL | TONGUE | FEMALE | 84 |
| B2.1 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | FEMALE | 61 |
| B2.1 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | LIP | FEMALE | 38 |
| B2.3 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | MALE | 46 |
| B2.4 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | GINGIVA | FEMALE | 12 |
| B2.5 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | FEMALE | 45 |
| B2.6 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | MALE | 67 |
| B2.7 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | BUCCAL MUCOSA | FEMALE | 60 |
| B2.8 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | FEMALE | 68 |
| B2.9 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | MALE | 69 |
| B2.10 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | TONGUE | FEMALE | 68 |
| B2.11 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | BUCCAL MUCOSA | FEMALE | 58 |
| B2.12 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | BUCCAL MUCOSA | FEMALE | 61 |
| B2.13 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | BUCCAL MUCOSA | MALE | 75 |
| B2.14 | MILDLY DYSPLASTIC AND NON-DYSPLASTIC OL | CORNER OF THE MOUTH | MALE | 37 |
| D.1 | NORMAL | TONGUE | FEMALE | 49 |
| D.2 | NORMAL | BUCCAL MUCOSA | MALE | 81 |
| D.3 | NORMAL | BUCCAL MUCOSA | FEMALE | 59 |
| D.4 | NORMAL | TONGUE | MALE | 69 |
| D.5 | NORMAL | TONGUE | FEMALE | 72 |
| 0–5% | 0 |
| 6–35% | 1 |
| 36–70% | 2 |
| >71% | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zisis, V.; Giannakopoulos, N.N.; Poulopoulos, A.; Schmitter, M.; Andreadis, D. Cancer Stem Cell Marker CD147 Expression in Erosive Oral Lichen Planus Compared to Moderately and Severely Dysplastic Leukoplakia. Reports 2024, 7, 77. https://doi.org/10.3390/reports7030077
Zisis V, Giannakopoulos NN, Poulopoulos A, Schmitter M, Andreadis D. Cancer Stem Cell Marker CD147 Expression in Erosive Oral Lichen Planus Compared to Moderately and Severely Dysplastic Leukoplakia. Reports. 2024; 7(3):77. https://doi.org/10.3390/reports7030077
Chicago/Turabian StyleZisis, Vasileios, Nikolaos Nikitas Giannakopoulos, Athanasios Poulopoulos, Marc Schmitter, and Dimitrios Andreadis. 2024. "Cancer Stem Cell Marker CD147 Expression in Erosive Oral Lichen Planus Compared to Moderately and Severely Dysplastic Leukoplakia" Reports 7, no. 3: 77. https://doi.org/10.3390/reports7030077
APA StyleZisis, V., Giannakopoulos, N. N., Poulopoulos, A., Schmitter, M., & Andreadis, D. (2024). Cancer Stem Cell Marker CD147 Expression in Erosive Oral Lichen Planus Compared to Moderately and Severely Dysplastic Leukoplakia. Reports, 7(3), 77. https://doi.org/10.3390/reports7030077






