Prevalence of Cardiotoxicity Secondary to Trastuzumab in Patients with HER-2-Positive Breast Cancer in Southeast Mexico
Abstract
1. Introduction
2. Results
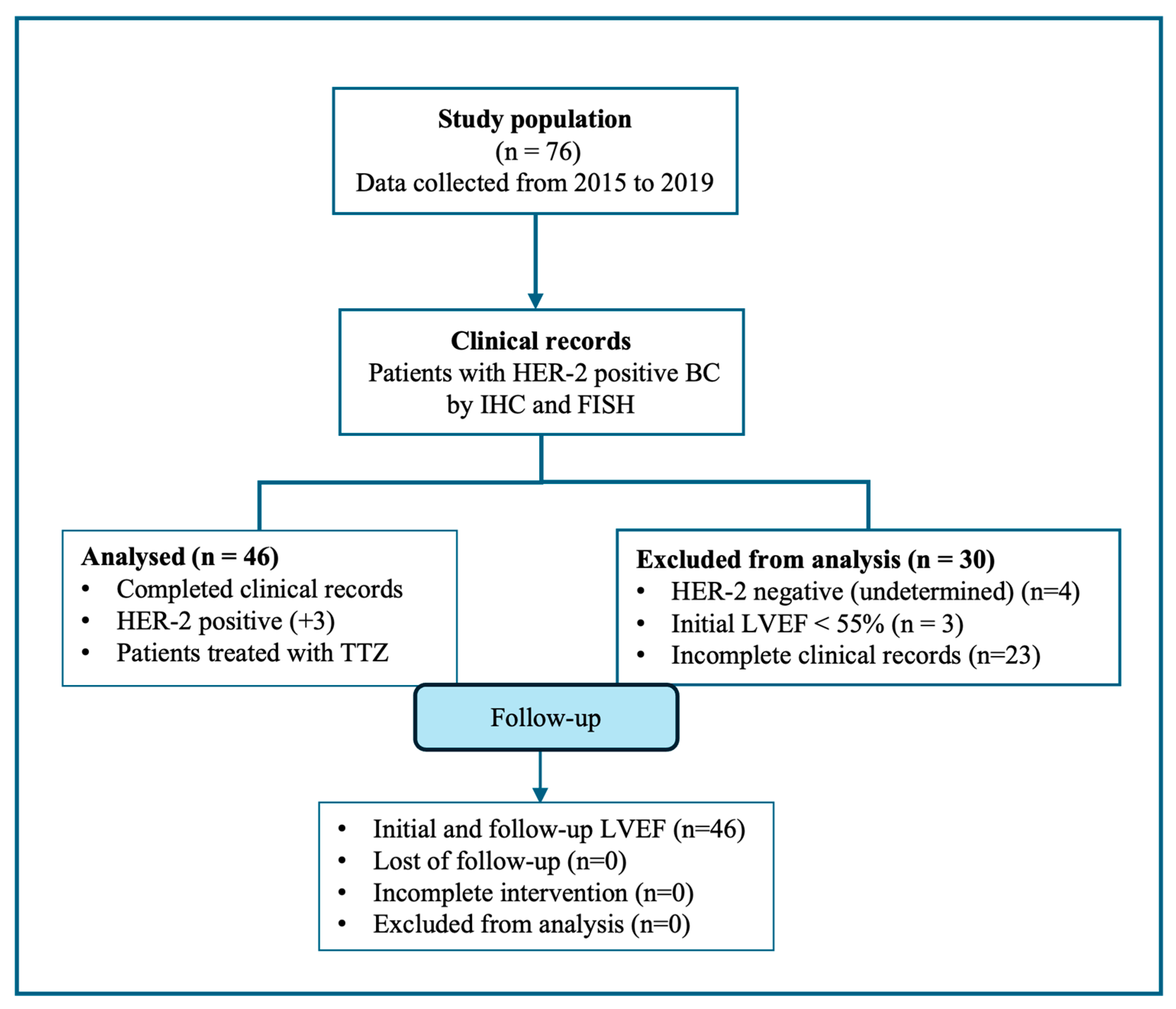
2.1. Characteristics of the Study Population
Diagnostics
2.2. Risk Factors of Cardiotoxicity in TTZ Patients
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
4.2. Study Setting
4.3. Echocardiogram and CT Evaluation
4.4. Ethical Considerations
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.who.int/today (accessed on 22 July 2024).
