Squamotransitional Cell Carcinoma of the Uterine Cervix with Ovarian Metastasis and Benign Brenner Tumor: A Case Report
Abstract
1. Introduction
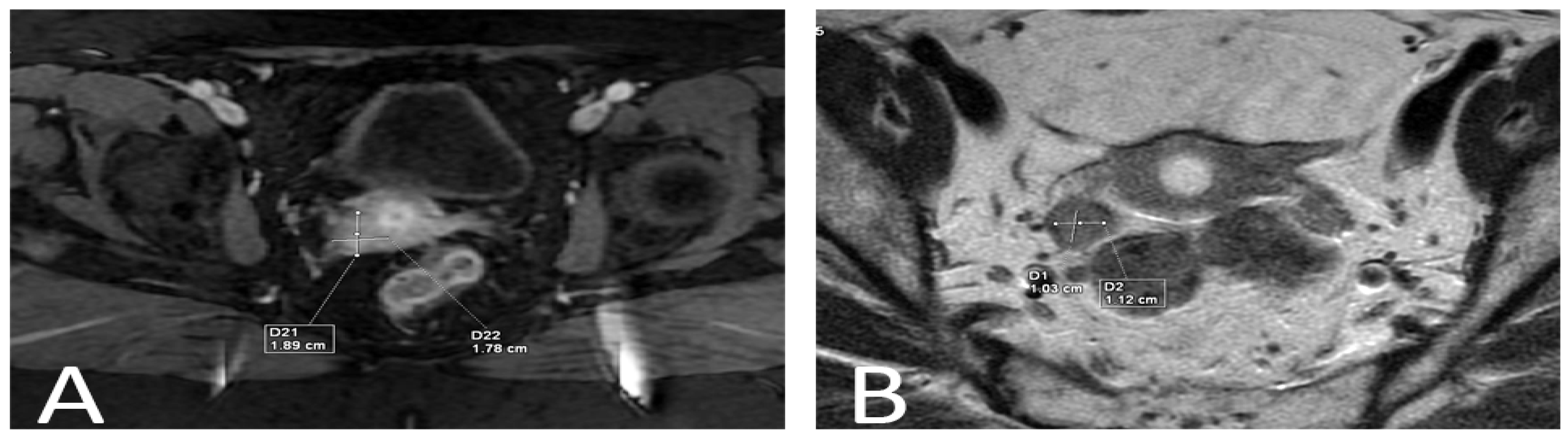
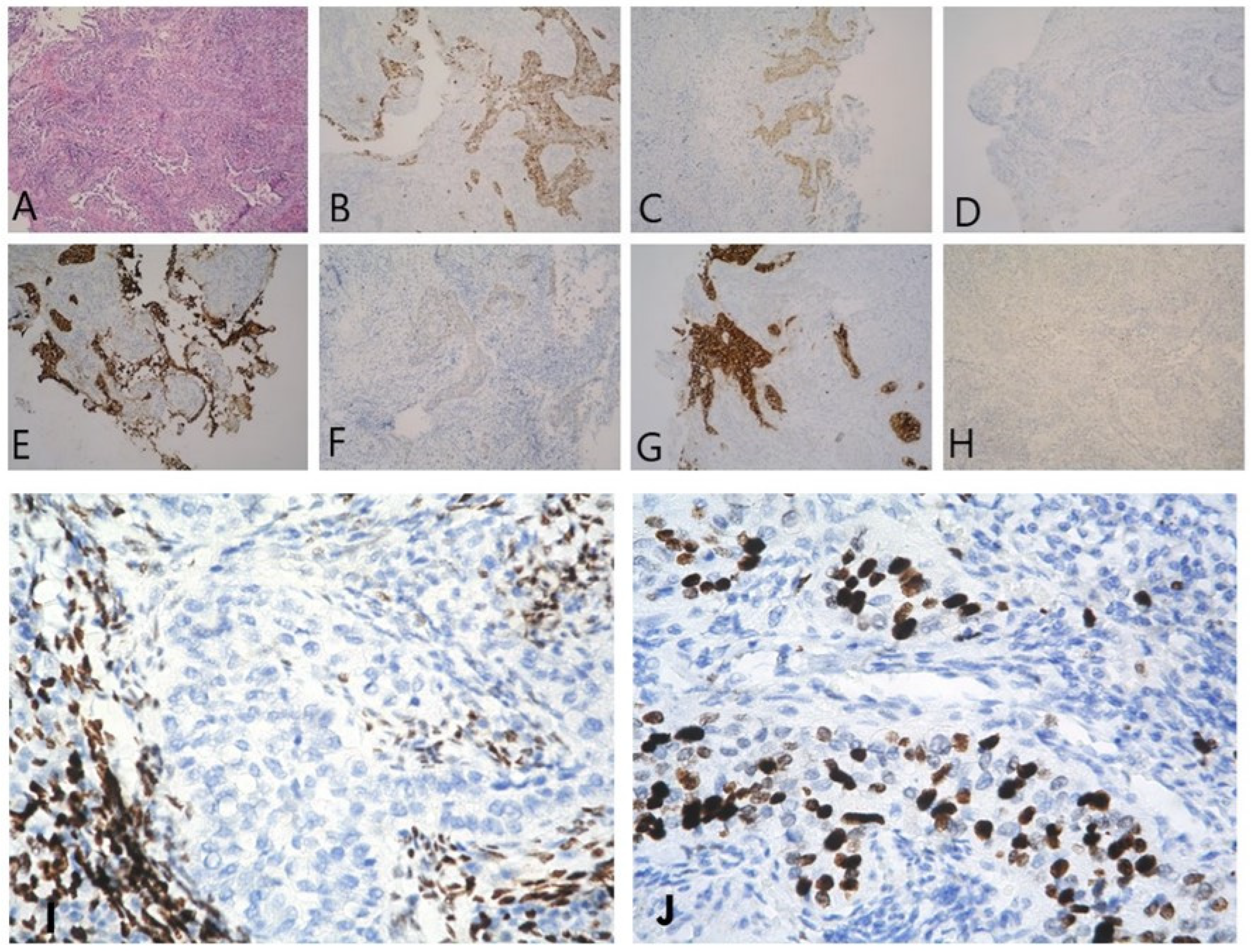
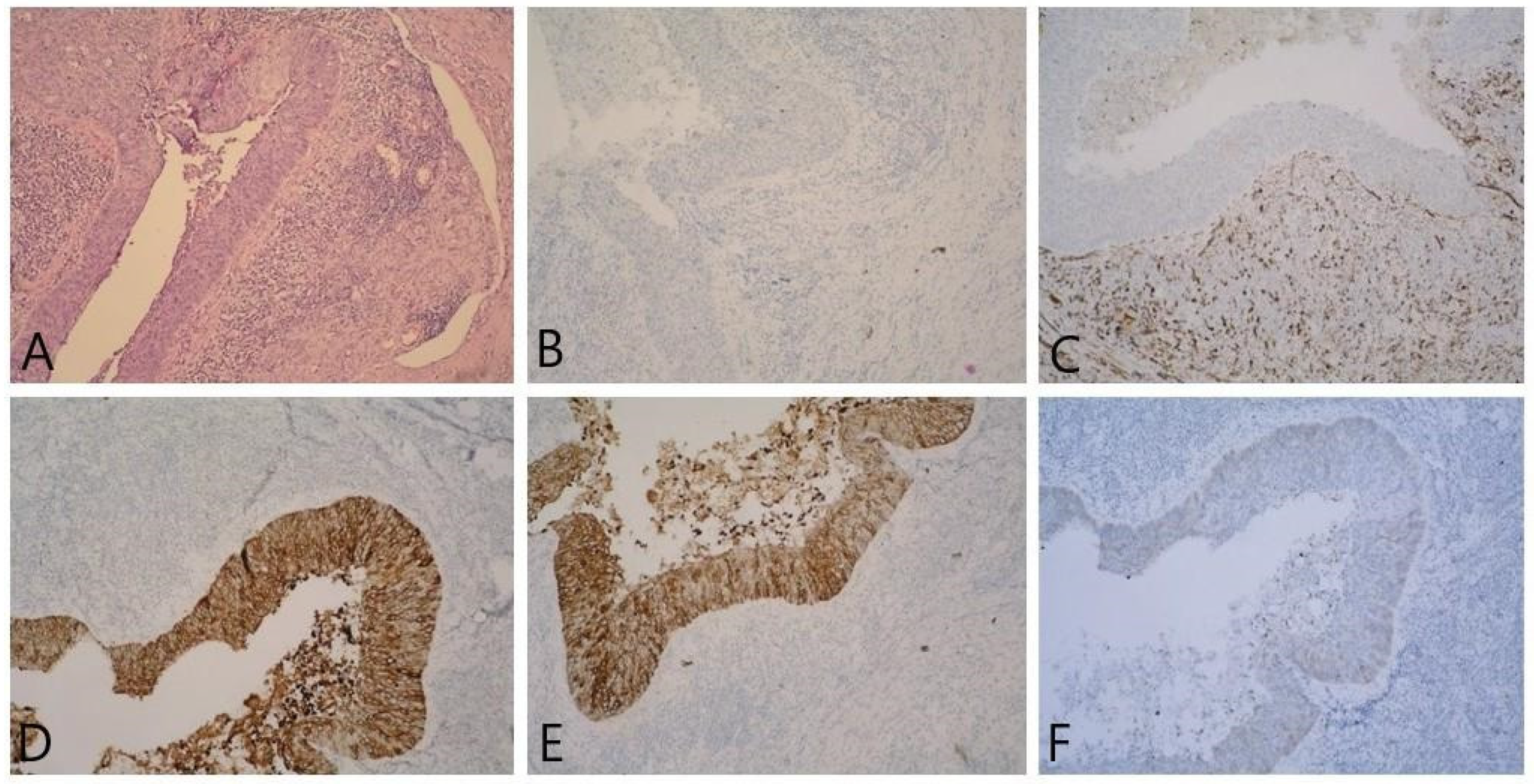
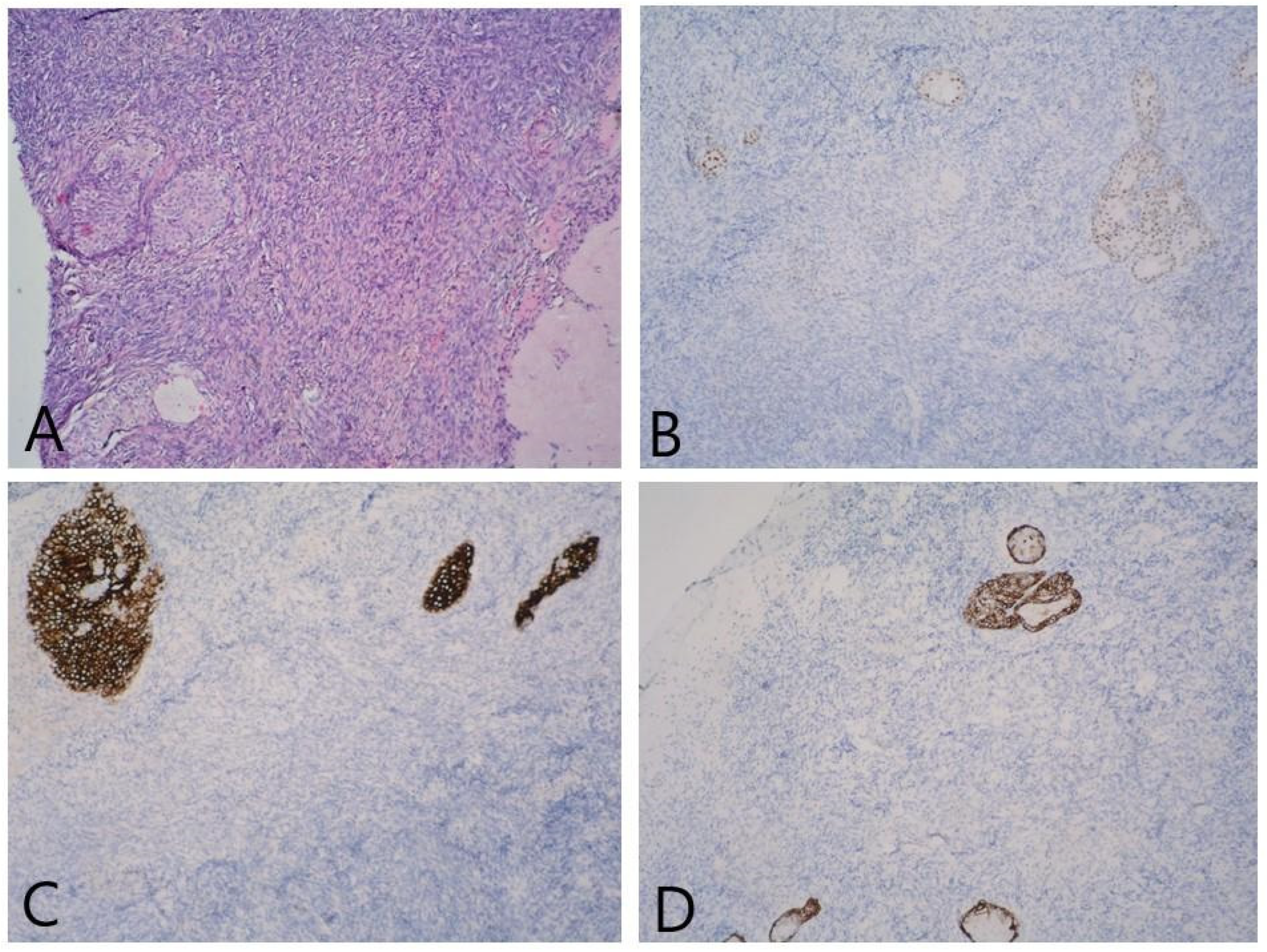
2. Detailed Case Description
3. Discussion
- Predominantly squamous (28.1%);
- Mixed squamous and transitional (50%);
- Predominantly transitional (21.9%).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuzick, J.; Du, R.; Adcock, R.; Kinney, W.; Joste, N.; McDonald, R.M.; English, K.; Torres, S.M.; Saslow, D.; Wheeler, C.M. The New Mexico HPV Pap Registry Steering Committee. Uptake of co-testing with HPV and cytology for cervical screening: A population-based evaluation in the United States. Gynecol Oncol. 2021, 162, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Intaraphet, S.; Kasatpibal, N.; Siriaunkgul, S.; Sogaard, M.; Patumanond, J.; Khunamornpong, S.; Chandacham, A.; Suprasert, P. Prognostic impact of histology in patients with cervical squamous cell carcinoma, adenocarcinoma and small cell neuroendocrine carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 5355–5360. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://screening.iarc.fr/atlasclassifwho.php (accessed on 10 April 2023).
