Infantile Hemangioma: Risk Factors and Management in a Preterm Patient—A Case Report
Abstract
1. Introduction
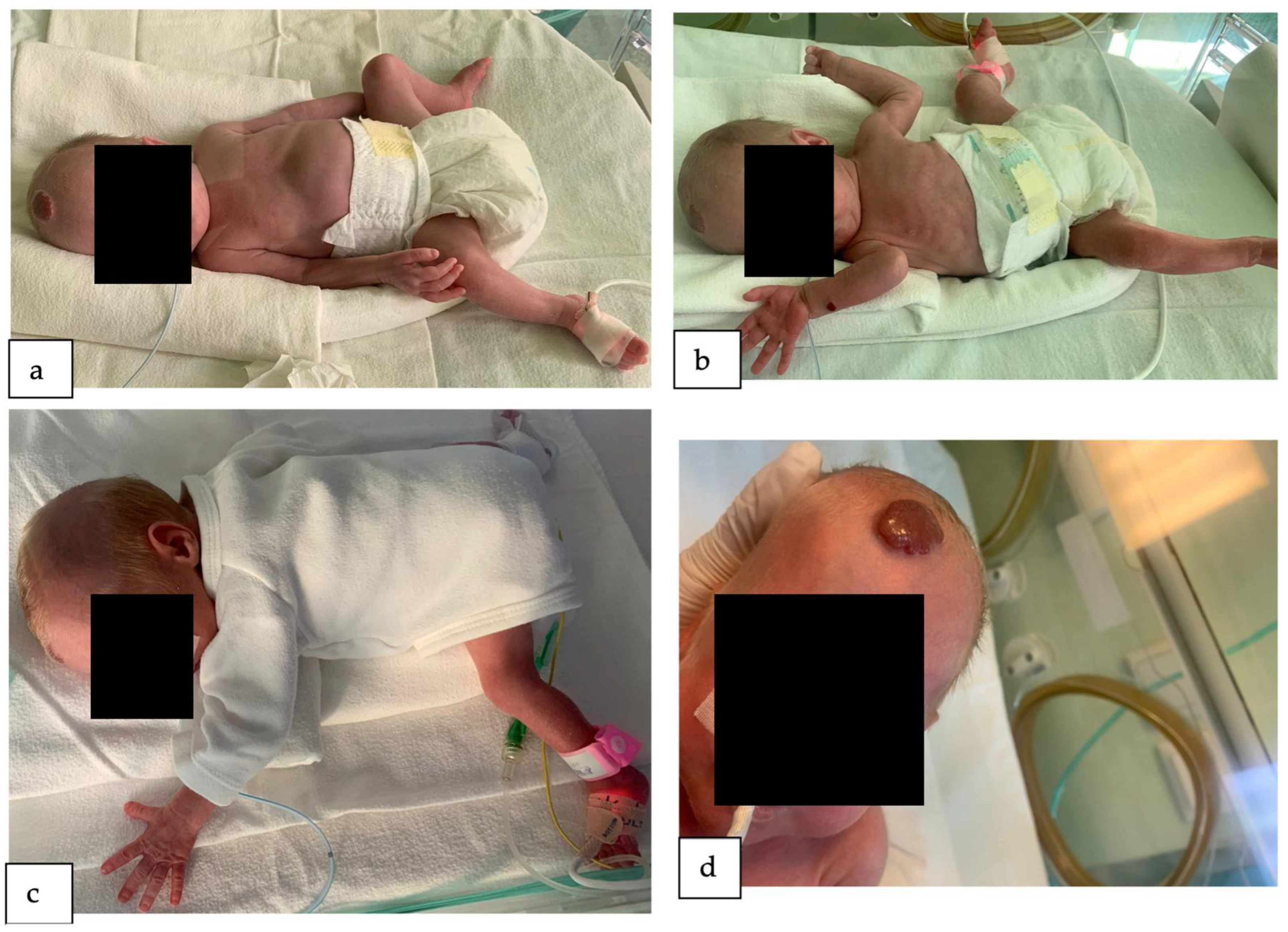
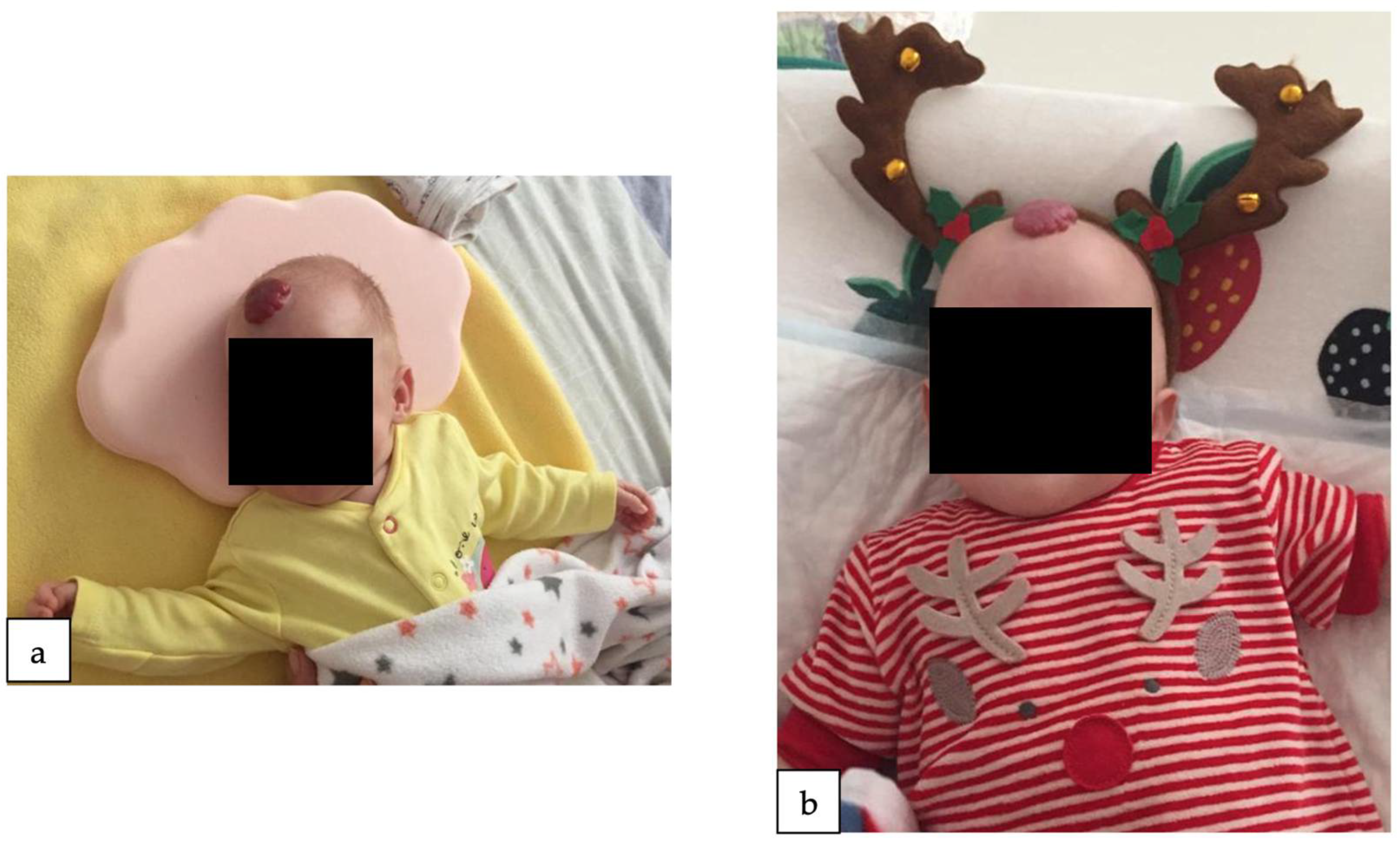
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickison, P.; Christou, E.; Wargon, O. A Prospective Study of Infantile Hemangiomas with a Focus on Incidence and Risk Factors. Pediatr. Dermatol. 2011, 28, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Kilcline, C.; Frieden, I.J. Infantile Hemangiomas: How Common Are They? A Systematic Review of the Medical Literature. Pediatr. Dermatol. 2008, 25, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Munden, A.; Butschek, R.; Tom, W.L.; Marshall, J.S.; Poeltler, D.M.; Krohne, S.E.; Aliõ, A.B.; Ritter, M.; Friedlander, D.F.; Catanzarite, V.; et al. Prospective Study of Infantile Haemangiomas: Incidence, Clinical Characteristics and Association with Placental Anomalies. Br. J. Dermatol. 2014, 170, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Hoornweg, M.J.; Smeulders, M.J.C.; Ubbink, D.T.; Van Der Horst, C.M.A.M. The Prevalence and Risk Factors of Infantile Haemangiomas: A Case-Control Study in the Dutch Population. Paediatr. Perinat. Epidemiol. 2012, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kanada, K.N.; Merin, M.R.; Munden, A.; Friedlander, S.F. A Prospective Study of Cutaneous Findings in Newborns in the United States: Correlation with Race, Ethnicity, and Gestational Status Using Updated Classification and Nomenclature. J. Pediatr. 2012, 161, 240–245. [Google Scholar] [CrossRef]
- Chiller, K.G.; Passaro, D.; Frieden, I.J. Hemangiomas of Infancy: Clinical Characteristics, Morphologic Subtypes, and Their Relationship to Race, Ethnicity, and Sex. Arch. Dermatol. 2002, 138, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, A.L.; Frieden, I.J. Hemangiomas of Infancy. J. Am. Acad. Dermatol. 2003, 48, 477–496. [Google Scholar] [CrossRef]
- Rodríguez Bandera, A.I.; Sebaratnam, D.F.; Wargon, O.; Wong, L.C.F. Infantile Hemangioma. Part 1: Epidemiology, Pathogenesis, Clinical Presentation and Assessment. J. Am. Acad. Dermatol. 2021, 85, 1379–1392. [Google Scholar] [CrossRef]