- American Cancer Society. 2021. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/9017.00.pdf (accessed on 26 July 2024).
- Estadísticas a Propósito del día Mundial Contra el Cáncer, 4 de Febrero; INEGI: Aguascalientes, Mexico, 2024.
- Carballo, T.D.; Lima, P.M.; Luperon, L.D.; Concepcion, I.R. Trastuzumab-Induced Cardiotoxicity in a Patient with HER-2 Positive Breast Carcinoma. Rev Cubana Med [online]. 2021, Vol.60, Suppl.1. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75232021000500007&lng=es&nrm=iso (accessed on 23 May 2024).
- Nicolazzi, M.A.; Carnicelli, A.; Fuorlo, M.; Scaldaferri, A.; Masetti, R.; Landolfi, R.; Favuzzi, A.M.R. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.W.K.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S. On behalf of the American Heart Association Cardiovascular Disease in Women and Special populations Committee of the Council of Clinical Cardiology. Cardiovascular Disease and Breast Cancer: Where these entities Intersect. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
- Chung, R.; Ghosh, A.K.; Banerjee, A. Cardiotoxicity: Precision medicine with imprecise definitions. Open Heart 2018, 5, e000774. [Google Scholar] [CrossRef]
- Jain, D.; Russell, R.R.; Schwartz, R.G.; Panjrath, G.S.; Aronow, W. Cardiac Complications of Cancer Therapy: Pathophysiology, Identification, Prevention, Treatment, and Future Directions. Curr. Cardiol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Mohan, N.; Jiang, J.; Dokmanovic, M.; Wu, W.J. Trastuzumab-mediated cardiotoxicity: Current understanding, challenges, and frontiers. Antib. Ther. 2018, 1, 13–17. [Google Scholar] [CrossRef]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. Br. J. Pharmacol. 2017, 174, 3727–3748. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Farolfi, A.; Melegari, E.; Aquilina, M.; Scarpi, E.; Ibrahim, T.; Maltoni, R.; Sarti, S.; Cecconetto, L.; Pietri, E.; Ferrario, C.; et al. Trastuzumab-induced cardiotoxiocity in early breast cancer patients: A retrospective study of possible risk and protective factors. Heart 2013, 99, 634–639. [Google Scholar] [CrossRef]
- Moilanen, T.; Jokimaki, A.; Tenhunen, O.; Koivunen, J.P. Trastuzumab-induced cardiotoxicity and its risk factors in real-world setting of breast cancer patients. J. Cancer Res. Clin. Oncol. 2018, 144, 1613–1621. [Google Scholar] [CrossRef]
- Virani, S.A.; Dent, S.; Brezden-Masley, C.; Clarke, B.; Davis, M.K.; Jassal, D.S.; Johnson, C.; Lemieux, J.; Paterson, I.; Sebag, I.A.; et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can. J. Cardiol. 