- Randall, M.E.; Andersen, W.A.; Mills, S.E.; Kim, J.A. Papillary squamous cell carcinoma of the uterine cervix: A clinicopathologic study of nine cases. Int. J. Gynecol. Pathol. 1986, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, A.; Karaivanov, M.; Kostov, S.; Kornovski, Y.; Ivanova, Y.; Slavchev, S.; Todorova, V.; Vasileva-Slaveva, M. Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix with Atypical Presentation: A Case Report with a Literature Review. Medicina 2022, 58, 1838. [Google Scholar] [CrossRef]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu–Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef]
- Gitas, G.; Ertan, K.; Rody, A.; Baum, S.; Tsolakidis, D.; Alkatout, I. Papillary squamotransitional cell carcinoma of the uterine cervix: A case report and review of the literature. J. Med. Case Rep. 2019, 13, 319. [Google Scholar] [CrossRef]
- Austin, R.M.; Norris, H.J. Malignant Brenner tumor and transitional cell carcinoma of the ovary: A comparison. Int. J. Gynecol. Pathol. 1987, 6, 29–39. [Google Scholar] [CrossRef]
- Bass, P.S.; Birch, B.; Smart, C.; Theaker, J.M.; Wells, M. Low-grade transitional cell carcinoma of the vagina--an unusual cause of vaginal bleeding. Histopathology 1994, 24, 581–583. [Google Scholar] [CrossRef]
- Marsh, M.R. Papilloma of the cervix. Am. J. Obstet. Gynecol. 1952, 64, 281–291. [Google Scholar] [CrossRef]
- Wells, M.; Östör, A.G.; Crum, C.P. Tumours of the uterine cervix. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs, 3rd ed.; Tavassoli, F.A., Devilee, P., Eds.; IARC: Lyon, France, 2003; pp. 259–289. [Google Scholar]
- Koenig, C.; Turnicky, R.P.; Kankam, C.F.; Tavassoli, F.A. Papillary squamotransitional cell carcinoma of the cervix: A report of 32 cases. Am. J. Surg. Pathol. 1997, 21, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Al-Nafussi, A.I.; Al-Yusif, R. Papillary squamotransitional cell carcinoma of the uterine cervix: An advanced stage disease despite superficial location: Report of two cases and review of the literature. Eur. J. Gynaecol. Oncol. 1998, 19, 455–457. [Google Scholar] [PubMed]
- Woo, H.Y.; Kim, H.-S. Local and Metastatic Relapses in a Young Woman with Papillary Squamous Cell Carcinoma of the Uterine Cervix. Diagnostics 2022, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Apte, S.M.; Campos, S.M.; Cho, K.R. Cervical cancer, version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lininger, R.A.; Ashfaq, R.; Albores-Saavedra, J.; Tavassoli, F.A. Transitional cell carcinoma of the endometrium and endometrial carcinoma with transitional cell differentiation. Cancer 1997, 79, 1933–1943. [Google Scholar] [CrossRef]
- Patrelli, T.S.; Silini, E.M.; Berretta, R.; Thai, E.; Gizzo, S.; Bacchi Modena, A.; Nardelli, G.B. Squamotransitional cell carcinoma of the vagina: Diagnosis and clinical management: A literature review starting from a rare case report. Pathol. Oncol. Res. 2011, 17, 149–153. [Google Scholar] [CrossRef]
- Tong, J.; Kasznica, J.; Habib, F. Transitional cell carcinoma of endometrium arising in a polypoid adenomyoma in the endometrial cavity. Am. J. Clin. Pathol. 2013, 140 (Suppl. 1), A188. [Google Scholar] [CrossRef][Green Version]
- Lee, D.-H.; Cho, D.-H.; Kim, K.M.; Yim, C.-Y.; Lee, N.-R. Primary transitional cell carcinoma of the fallopian tube: A case report and literature review. Medicine 2020, 99, e20499. [Google Scholar] [CrossRef]
- Dum, D.; Menz, A.; Völkel, C.; De Wispelaere, N.; Hinsch, A.; Gorbokon, N.; Lennartz, M.; Luebke, A.M.; Hube-Magg, C.; Kluth, M.; et al. Cytokeratin 7 and cytokeratin 20 expression in cancer: A tissue microarray study on 15,424 cancers. Exp. Mol. Pathol. 2022, 126, 104762. [Google Scholar] [CrossRef]