- Chang, L.C.; Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.L.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Mancini, A.J.; Metry, D.W.; et al. Growth Characteristics of Infantile Hemangiomas: Implications for Management. Pediatrics 2008, 122, 360–367. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L. Infantile Hemangioma: An Updated Review. Curr. Pediatr. Rev. 2020, 17, 55–69. [Google Scholar] [CrossRef]
- Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.L.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Mancini, A.J.; Metry, D.W.; Newell, B.; et al. Prospective Study of Infantile Hemangiomas: Clinical Characteristics Predicting Complications and Treatment. Pediatrics 2006, 118, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Boye, E.; Jinnin, M.; Olsen, B.R. Infantile Hemangioma: Challenges, New Insights, and Therapeutic Promise. J. Craniofac. Surg. 2009, 20 (Suppl. S1), 678–684. [Google Scholar] [CrossRef] [PubMed]
- Mizawa, M.; Matsumura, K.; Hamazaki, K.; Furukawa, F.; Makino, T.; Shimizu, T.; Inadera, H.; Kamijima, M.; Yamazaki, S.; Ohya, Y.; et al. Infantile Hemangioma and the Risk Factors in a Japanese Population: A Nationwide Longitudinal Study-The Japan Environment and Children’s Study. J. Investig. Dermatol. 2021, 141, 2745–2748.e2. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Qiu, T.; Feng, L.; Yang, K.; Dai, S.; Zhou, J.; Zhang, X.; Chen, S.; Ji, Y. Maternal and Perinatal Risk Factors for Infantile Hemangioma: A Matched Case-Control Study with a Large Sample Size. Dermatol. Ther. 2022, 12, 1659. [Google Scholar] [CrossRef]
- Petca, A.; Miron, B.C.; Pacu, I.; Dumitrașcu, M.C.; Mehedințu, C.; Șandru, F.; Petca, R.C.; Rotar, I.C. HELLP Syndrome—Holistic Insight into Pathophysiology. Medicina 2022, 58, 326. [Google Scholar] [CrossRef] [PubMed]
- Cristian Dumitrascu, M.; Maria Alexandra Stanescu, A.; Bejan, C.; Sandru, F.; Oana Toader, D.; George Radavoi, D.; Cotirlet, A.; Teodora Judea Pusta, C.; Cristina Diaconu, C. Obesity and Its Implications on Stress Urinary Incontinence. Rev. Chim. 2019, 70, 3660–3662. [Google Scholar] [CrossRef]