2016, 32, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Luna-Alcala, S.; Espejel-Guzmán, A.; Lerma, C.; Leon, P.; Guerra, E.C.; Fernández, J.R.E.; Martinez-Dominguez, P.; Serrano-Roman, J.; Cabello-Ganem, A.; Aparicio-Ortiz, A.D.; et al. Heart rate variability-based prediction of early cardiotoxicity in breast-cancer patients treated with anthracyclines and trastuzumab. Cardiooncology 2024, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, G.; Yung, A.E.; Yung, D.E.; Skonieczna-Żydecka, K.; Marlicz, W.; Koulaouzidis, A.; Charisopoulou, D. Conventional cardiac risk factors associated with trastuzumab-induced cardiotoxicity in breast cancer: Systematic review and meta-analysis. Curr. Probl. Cancer 2021, 45, 100723. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Stankowski, R.V. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: Prevalence, patient characteristics, and risk factors. Ther. Adv. Drug Saf. 2014, 5, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, M. Cardiotoxicity associated with trastuzumab treatment of HER2+ breast cancer. Adv. Ther. 2009, 26 (Suppl. S1), 9–17. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, K.H.; Kim, H.Y.; Park, H.; Cho, J.Y.; Hong, Y.J.; Park, H.W.; Kim, J.H.; Ahn, Y.; Jeong, M.H.; et al. Impacts of non-recovery of trastuzumab-induced cardiomyopathy on clinical outcomes in patients with breast cancer. Clin. Res. Cardiol. 2019, 108, 892–900. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Aguilar-Cordero, M.J.; González-Jiménez, E.; García-López, A.P.; Álvarez-Ferré, J.; Padilla-López, C.A.; Guisado-Barrilao, R.; Rizo Baeza, M. Obesidad y su implicación en el cáncer de mama. Nutr. Hosp. 2011, 26, 899–903. [Google Scholar] [CrossRef]
- Gomez, A.; Robello, E.; Americo, C.; Janssen, B.; Pazos, A.; Castillo, C.; Parma, G.; Florio, L. Cardiotoxicidad por trastuzumab en pacientes con Cancer de mama. Serie de Casos. Rev. Urug. Cardiol. 2019, 34, 85–107. [Google Scholar]
- Cardinale, D.M.; Zaninotto, M.; Cipolla, C.M.; Passino, C.; Plebani, M.; Clerico, A. Cardiotoxic effects and myocardial injury: The search for a more precise definition of drug cardiotoxicity. Clin. Chem. Lab. Med. 2020, 59, 51–57. [Google Scholar] [CrossRef]
- Maffuz-Aziz, A.L.-A.; Espejo-Fonseca, A.; Rodriguez-Cuevas, S. Caracteristicas clinicopatologicas del cancer de mama en una poblacion de mujeres en Mexico. Cir. Cir. 2017, 85, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Diaz, M.; Camejo, N.; Reborido, N.; Vazquez, H.; Parma, G.; Vazquez, A.; Castillo, C.; Krygier, G.D.; Delgado, L.B. Trastuzumab-induced cardiotoxicity in Uruguayan HER2-positive breast cancer patients. Arch. Med. Interna 2015, 37, 109–113. [Google Scholar] [CrossRef]
- Tarantini, L.; Cioffi, G.; Gori, S.; Tuccia, F.; Boccardi, L.; Bovelli, D.; Lestuzzi, C.; Maurea, N.; Oliva, S.; Russo, G.; et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J. Card. Fail. 2012, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.-L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.-L.; Simcock, R.; Rea, D.; et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef]