- Roma, A.A.; Masand, R.P. Different staining patterns of ovarian Brenner tumor and the associated mucinous tumor. Ann. Diagn. Pathol. 2015, 19, 29–32. [Google Scholar] [CrossRef]
- Giordano, G.; D’Adda, T.; Gnetti, L.; Merisio, C.; Raboni, S. Transitional cell carcinoma of the endometrium associated with benign ovarian brenner tumor: A case report with immunohistochemistry molecular analysis and a review of the literature. Int. J. Gynecol. Pathol. 2007, 26, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Chen, J.-F.; Al-Ahmadie, H. Urothelial Carcinoma: Divergent Differentiation and Morphologic Subtypes. Surg. Pathol. Clin. 2022, 15, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Terzic, T.; Mills, A.M.; Zadeh, S.; Atkins, K.A.; Hanley, K.Z. GATA3 Expression in Common Gynecologic Carcinomas: A Potential Pitfall. Int. J. Gynecol. Pathol. 2019, 38, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Drew, P.A.; Hong, B.; Massoll, N.A.; Ripley, D.L. Characterization of Papillary Squamotransitional Cell Carcinoma of the Cervix. J. Low. Genit. Tract Dis. 2005, 9, 149–153. [Google Scholar] [CrossRef]
- Schwartz, L.E.; Khani, F.; Bishop, J.A.; Vang, R.; Epstein, J.I. Carcinoma of the Uterine Cervix Involving the Genitourinary Tract: A Potential Diagnostic Dilemma. Am. J. Surg. Pathol. 2016, 40, 27–35. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours WHO Classification of Tumours, 5th ed.; IARC: Lyon, France, 2020; Volume 4, pp. 342–351. [Google Scholar]

| Author/Article | Type of Reported Tumor (as Reported) | Primary Site | Site(s) Involved | Positive Expression | Negative Expression |
|---|---|---|---|---|---|
| Lininger RA et al. [17] | transitional cell carcinomas | transitional cell carcinomas can affect the ovaries, fallopian tubes, cervix, and endometrium | tumors originating from the endometrium can metastasize to the ovaries, ovarian transitional cell carcinoma may metastasize to the myometrium | CK 7 (some cases might be negative) | CK 20 |
| Patrelli TS et al. [18] | squamous urothelial carcinoma | vagina | one site was involved (no spread of the disease) | CK 7, p16 ink4a, p63 | CK 20 |
| Tong J et al. [19] | transitional cell carcinoma | endometrium (arising in a polypoid adenomyoma) | one site was involved (urinary tract carcinoma was excluded) | CK AE1/AE3, CAM5.2, CK7, EMA, PAX-8, CD56 | CK20 |
| Gitas et al. [8] | papillary squamotransitional carcinoma | uterine cervix | ovarian involvement | CK7, p63, CK5, CK14, p16 | CK20 and ER |
| Lee DH et al. [20] | transitional cell carcinoma | fallopian tube | pelvic rectal peritoneum involvement | CK7 and EMA | p53, CK20, p63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Karaivanov, M.; Ivanov, I.; Kostov, S.; Todorova, V.; Iliev, I.; Tzoneva, E.; Strateva, D. Squamotransitional Cell Carcinoma of the Uterine Cervix with Ovarian Metastasis and Benign Brenner Tumor: A Case Report. Reports 2023, 6, 54. https://doi.org/10.3390/reports6040054
Yordanov A, Karaivanov M, Ivanov I, Kostov S, Todorova V, Iliev I, Tzoneva E, Strateva D. Squamotransitional Cell Carcinoma of the Uterine Cervix with Ovarian Metastasis and Benign Brenner Tumor: A Case Report. Reports. 2023; 6(4):54. https://doi.org/10.3390/reports6040054
Chicago/Turabian StyleYordanov, Angel, Milen Karaivanov, Ivan Ivanov, Stoyan Kostov, Venelina Todorova, Ilko Iliev, Eva Tzoneva, and Diana Strateva. 2023. "Squamotransitional Cell Carcinoma of the Uterine Cervix with Ovarian Metastasis and Benign Brenner Tumor: A Case Report" Reports 6, no. 4: 54. https://doi.org/10.3390/reports6040054
APA StyleYordanov, A., Karaivanov, M., Ivanov, I., Kostov, S., Todorova, V., Iliev, I., Tzoneva, E., & Strateva, D. (2023). Squamotransitional Cell Carcinoma of the Uterine Cervix with Ovarian Metastasis and Benign Brenner Tumor: A Case Report. Reports, 6(4), 54. https://doi.org/10.3390/reports6040054