- Paredes, C.; Hsu, R.C.; Tong, A.; Johnson, J.R. Obesity and Pregnancy. Neoreviews 2021, 22, e78–e87. [Google Scholar] [CrossRef]
- Sandru, F.; Turenschi, A.; Constantin, A.T.; Dinulescu, A.; Radu, A.-M.; Rosca, I. Infantile Hemangioma: A Cross-Sectional Observational Study. Life 2023, 13, 1868. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Bandera, A.I.; Sebaratnam, D.F.; Feito Rodríguez, M.; de Lucas Laguna, R. Cutaneous Ultrasound and Its Utility in Pediatric Dermatology: Part II-Developmental Anomalies and Vascular Lesions. Pediatr. Dermatol. 2020, 37, 40–51. [Google Scholar] [CrossRef]
- Frieden, I.J.; Reese, V.; Cohen, D. PHACE Syndrome. The Association of Posterior Fossa Brain Malformations, Hemangiomas, Arterial Anomalies, Coarctation of the Aorta and Cardiac Defects, and Eye Abnormalities. Arch. Dermatol. 1996, 132, 307–311. [Google Scholar] [CrossRef]
- Iacobas, I.; Burrows, P.E.; Frieden, I.J.; Liang, M.G.; Mulliken, J.B.; Mancini, A.J.; Kramer, D.; Paller, A.S.; Silverman, R.; Wagner, A.M.; et al. LUMBAR: Association between Cutaneous Infantile Hemangiomas of the Lower Body and Regional Congenital Anomalies. J. Pediatr. 2010, 157, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Chamlin, S.L.; Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Frieden, I.J.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Metry, D.W.; Newell, B.; et al. Multicenter Prospective Study of Ulcerated Hemangiomas. J. Pediatr. 2007, 151, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, P.; Wu, J.K. Current Perspectives on the Optimal Management of Infantile Hemangioma. Pediatr. Health Med. Ther. 2017, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.A.; Adams, S.; Wargon, O. Segmental Haemangiomas of Infancy: A Review of 14 Cases. Australas. J. Dermatol. 2006, 47, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Hogeling, M.; Adams, S.; Wargon, O. A Randomized Controlled Trial of Propranolol for Infantile Hemangiomas. Pediatrics 2011, 128, e259–e266. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.Z.; Yu, S.R.; Xiang, F.; Kang, X.J. Risk Factors for Infantile Hemangioma: A Meta-Analysis. World J. Pediatr. 2020, 16, 377–384. [Google Scholar] [CrossRef]