| General | Cardiotoxicity | |||
|---|---|---|---|---|
| Characteristics | (n = 46) | Yes (n = 19) | No (n = 27) | p Value * |
| Age (years) [mean ± SD] | 53.6 ± 8.7 | 54.9 ± 10.3 | 52.33 ± 7.4 | 0.320 |
| Medical history | ||||
| Tobacco consumption, n (%) | 5 (10.9%) | 4 (21.1%) | 1 (3.7%) | 0.144 |
| Diabetes mellitus II, n (%) | 6 (13%) | 4 (21.1%) | 2 (7.4%) | 0.213 |
| Hypertension, n (%) | 15 (32.6%) | 7 (36.8%) | 8 (29.6%) | 0.607 |
| Menopause, n (%) | 30 (65.2%) | 13 (68.4%) | 17 (63.0%) | 0.783 |
| Hypercholesterolemia, n (%) | 26 (56.5%) | 12 (63.2%) | 14 (51.9%) | 0.532 |
| Blood cholesterol (mg/dL) [mean ± SD] | 209.3 ± 52.1 | 212.8 ± 56.4 | 206.7 ± 9.7 | 0.704 |
| Physical examination | ||||
| Weight (kg) [mean ± SD] | 67.2 ± 13.4 | 72.7 ± 16 | 63.2 ± 9.4 | 0.028 ** |
| Height [mean ± SD] | 153.4 ± 6.2 | 154.7 ± 5.1 | 152.4 ± 6.8 | 0.223 |
| Body surface (m2) [mean ± SD] | 1.7 ± 0.2 | 1.8 ± 0.2 | 1.6 ± 0.1 | 0.014 ** |
| BMI | ||||
| Underweight | 1 (2.2%) | 0 | 1 (3.7%) | 0.508 |
| Healthy weight | 13 (28.2%) | 5 (26.3%) | 7 (25.9%) | |
| Overweight | 16 (34.8%) | 5 (26.3%) | 11 (40.8%) | |
| Obesity | 17 (36.9%) | 9 (47.4%) | 8 (29.6%) | |
| Cancer description | ||||
| Infiltrating ductal carcinoma, n (%) | 33 (71.7%) | 13 (68.4%) | 20 (74.1%) | 0.675 |
| Clinical stage, n (%) | ||||
| I | 3 (6.5%) | 1 (5.3%) | 2 (7.4%) | 0.461 |
| II | 22 (47.8%) | 8 (42.1%) | 14 (51.8%) | |
| III | 16 (34.8%) | 7 (36.8%) | 9 (33.3%) | |
| IV | 1 (2.2%) | 1 (5.3%) | 0 | |
| Not specified | 4 (8.7%) | 2 (10.5%) | 2 (7.4%) | |
| Tumor side | ||||
| Left breast cancer, n (%) | 20 (43.5%) | 7 (36.8%) | 13 (48.1%) | 0.446 |
| Hormonal receptors | ||||
| ER/PRR positive | 11 (23.9%) | 4 (21.1%) | 7 (25.9%) | 0.451 |
| ER/PRR negative | 24 (52.2%) | 10 (52.6%) | 14 (51.9%) | |
| ER−/PRR+ | 5 10.9%) | 1 (5.3%) | 4 (14.8%) | |
| ER+/PRR− | 6 (13.0%) | 4 (21.1%) | 2 (7.4%) | |
| Oncological treatment | ||||
| Mastectomy | 46 (100%) | 19 (100%) | 27 (100%) | 1 |
| Radiotherapy | 44 (95.7%) | 18 (94.7%) | 26 (96.3%) | 0.798 |
| Radiotherapy + chemotherapy [n, %] | 41 (89.1%) | 16 (84.2%) | 25 (92.6%) | 0.368 |
| Anthracycline | ||||
| No | 4 (8.7%) | 3 (15.8%) | 1 (3.7%) | 0.292 |
| Epirubicin + fluorouracil + ciclophosphamide | 41 (89.1%) | 15 (78.9%) | 26 (96.3%) | 0.197 |
| Epirubicin + ciclophosphamide | 1 (2.2%) | 1 (5.3%) | 0 | |
| Taxane | ||||
| No | 9 (19.6%) | 4 (21.1%) | 5 (18.5%) | 0.831 |
| Docetaxel | 34 (73.9%) | 12 (63.2%) | 22 (81.5%) | 0.060 |
| Paclitaxel | 3 (6.5%) | 3 (15.8%) | 0 | |
| Both anthracycline and taxane | 33 (71.7%) | 12 (63.2%) | 21 (77.8%) | 0.278 |
| TTZ loading dose (mg) [median, IR] | 452, 143 | 540, 200 | 440, 73 | 0.041 ** |
| TTZ loading dose (mg/Kg) [median, IR] | 7.9, 0.6 | 7.8, 0.5 | 7.9, 1.0 | 0.849 |