- Janmohamed, S.R.; Madern, G.C.; de Laat, P.C.J.; Oranje, A.P. Educational Paper: Pathogenesis of Infantile Haemangioma, an Update 2014 (Part I). Eur. J. Pediatr. 2015, 174, 97–103. [Google Scholar] [CrossRef]
- Greenberger, S.; Bischoff, J. Pathogenesis of Infantile Haemangioma. Br. J. Dermatol. 2013, 169, 12–19. [Google Scholar] [CrossRef]
- Steer, P.J. Maternal and Perinatal Morbidity and Mortality Associated With Anemia in Pregnancy. Obstet. Gynecol. 2020, 135, 731. [Google Scholar] [CrossRef]
- Castrén, E.; Salminen, P.; Vikkula, M.; Pitkäranta, A.; Klockars, T. Inheritance Patterns of Infantile Hemangioma. Pediatrics 2016, 138, e20161623. [Google Scholar] [CrossRef] [PubMed]
- Metry, D.W.; Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.; Garzon, M.; Horii, K.; Lucky, A.; Mancini, A.J.; Newell, B.; et al. A Prospective Study of PHACE Syndrome in Infantile Hemangiomas: Demographic Features, Clinical Findings, and Complications. Am. J. Med. Genet. A 2006, 140, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Krowchuk, D.P.; Frieden, I.J.; Mancini, A.J.; Darrow, D.H.; Blei, F.; Greene, A.K.; Annam, A.; Baker, C.N.; Frommelt, P.C.; Hodak, A.; et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 2019, 143, e20183475. [Google Scholar] [CrossRef] [PubMed]
- Stockman, A.; Boralevi, F.; Taïeb, A.; Léauté-Labrèze, C. SACRAL Syndrome: Spinal Dysraphism, Anogenital, Cutaneous, Renal and Urologic Anomalies, Associated with an Angioma of Lumbosacral Localization. Dermatology 2007, 214, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Rosca, I.; Turenschi, A.; Nicolescu, A.; Constantin, A.T.; Canciu, A.M.; Dica, A.D.; Bratila, E.; Coroleuca, C.A.; Nastase, L. Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report. Medicina 2023, 59, 856. [Google Scholar] [CrossRef] [PubMed]