| TTZ maintenance doses (mg) [median, IR] | 390, 105 | 432, 140 | 375, 77 | 0.100 |
| TTZ duration of treatment (months) [median, IR] | 11, 1 | 11, 3 | 11, 0 | 0.137 |
| TTZ doses administered [median, IR] | 17, 1 | 17, 2 | 17, 1 | 0.725 |
| Heart function | ||||
| Basal LVEF [mean ± SD] | 63.6 ± 6.4 | 64.5 ± 8.0 | 62.9 ± 4.9 | 0.459 |
| Follow-up LVEF [mean ± SD] | 59.7 ± 5.7 | 56.1 ± 5.5 | 62.3 ± 4.2 | <0.001 * |
| Characteristics | CT (n = 19) | No CT (n = 27) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 15 (78.9%) | 24 (88.9%) | 1 | |
| >60 | 4 (21.1%) | 3 (11.1%) | 4.9 (0.4–64.8) | 0.228 |
| Tobacco consumption | ||||
| No | 15 (78.9%) | 26 (96.3%) | 1 | |
| Yes | 4 (21.1%) | 1 (3.7%) | 13.6 (0.9–209) | 0.061 |
| Diabetes mellitus II | ||||
| No | 15 (78.9%) | 25 (9.26%) | 1 | |
| Yes | 4 (21.1%) | 2 (7.4%) | 1.3 (0.08–19.4) | 0.865 |
| Hypertension | ||||
| No | 12 (63.2%) | 19 (70.4%) | 1 | |
| Yes | 7 (36.8%) | 8 (29.6%) | 0.9 (0.1–5.8) | 0.868 |
| Obesity | ||||
| No | 10 (52.6%) | 19 (70.4%) | 1 | |
| Yes | 9 (47.4%) | 8 (29.6%) | 1.5 (0.3–6.8) | 0.597 |
| Hypercholesterolemia | ||||
| No | 7 (36.8%) | 13 (48.1%) | 1 | |
| Yes | 12 (63.2%) | 14 (51.8%) | 2.0 (0.4–10.6) | 0.406 |
| Tumor side | ||||
| Right breast cancer, n (%) | 12 (63.2%) | 14 (51.9%) | 1 | |
| Left breast cancer, n (%) | 7 (36.8%) | 13 (48.1%) | 0.6 (0.1–3.6) | 0.550 |
| Chemotherapy and taxane | ||||
| No | 7 (36.8%) | 6 (22.2%) | 1 | |
| Yes | 12 (63.5%) | 21 (77.8%) | 0.2 (0.1–3.4) | 0.276 |
| Taxane | ||||
| No | 4 (21.1%) | 5 (18.5%) | 1 | |
| Yes | 15 (78.9%) | 22 (18.5%) | 3.4 (0.2–98.6) | 0.478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Mathey, L.I.; Velez-Figueroa, M.I.; Díaz-Vallejo, J.J.; Mendez-Hirata, G.; Mendez-Machado, G.F. Prevalence of Cardiotoxicity Secondary to Trastuzumab in Patients with HER-2-Positive Breast Cancer in Southeast Mexico. Reports 2024, 7, 76. https://doi.org/10.3390/reports7030076
Pascual-Mathey LI, Velez-Figueroa MI, Díaz-Vallejo JJ, Mendez-Hirata G, Mendez-Machado GF. Prevalence of Cardiotoxicity Secondary to Trastuzumab in Patients with HER-2-Positive Breast Cancer in Southeast Mexico. Reports. 2024; 7(3):76. https://doi.org/10.3390/reports7030076
Chicago/Turabian StylePascual-Mathey, Luz I., Midory I. Velez-Figueroa, Joel J. Díaz-Vallejo, Gustavo Mendez-Hirata, and Gustavo F. Mendez-Machado. 2024. "Prevalence of Cardiotoxicity Secondary to Trastuzumab in Patients with HER-2-Positive Breast Cancer in Southeast Mexico" Reports 7, no. 3: 76. https://doi.org/10.3390/reports7030076
APA StylePascual-Mathey, L. I., Velez-Figueroa, M. I., Díaz-Vallejo, J. J., Mendez-Hirata, G., & Mendez-Machado, G. F. (2024). Prevalence of Cardiotoxicity Secondary to Trastuzumab in Patients with HER-2-Positive Breast Cancer in Southeast Mexico. Reports, 7(3), 76. https://doi.org/10.3390/reports7030076