- Garzon, M.C.; Frieden, I.J. Hemangiomas: When to Worry. Pediatr. Ann. 2000, 29, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; Frieden, I.; Delarue, A. Early Initiation of Treatment with Oral Propranolol for Infantile Hemangioma Improves Success Rate. Pediatr. Dermatol. 2023, 40, 261–264. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S.; Yang, K.; Zhang, X.; Zhou, J.; Li, L.; Xiang, B.; Qiu, T.; Dai, S.; Jiang, X.; et al. Efficacy and Safety of Propranolol vs Atenolol in Infants With Problematic Infantile Hemangiomas: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 599–607. [Google Scholar] [CrossRef]
- Pope, E.; Lara-Corrales, I.; Sibbald, C.; Liy-Wong, C.; Kanigsberg, N.; Drolet, B.; Ma, J. Noninferiority and Safety of Nadolol vs Propranolol in Infants with Infantile Hemangioma: A Randomized Clinical Trial. JAMA Pediatr. 2022, 176, 34–41. [Google Scholar] [CrossRef]
- Dahan, E.; Jaoude, L.A. Infantile Hemangiomas: A Review of Current Treatment Options. Pediatr. Ann. 2023, 52, 192–197. [Google Scholar] [CrossRef]
- Khamaysi, Z.; Pam, N.; Zaaroura, H.; Avitan-Hersh, E. Nd:YAG 1064-Nm Laser for Residual Infantile Hemangioma after Propranolol Treatment. Sci. Rep. 2023, 13, 7474. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandru, F.; Petca, A.; Radu, A.-M.; Preda, A.G.; Turenschi, A.; Constantin, A.T.; Miulescu, R.-G. Infantile Hemangioma: Risk Factors and Management in a Preterm Patient—A Case Report. Reports 2024, 7, 3. https://doi.org/10.3390/reports7010003
Sandru F, Petca A, Radu A-M, Preda AG, Turenschi A, Constantin AT, Miulescu R-G. Infantile Hemangioma: Risk Factors and Management in a Preterm Patient—A Case Report. Reports. 2024; 7(1):3. https://doi.org/10.3390/reports7010003
Chicago/Turabian StyleSandru, Florica, Aida Petca, Andreea-Maria Radu, Andrei Gheorghe Preda, Alina Turenschi, Andreea Teodora Constantin, and Raluca-Gabriela Miulescu. 2024. "Infantile Hemangioma: Risk Factors and Management in a Preterm Patient—A Case Report" Reports 7, no. 1: 3. https://doi.org/10.3390/reports7010003
APA StyleSandru, F., Petca, A., Radu, A.-M., Preda, A. G., Turenschi, A., Constantin, A. T., & Miulescu, R.-G. (2024). Infantile Hemangioma: Risk Factors and Management in a Preterm Patient—A Case Report. Reports, 7(1), 3. https://doi.org/10.3390/reports7010003





